Abstract
Aims
Low birth weight and low placental weight predict later coronary heart disease and hypertension. This has led to the hypothesis that these diseases are initiated by foetal programming, the process by which foetal malnutrition leads to permanent changes in the body in ways that lead to chronic disease in later life. Here we examine the association between body and placental size at birth and later chronic heart failure.
Methods and results
We identified 187 patients taking medications for chronic heart failure in a birth cohort of 13 345 people born in Helsinki, Finland during 1934–44. Chronic heart failure was associated with a small placental surface area. In people born with a placental area less than 225 cm2, the odds ratio for chronic heart failure was 1.7 (1.1–2.5), compared with people born with a placental area greater than 295 cm2. The risk of heart failure was further increased by rapid gain in body mass index after the age of 2 years, a path of growth known to be linked to insulin resistance in later life. In a simultaneous regression, low body mass index at 2 years and high body mass index at 11 years were each associated with chronic heart failure (P = 0.008 and 0.001, respectively).
Conclusion
Chronic heart failure in adult life may be initiated by impaired placental growth which adversely affects cardiac development. People born with a vulnerable heart are more likely to develop chronic heart failure if they become insulin resistant.
Keywords: Heart failure, Placenta, Type 2 diabetes, Maternal stature, Childhood growth
Introduction
Nearly five million people in the USA are being treated for chronic heart failure: some 500 000 new cases are diagnosed every year.1,2 Heart failure is the most common reason for hospitalization among elderly adults.1 It results from functional cardiac disorders that compromise the ability of the ventricle to fill with blood or eject it.1 The initial haemodynamic and neurohormonal responses to ventricular dysfunction are compensatory. Over time, however, they become maladaptive and lead to myocardial fibrosis and further myocardial dysfunction.1,2
Half of all cases of heart failure are the result of coronary heart disease.1 Type 2 diabetes predisposes to the disorder because it increases the risk of coronary heart disease and because it has adverse effects on the myocardium.3 There is now a body of evidence that coronary heart disease, type 2 diabetes, and hypertension originate through malnutrition during prenatal life. The disorders are more common in people who had low birth weight,4–7 which is usually the result of inadequate foetal nutrition.8 The association between the disorders and low birth weight has led to the hypothesis that the disorders are initiated by foetal programming. This is the process by which foetal malnutrition leads to permanent changes in the body's structure and function in ways that lead to chronic disease in later life.9 These changes include permanent alterations in organ structure, in glucose-insulin and lipid metabolism, and in hormonal feedback systems.
The placenta regulates the transport of nutrients from mother to baby.8 Low birth weight is generally correlated with low placental weight.10 Coronary heart disease is associated with low placental weight as well as low birth weight.11 This association could reflect the effects of foetal malnutrition on cardiac development or it could be the result of a reduced placental vasculature. The placenta receives 40% of the foetal cardiac output. The architecture of the placental vasculature has a major effect on loading the foetal heart. Excessive loading associated with a small placenta programs permanent changes in the developing myocardium.12–15
Usually weight is the only measurement of placental size recorded in birth records. Variations in the weight of the placenta at birth may reflect differences in either its surface area or its thickness. The surface area reflects the spread of nutrient exchange tissue across the inner wall of the uterus: its thickness represents the volume of tissue.16,17 In the Helsinki Birth Cohort, the records of each subject include not only the weight of the placenta but also two measures of the width of its surface, a maximal width, and a lesser one at right angles to it. These widths allow the placental surface area to be estimated. We have previously reported that a small placental surface area predicts hypertension in the offspring in later life. This association was strongest in people whose mothers were poorly nourished as indicated by short stature and low socioeconomic status.18
We have examined placental size among people taking medication for chronic heart failure. We hypothesized that small placental surface area at birth would be linked to chronic heart failure. Because the risk of coronary heart disease and hypertension associated with small placental size is further increased by rapid weight gain after birth, we have examined the effects of childhood weight gain on later heart failure.7,19
Methods
Study population
The Helsinki Birth Cohort comprises 13 345 men and women who were born between 1934 and 1944 in either the Helsinki University Central Hospital or the Helsinki City Maternity Hospital and who visited infant welfare clinics in the city. Most of them also went to school in the city. Details of the birth records, child welfare clinic records, and school health records have been described.19 The birth records included the baby's body size at birth, the mother's height and weight in late pregnancy, and the date of her last menstrual period. The records also included the weight of the placenta, together with the maximal width of its surface, and a lesser width measured at right angles to it. These widths were referred to as diameters and we have preserved this terminology. Assuming an elliptical surface, we estimated the surface area of the placenta as maximal × lesser diameter × π/4. Assuming a constant density, we estimated the thickness of the placenta as weight divided by area.
Socioeconomic status
Each of the birth records included the father's occupation which, using a classification from Statistics Finland, we classified as either middle class or manual worker. In order to obtain data on the people's own occupations in their adult lives, we accessed the 1980 census data, through Statistics Finland.
Chronic heart failure
Tracing of the cohort assembled from the birth and infant welfare records was made possible by the unique personal identification number assigned to all residents of Finland by 1971. One source of information on chronic disease in the cohort is the Social Insurance Institution's Register of people on medication for chronic disease. In Finland, the cost of medication, including drugs for chronic heart failure, type 2 diabetes, coronary heart disease, and hypertension, is partly reimbursed by the government. This is subject to the approval of a physician who reviews each case and writes a statement about the patient. The statement must describe the aetiology of chronic heart failure, its clinical presentation, and the clinical situation before the start of the treatment. In addition, the statement must describe the diagnostic criteria and a treatment plan based on good clinical practice. If the diagnosis is unclear, a second specialist opinion is required. The statements may be re-evaluated. For example, