Abstract
Purpose
Depression symptoms are common among patients with lung cancer; however, longitudinal changes and their impact on survival are understudied.
Methods
This was a prospective, observational study from the Cancer Care Outcomes Research and Surveillance Consortium from five US geographically defined regions from September 2003 through December 2005. Patients enrolled within 3 months of their lung cancer diagnosis were eligible. The eight-item Center for Epidemiologic Studies Depression scale was administered at diagnosis and 12 months’ follow-up. The main outcome was survival, which was evaluated using Kaplan-Meyer curves and adjusted Cox proportional hazards modeling.
Results
Among 1,790 participants, 681 (38%) had depression symptoms at baseline and an additional 105 (14%) developed new-onset depression symptoms during treatment. At baseline, depression symptoms were associated with increased mortality (hazard ratio [HR], 1.17; 95% CI, 1.03 to 1.32; P = .01). Participants were classified into the following four groups based on longitudinal changes in depression symptoms from baseline to follow-up: never depression symptoms (n = 640), new-onset depression symptoms (n = 105), depression symptom remission (n = 156), and persistent depression symptoms (n = 254) and HRs were calculated. Using the never-depression symptoms group as a reference group, HRs were as follows: new-onset depression symptoms, 1.50 (95% CI, 1.12 to 2.01; P = .006); depression symptom remission, 1.02 (95% CI, 0.79 to 1.31; P = .89), and persistent depression symptoms, 1.42 (95% CI, 1.15 to 1.75; P = .001). At baseline, depression symptoms were associated with increased mortality among participants with early-stage disease (stages I and II; HR, 1.61; 95% CI, 1.26 to 2.04), but not late-stage disease (stages III and IV; HR, 1.05; 95% CI, 0.91 to 1.22). At follow-up, depression symptoms were associated with increased mortality among participants with early-stage disease (HR, 1.71; 95% CI, 1.27 to 2.31) and those with late-stage disease (HR, 1.32; 95% CI, 1.04 to 1.69).
Conclusion
Among patients with lung cancer, longitudinal changes in depression symptoms are associated with differences in mortality, particularly among patients with early-stage disease. Symptom remission is associated with a similar mortality rate as never having had depression.
INTRODUCTION
A life-threatening diagnosis such as cancer evokes distress in many patients and may result in depression symptoms or clinical depression.1,2 Additional stressors contribute, including the effects of chemotherapeutic agents, surgical procedures, radiotherapy, and the consequences of physical symptoms and paraneoplastic syndromes. Rates of depression vary by diagnostic criteria, but among patients with lung cancer, the prevalence of major depressive disorder ranges from 5% to 13%, whereas up to 44% of patients with lung cancer may experience depression symptoms. Both estimates are consistently higher than those associated with other cancers types.3-6
Among patients with cancer, more severe and persistent depression symptoms are associated with prolonged hospital stays, worse treatment adherence, lower quality of life (QOL), physical distress and pain, and increased desire for hastened death.7-9 Depression may amplify physical symptoms and interfere with effective coping during treatment.10,11 Most important, depression present at cancer diagnosis is associated with increased mortality among patients with lung cancer.12-15
Recognizing the negative consequences of psychological distress, professional oncologic societies have begun developing guidelines regarding the assessments and care of patients with cancer who are experiencing distress.9,16,17 Guidelines include periodic assessments across the trajectory of cancer care, and institutional and community resources for treatment. Integrated multicomponent, collaborative depression care is effective and improves QOL and role functioning in patients with cancer.18
Treating depression symptoms is recommended to improve QOL among patients with cancer.16 If decreased depression symptoms are associated with improved mortality as well, depression care may need to be considered as important as other adjuvant oncologic therapies. In addition, the high prevalence of persistent depression symptoms in patients with lung cancer during treatment affirms the need to better understand its impact on outcomes.19,20 In other cancer populations without lung cancer, outcomes have been inconsistent. Among patients with breast cancer, health-related QOL and psychological distress at follow-up had no impact on survival.21 In a mixed group of patients with cancer who were assessed during survivorship, depression symptoms were associated with increased mortality.22 Overall, describing the impact of depression symptoms on outcomes of patients with lung cancer may support the development and implementation of high-quality depression care.
METHODS
Data were used from the National Cancer Institute-funded Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium, a prospective, observational, national study of practices and outcomes for patients with newly diagnosed lung and colorectal cancer. The total lung cancer cohort was composed of 5,150 participants. The consortium enrolled patients from five geographically defined regions, five integrated health-care delivery systems in the National Cancer Institute -funded Cancer Research Network, or 15 Veterans Affairs (VA) health-care systems from September 2003 through December 2005. CanCORS study methods have been described.23,24 This study was approved by the institutional review boards at the VA-Portland Health Care System and the VA-Puget Sound Health Care System.
Cohort Selection
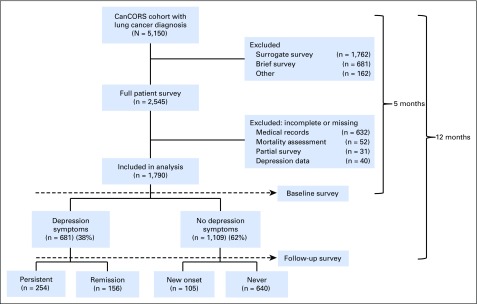
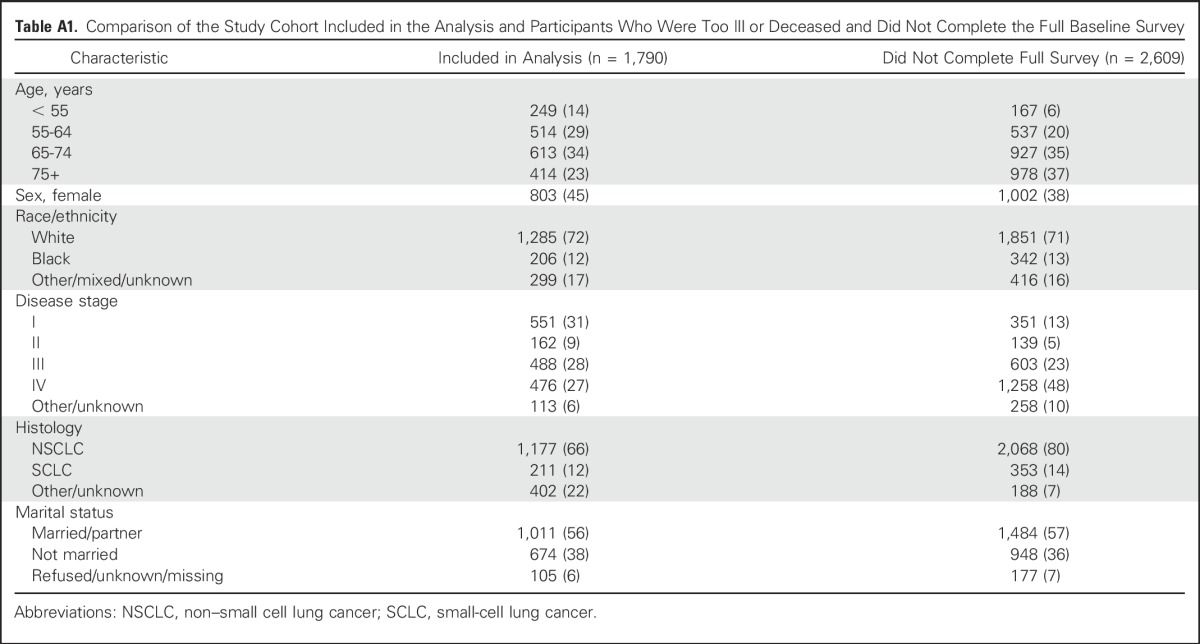
Patients were 21 years of age or older with newly diagnosed invasive non–small cell or small-cell lung cancer were eligible if they were identified within 3 months of cancer diagnosis. Several sites oversampled black, Hispanic, and Asian or Pacific Islander patients to increase inclusivity. Participants completed a baseline and follow-up survey via telephone approximately 5 and 12 months after cancer diagnosis, respectively. CanCORS enrolled a demographically and clinically representative cohort, reflective of newly diagnosed patients with lung cancer in all SEER regions.25 The response rate was 49% and the cooperation rate was 59%.26 Differences between responders and nonresponders have been described, as have methods to mitigate nonresponse bias.23,27 For this analysis, CanCORS participants who completed the Center for Epidemiologic Studies Depression (CES-D) scale and had medical records available for review were included. Between the time of case ascertainment and baseline survey, some participants were deceased or too ill to complete full patient surveys that included the CES-D scale; therefore, these participants were excluded (Fig 1; Appendix Table A1, online only).
Fig 1.
CONSORT diagram showing the flow of patients from enrollment to baseline and follow-up survey based on depression symptoms in the CanCORS study. CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
Variables and Measures
Baseline and follow-up patient surveys, physician surveys, and data from medical records and cancer registries were abstracted. Research staff were able to contact hospitals and physicians for missing information. Validated scales were used from published surveys.23,24 A brief, eight-item version of the CES-D scale28,29 was administered to measure depression symptoms at baseline and follow-up. The CES-D shows very high internal consistency and adequate test-retest repeatability.30 The CES-D has good factor structure and internal consistency in patients with cancer,31 community-dwelling older adults,28 and ethnically diverse adults.32 Scores ≥4 on the CES-D indicate elevated depression symptoms.33-35 The CES-D includes somatic complaint factors that are not typical lung cancer symptoms.28,36 Participants were classified based on longitudinal changes in depression symptoms from baseline to follow-up. Those without depression symptoms (labeled “never”), those with new depression symptoms at follow-up (labeled “new onset”), those with depression symptom remission at follow-up (labeled “remission”), and those with persistent depression symptoms from baseline to follow-up (labeled “persistent”).
Patient-reported psychiatric history was collected at the time of the baseline patient survey. Patients responded yes or no to the following: “Have you ever suffered from depression or any other emotional, nervous, or psychiatric problems?” Patients who answered yes were asked a follow-up question: “Was it before or after you were diagnosed with lung cancer?” Possible responses included before, after, or both. Vital status was collected from established sources and the end date of vital status query was April 2012. Further vital status ascertainment information is available in the Appendix.
Covariates
Covariates determined at the baseline survey included age, sex, race/ethnicity, lung cancer stage and histology at diagnosis, income, education, marital status, smoking and alcohol use, lung cancer therapies received, and comorbidities. Stage at diagnosis was determined using collaborative staging when possible, or from registry or medical record review. Comorbidities were determined by using the Adult Comorbidity Evaluation-27 (ACE-27) index. The ACE-27 is a 27-item, validated comorbidity index for use in patients with cancer.37,38
Statistical Analysis
Descriptive statistics at the time of the baseline survey summarized participants’ demographics categorized by depression symptoms status. At baseline, participants were categorized as having depression symptoms or not having symptoms, based on CES-D scores. At follow-up, participants were categorized by the longitudinal changes in their depression symptoms from baseline and follow-up CES-D scores, and were classified as never, new-onset, remission, or persistent depression symptoms. Item nonresponse rate was negligible across all variables. Incidence of death by baseline and follow-up depression symptoms status, per 100-person years, was calculated as the number of deaths divided by the total number of person-years at risk for death.
The primary outcome was survival measured from the date of the initial baseline survey until the date of death or censoring. To eliminate immortal time, survival for follow-up analyses was measured from the date of the follow-up survey until the date of death or censoring. Hazard ratios, 95% CIs, and P values are reported. To compare participants’ mean CES-D scores, t tests with equal variance were used. The overall drop-out rate at follow-up, not including deaths, was < 10% and not significantly different between groups. Participants without depression symptoms at baseline and never participants were used as reference groups for baseline and follow-up analyses, respectively. Cox’s proportional-hazards regression models were fitted to obtain hazard ratios and corresponding CIs. Regression models were adjusted for age, sex, race/ethnicity, cancer stage and histology at diagnosis, income, education, marital status, smoking and alcohol use, and ACE-27 index. Stage-specific regression models were also adjusted for receipt of National Comprehensive Cancer Network–recommended stage-specific lung cancer treatments.39 The interaction effect between stage and depression symptoms was similarly explored. Psychiatric history was used to dichotomize participants (yes/no) with depression symptoms at baseline; regression models are presented using participants with depression symptoms and without a psychiatric history as a reference group.
Several sensitivity analyses for survival models were conducted. These adjusted for time from cancer diagnosis to baseline or follow-up survey completion; excluded somatic items (everything was effort, sleep was restless, could not get going) from CES-D scoring; and considered CES-D scores as a continuous variable, which did not affect results. Survival functions for baseline or follow-up analyses were estimated from the date of the baseline survey or the date of the follow-up survey, respectively, until the date of death, using the Kaplan-Meier method. All analyses were performed using Stata version 14 (StataCorp, College Station, TX) and two-sided statistical significance was defined as a resultant P value of .05 or less.
RESULTS
Among 1,790 participants who completed the baseline survey and CES-D, 57% were older than 65 years, 45% were female, 72% were white, 29% were current tobacco users, and 15% received care at VA health-care systems (Table 1). Considering tumor characteristics, 66% of participants were diagnosed with non–small cell lung cancer (NSCLC) and 47% of these participants had early-stage disease (stages I and II). Overall, 681 (38%) participants had depression symptoms at baseline and 254 of these participants (62%) still alive remained persistently depressed at follow-up. At follow-up, 105 participants (14%) had new-onset depression symptoms. Among all participants, 44% had depression symptoms at some point during the study. Among participants with depression symptoms at baseline, 288 (42%) reported a prior psychiatric history and, among these, 179 (62%), 75 (26%), and 34 (12%) participants reported problems before, both, or after cancer diagnosis, respectively.
Table 1.
Participant Characteristics at Baseline and Follow-up by Depression Symptoms

Baseline
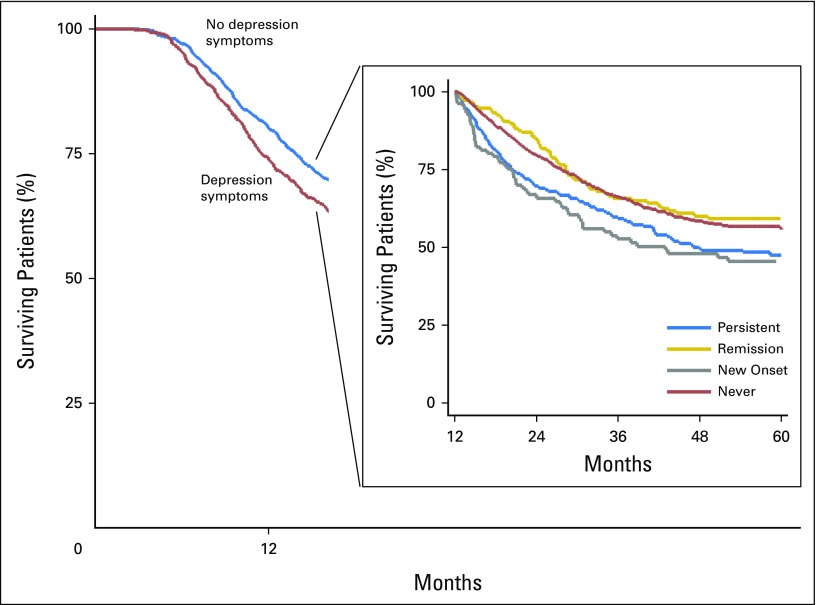
The incidence of death was 27.0 per 100 person-years (95% CI, 25.1 to 29.1) for participants without depression symptoms and 34.2 per 100 person-years (95% CI, 31.3 to 37.4) for participants with depression symptoms (Table 2). Depression symptoms were associated with increased mortality (adjusted HR [AdjHR], 1.17; 95% CI, 1.03 to 1.32; P = .01; Table 3). Median estimates of survival were 797 days in the no-depression symptoms group and 604 days in the depression symptoms group (difference, 193 days; Fig 2). There was a significant interaction effect between stage and depression symptoms (P = .003). Depression symptoms were associated with increased mortality among participants with early-stage disease (stages I and II; AdjHR, 1.61; 95% CI, 1.26 to 2.04; P < .001) but not among those with late-stage disease (stages III and IV) (AdjHR, 1.05; 95% CI, 0.91 to 1.22; P = .47). Results were comparable when analyses were restricted to participants with NSCLC and early-stage disease (AdjHR, 1.49; 95% CI, 1.13 to 1.97; P = .005) or late-stage disease (AdjHR, 1.05; 95% CI, 0.86 to 1.29; P = .63). Among participants with depression symptoms, prior psychiatric history was not associated with increased mortality (AdjHR, 1.1.2; 95% CI, 0.93 to 1.35; P = .25).
Table 2.
Incidence of Death Based on Depression Symptoms Status
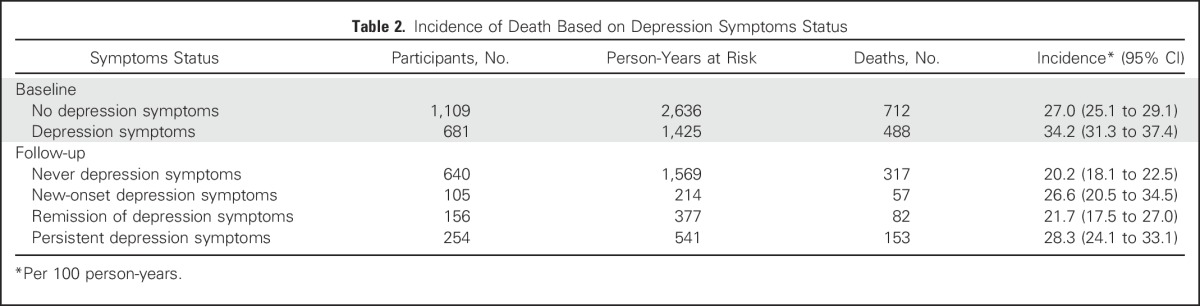
Table 3.
Hazard Ratios Based on Depression Symptoms Status
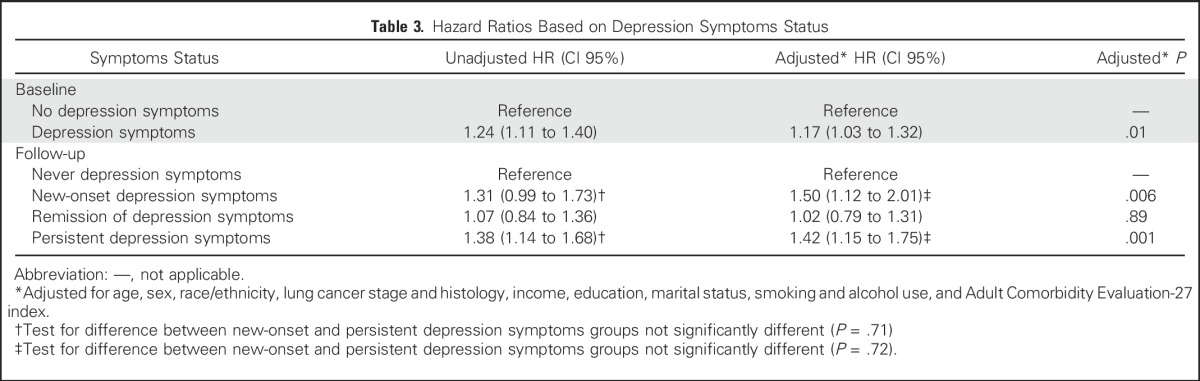
Fig 2.
Kaplan-Meier estimates of survival based on depression status. Survival was calculated from baseline survey to the time of death, if it occurred during the study period, or to the time of data censoring. The difference in median estimates of survival between the no depression symptoms group and the depression symptoms group was 193 days. Depression symptoms were a significant predictor of mortality (hazard ratio for death in the depression symptoms group, 1.17; 95% CI, 1.03 to 1.32) after adjustment for age, sex, race/ethnicity, lung cancer stage and histology at diagnosis, income, education, marital status, smoking and alcohol use, and Adult Comorbidity Evaluation-27 (ACE-27) index. (Insert): Survival was calculated from follow-up survey to the time of death, if it occurred during the study period, or to the time of data censoring. Median estimates of survival from cancer diagnosis were 896 days in the never depression symptoms group, 889 days in the remission of depression symptoms group, 746 days in the new-onset depression symptoms group, and 778 days in the persistent depression symptoms group. At follow-up, the difference in median survival between depression symptoms (the new-onset and persistent groups) and no depression symptoms groups (includes the never and remission groups) was 130 days. Persistent depression symptoms (hazard ratio for death 1.42; 95% CI, 1.15 to 1.75) and new-onset depression symptoms at follow-up (hazard ratio for death, 1.50; 95% CI, 1.12 to 2.01) were significant predictors of mortality after adjustment for age, sex, race/ethnicity, income, education, marital status, smoking and alcohol use, cancer stage and histology, and ACE-27 index.
Follow-Up
The incidence of death was 20.2 per 100 person-years (95% CI, 18.1 to 22.5) for never participants, 26.6 per 100 person-years (95% CI, 20.5 to 34.5) for new-onset participants, 21.7 per 100 person-years (95% CI, 17.5 to 27.0) for remission participants, and 28.3 per 100 person-years (95% CI, 24.1 to 33.1) for persistent participants at follow-up (Table 2). At baseline, persistent participants had a mean CES-D score (5.9; standard deviation [SD], 1.4) which was significantly different than that of remission participants (5.2; SD, 1.2; P < .001). At follow-up, persistent participants (5.8; SD, 1.4) had a mean CES-D score that was significantly different than that of new-onset participants (5.1; SD, 1.3; P < .001).
Using never participants as a reference group, among new-onset participants and persistent participants, there was an increased risk of mortality (AdjHR, 1.50; 95% CI, 1.12 to 2.01; P = .006; and AdjHR, 1.42; 95% CI, 1.15 to 1.75; P = .001, respectively). Among remission participants, there was no associated increased risk of mortality (AdjHR, 1.02; 95% CI, 0.79 to 1.31; P = .89). The adjusted HRs between the new-onset and persistent groups were not significantly different (P = .72; Table 3). The difference at follow-up in median survival between the depression symptoms (includes the new-onset and persistent groups) and no-depression symptoms (includes the never and remission groups) groups was 130 days (Fig 2). Depression symptoms at follow-up (includes the new-onset and persistent groups) was associated with increased mortality among both participants with early-stage disease (AdjHR, 1.71; 95% CI, 1.27 to 2.31; P < .001) and those with late-stage disease (AdjHR, 1.32; 95% CI, 1.04 to 1.69; P = .025). Results were comparable when analyses were restricted to participants with NSCLC histology and early-stage disease (AdjHR,1.50; 95% CI, 1.07 to 2.12; P = .02) or late-stage disease (AdjHR, 1.43; 95% CI, 1.04 to 1.99; P = .03).
DISCUSSION
Among patients with lung cancer who received treatment in multiple care settings, depression symptoms were common around the time of cancer diagnosis and were often persistent during treatment. Depression symptoms present at the time of cancer diagnosis were associated with increased mortality, with the predominant effect seen among patients with early-stage disease. Presence of a prior psychiatric history was not associated with increased mortality among those with depression symptoms at baseline. At follow-up, new-onset or persistent depression symptoms were associated with increased mortality. Importantly, remission of depression symptoms at follow-up was associated with comparable mortality as never having had depression symptoms. These results provide further evidence that depression symptoms are associated with increased mortality and that longitudinal changes are significant.
Depression is the most common psychologic symptom in patients with cancer,40 and patients with lung cancer are at particularly high risk.5,41 Some patients experience transient symptoms as an initial reaction to their diagnosis,42 whereas others experience persistent symptoms for years during survivorship.19,20,43,44 Approximately half of the study cohort experienced depression symptoms at some point during the study and symptoms persisted in most patients. In other cancer populations, persistent symptoms are associated with poor adherence to anticancer treatments,7,45 and significant cognitive46 and functional impairment,47,48 which may contribute to increased mortality. Receipt of guideline-recommended lung cancer treatment did not significantly alter study results. Cognitive and functional impairments are aspects of QOL that likely interact with the depression-mortality causal pathway in ways not fully elucidated or adjusted for in study analyses. Survival differences may also be related to additional comorbidities and outcomes associated with depression symptoms but not cancer, such as suicide.
Participants who experienced remission of depression symptoms at follow-up had a similar mortality rate as those who were never depressed. The potential reversibility of the negative effects of depression symptoms deserves further study; if confirmed, this would support the importance of effective mental health treatment. Remission may be achieved with mental health treatment, although studies of individual pharmacologic and psychotherapeutic treatments in patients with cancer have yielded mixed outcomes.2,49,50 More recent studies of integrated, collaborative, multicomponent depression interventions for cancer care have impressive outcomes. In a study of patients with lung cancer who had major depressive disorder, a multicomponent intervention including evidence-based psychologic therapies such as behavioral activation was associated with significantly improved depression scores and remission rates. This intervention led to significantly better QOL, role functioning, and perceived quality of care compared with usual depression care.18 In a larger study of this intervention in patients with various cancer types, there was an absolute difference in depression remission of 45% at 24 weeks compared with usual care.51 There was no survival benefit conferred with this intervention in either study. Overall, there is a paucity of evidence that psychologic or depression treatment prolongs survival in patients with cancer52,53; however, the limited number of high-quality intervention studies has been noted.50,54 There is growing recognition that psychosocial support is an essential component of comprehensive cancer care, yet it remains uncertain if effective depression treatment can prolong survival.16,39,50
Recognizing the substantial impact of depression on adults with cancer, ASCO has developed a set of practice guidelines for all professional health-care providers.16 These guidelines recommend systematic depression screening across the trajectory of cancer care. If screening instruments indicate the presence of moderate or severe symptoms, the American Society of Clinical Oncology recommends patients undergo further diagnostic assessments to identify the nature and extent of disease. Regular depression assessments throughout the continuum of cancer care, including survivorship, may help providers identify patients who may most benefit from treatment. The increased mortality rate among patients with lung cancer who had depression symptoms was observed predominantly in patients with early-stage disease. Because these patients are the ones most likely to be cured, attention should focus on providing support posttreatment into survivorship. Patients report unmet emotional needs and a desire for psychologic support during and after completion of cancer treatment.55,56,57 Early detection and prompt treatment can promote remission, prevent relapse, and reduce the emotional and financial burden of depression. This approach may diminish the prevalence and persistence of depression symptoms, and decrease the associated negative effects of this debilitating disease.
This study has several strengths, including the large sample size, prospective national ascertainment of patients, oversampling of nonwhite races/ethnicities, and inclusion of patients from diverse health-care settings. The unique inclusion of surveys conducted at two time points, soon after and at 1-year follow-up from cancer diagnosis, allowed an assessment of how depression symptoms changed over time. There are limitations. Patients were surveyed soon after diagnosis; however, some died or were too ill to complete surveys, which may limit generalizability. Exclusion of these patients likely underestimates the prevalence of depression symptoms because low performance status19 and advanced-stage disease49 are both associated with depression in patients with cancer. Although validated and used extensively in research, the use of a brief depression screen may have led to misclassification of psychologic status for some patients. Gold standard interview-based assessments for major depressive disorder, in general, report a lower prevalence of illness than found on screening instruments. We did not have complete information about patients’ history or their family history of psychiatric disorders before study entry. The increased mortality rate among depressed patients during treatment could have been accounted for by differences in cancer progression, although cancer characteristics (eg, stage and histology) and cancer treatments received, most likely to predict cancer activity, were adjusted for in analyses. In addition, a meta-analysis found no evidence that depression predicts progression in patients with cancer.58 Among included studies, there was no evidence of an effect of illness severity, overlapping symptoms, advanced stage, or differences in effect sizes depending on whether studies controlled for cancer-related confounders.58 Other possible mechanisms for increased mortality rates that are associated with depression, such as suicide, were not available. Our results cannot prove causation and it is possible patients’ anticipated mortality is associated with depression symptoms.
In conclusion, depression symptoms are common among patients with lung cancer at cancer diagnosis and often persist during cancer treatment. Longitudinal changes in depression symptoms are associated with significant differences in patient mortality rates. Depression remission was associated with similar mortality as never having had depression. These results underscore the importance of integrated systems of depression treatment of patients with cancer as part of comprehensive cancer care. Research is needed to explore depression treatment delivery and determine whether treatment can actually improve survival.
ACKNOWLEDGMENT
This work was supported by Grant No. SB-164388-N from the American Lung Association (C.G.S.). D.R.S. was supported by Grant No. 5KL2TR000152-08 funded through the National Center for Advancing Translational Sciences of the National Institutes of Health and National Center for Research Resources through the Oregon Health & Science University Oregon Clinical & Translational Research Institute Grants No. UL1TR000128 and 1K07CA190706-01A1. C.G.S. was supported by Veterans Affairs (VA) Health Services Research and Development Career Development Awards No. CDA 09-025 and CDP 11-227. D.R.S., L.G., and C.G.S. are supported by resources from the Portland VA Portland Health Care System, Oregon. The work of the CanCORS consortium was supported by Grant No. U01 CA093344 from the National Cancer Institute to the Statistical Coordinating Center and the National Cancer Institute-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network, Grant No. U01 CA093332; Harvard Medical School/Northern California Cancer Center, Grant No. U01 CA093324; RAND/UCLA, Grant No. U01 CA093348; University of Alabama at Birmingham, Grant No. U01 CA093329; University of Iowa, Grant No. U01 CA093339; and University of North Carolina, Grant No. U01 CA 093326) and by Department of Veteran's Affairs Grant No. HSRD CRS-02-164 to the Durham VA Medical Center. The Department of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US Government.
Appendix
Supplemental Vital Status Ascertainment Information
Vital status was collected from these sources: baseline and follow-up survey, medical record abstraction, database updates, Social Security Death Index, or the National Death Index. Death was determined based on documentation of known date of death or date assumed alive based on either no death reported in national records or date of last vital status in plan records for managed care sites (ie, a patient encounter). Local adjudication of vital status occurred at each site, using a source-specific algorithm. Vital status data were matched among data sources using the participants’ social security number, sex, and date of birth.
Table A1.
Comparison of the Study Cohort Included in the Analysis and Participants Who Were Too Ill or Deceased and Did Not Complete the Full Baseline Survey
Footnotes
Listen to the podcast by Dr Duberstein at www.jco.org/podcasts
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Donald R. Sullivan, Dawn Provenzale, Christopher G. Slatore
Financial support: Christopher G. Slatore
Provision of study materials or patients: Christopher G. Slatore
Collection and assembly of data: Donald R. Sullivan, Christopher W. Forsberg, David H. Au, Christopher G. Slatore
Data analysis and interpretation: Donald R. Sullivan, Christopher W. Forsberg, Linda Ganzini, David H. Au, Michael K. Gould, Christopher G. Slatore
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Longitudinal Changes in Depression Symptoms and Survival Among Patients With Lung Cancer: A National Cohort Assessment
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Donald R. Sullivan
No relationship to disclose
Christopher W. Forsberg
No relationship to disclose
Linda Ganzini
No relationship to disclose
David H. Au
Consulting or Advisory Role: Novartis
Michael K. Gould
Honoraria: UpToDate
Dawn Provenzale
No relationship to disclose
Christopher G. Slatore
No relationship to disclose
REFERENCES
- 1.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 2.Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: A systematic review. Ann Oncol. 2013;24:895–900. doi: 10.1093/annonc/mds575. [DOI] [PubMed] [Google Scholar]
- 3.Brown Johnson CG, Brodsky JL, Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32:59–73. doi: 10.1080/07347332.2013.855963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16:264–269. doi: 10.1016/j.ejon.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–3991. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 6.Linden W, Vodermaier A, Mackenzie R, et al. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 7. Li M, Boquiren V, Lo C, et al: Depression and anxiety in supportive oncology. In Davis M et al (eds): Supportive Oncology (ed 1). Philadelphia, PA, Elsevier, 2011, pp 528-540. [Google Scholar]
- 8.Brown LF, Kroenke K, Theobald DE, et al. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19:734–741. doi: 10.1002/pon.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazenby M, Ercolano E, Grant M, et al. Supporting commission on cancer-mandated psychosocial distress screening with implementation strategies. J Oncol Pract. 2015;11:e413–e420. doi: 10.1200/JOP.2014.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mystakidou K, Tsilika E, Parpa E, et al. Psychological distress of patients with advanced cancer: Influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29:400–405. doi: 10.1097/00002820-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Passik SD, Dugan W, McDonald MV, et al. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol) 2014;26:25–31. doi: 10.1016/j.clon.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Pirl WF, Temel JS, Billings A, et al. Depression after diagnosis of advanced non-small cell lung cancer and survival: A pilot study. Psychosomatics. 2008;49:218–224. doi: 10.1176/appi.psy.49.3.218. [DOI] [PubMed] [Google Scholar]
- 14.Buccheri G. Depressive reactions to lung cancer are common and often followed by a poor outcome. Eur Respir J. 1998;11:173–178. doi: 10.1183/09031936.98.11010173. [DOI] [PubMed] [Google Scholar]
- 15.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94:2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: Screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20:e233–e246. doi: 10.3747/co.20.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker J, Hansen CH, Martin P, et al. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): A multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15:1168–1176. doi: 10.1016/S1470-2045(14)70343-2. [DOI] [PubMed] [Google Scholar]
- 19.Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 20.Lo C, Zimmermann C, Rydall A, et al. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. J Clin Oncol. 2010;28:3084–3089. doi: 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ, Ennis M, Bordeleau LJ, et al. Health-related quality of life and psychosocial status in breast cancer prognosis: Analysis of multiple variables. J Clin Oncol. 2004;22:4184–4192. doi: 10.1200/JCO.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 22.Mols F, Husson O, Roukema JA, et al. Depressive symptoms are a risk factor for all-cause mortality: Results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. 2013;7:484–492. doi: 10.1007/s11764-013-0286-6. [DOI] [PubMed] [Google Scholar]
- 23.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients’ experience and outcomes: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 24.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The cancer care outcomes research and surveillance consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 25. doi: 10.1097/MLR.0b013e318222a711. Catalano PJ, Ayanian JZ, Weeks JC, et al: Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the Surveillance, Epidemiology, and End Results program. Med Care 51:e9-e15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Groves R, Dillma D, Eltinge J, et al. Survey Nonresponse. Wiley-Interscience, New York, 2001. [Google Scholar]
- 27.Huskamp HA, Keating NL, Malin JL, et al. Discussions with physicians about hospice among patients with metastatic lung cancer. Arch Intern Med. 2009;169:954–962. doi: 10.1001/archinternmed.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 29. Melchior L, Huba G, Brown V, et al: A short depression index for women. Educ Psychol Meas 53:1117-1125, 1993. [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Bardwell WA, Natarajan L, Dimsdale JE, et al. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol. 2006;24:2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- 32.Wight RG, Aneshensel CS, Barrett C, et al. Urban neighbourhood unemployment history and depressive symptoms over time among late middle age and older adults. J Epidemiol Community Health. 2013;67:153–158. doi: 10.1136/jech-2012-201537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: Prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34:623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 34. Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study. Ann Arbor, MI, HRS/AHEAD, 2000. [Google Scholar]
- 35.Zivin K, Pirraglia PA, McCammon RJ, et al. Trends in depressive symptom burden among older adults in the United States from 1998 to 2008. J Gen Intern Med. 2013;28:1611–1619. doi: 10.1007/s11606-013-2533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foelker GA, Jr, Shewchuk RM. Somatic complaints and the CES-D. J Am Geriatr Soc. 1992;40:259–262. doi: 10.1111/j.1532-5415.1992.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 37. Bang D, Piccirillo JF, Littenberg B: The Adult Comorbidity Evaluation-27 Test–a new comorbidity index for patients with cancer. J Clin Oncol 19:abstr 1701, 2000 (suppl) [Google Scholar]
- 38.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 39. National Comprehensive Cancer Network: NCCN guidelines updates: Non-small cell lung cancer (Version 3.2016). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed May 15, 2016. [Google Scholar]
- 40.Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary-care research. Psychooncology. 2011;20:572–584. doi: 10.1002/pon.1943. [DOI] [PubMed] [Google Scholar]
- 41.Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Montazeri A, Milroy R, Hole D, et al. Anxiety and depression in patients with lung cancer before and after diagnosis: Findings from a population in Glasgow, Scotland. J Epidemiol Community Health. 1998;52:203–204. doi: 10.1136/jech.52.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyes AW, Girgis A, D’Este CA, et al. Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: A population-based longitudinal study. J Clin Oncol. 2013;31:2724–2729. doi: 10.1200/JCO.2012.44.7540. [DOI] [PubMed] [Google Scholar]
- 44.Brant JM, Beck S, Dudley WN, et al. Symptom trajectories in posttreatment cancer survivors. Cancer Nurs. 2011;34:67–77. doi: 10.1097/NCC.0b013e3181f04ae9. [DOI] [PubMed] [Google Scholar]
- 45.Colleoni M, Mandala M, Peruzzotti G, et al. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356:1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 46.Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Miller IW, Keitner GI, Schatzberg AF, et al. The treatment of chronic depression, part 3: Psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59:608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- 48. Bakish D: New standard of depression treatment: Remission and full recovery. J Clin Psychiatry 62:5-9, 2001 (Suppl 26) [PubMed] [Google Scholar]
- 49.Rodin G, Lloyd N, Katz M, et al. The treatment of depression in cancer patients: A systematic review. Support Care Cancer. 2007;15:123–136. doi: 10.1007/s00520-006-0145-3. [DOI] [PubMed] [Google Scholar]
- 50.Walker J, Sawhney A, Hansen CH, et al. Treatment of depression in adults with cancer: A systematic review of randomized controlled trials. Psychol Med. 2014;44:897–907. doi: 10.1017/S0033291713001372. [DOI] [PubMed] [Google Scholar]
- 51.Sharpe M, Walker J, Holm Hansen C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet. 2014;384:1099–1108. doi: 10.1016/S0140-6736(14)61231-9. [DOI] [PubMed] [Google Scholar]
- 52.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 53.Chow E, Tsao MN, Harth T. Does psychosocial intervention improve survival in cancer? A meta-analysis. Palliat Med. 2004;18:25–31. doi: 10.1191/0269216304pm842oa. [DOI] [PubMed] [Google Scholar]
- 54.Smedslund G, Ringdal GI. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. J Psychosom Res. 2004;57:123–131, discussion 133-135. doi: 10.1016/S0022-3999(03)00575-0. [DOI] [PubMed] [Google Scholar]
- 55.Institute of Medicine of the National Academies . Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 56.Merckaert I, Libert Y, Messin S, et al. Cancer patients’ desire for psychological support: Prevalence and implications for screening patients’ psychological needs. Psychooncology. 2010;19:141–149. doi: 10.1002/pon.1568. [DOI] [PubMed] [Google Scholar]
- 57.Harrison JD, Young JM, Price MA, et al. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117–1128. doi: 10.1007/s00520-009-0615-5. [DOI] [PubMed] [Google Scholar]
- 58.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]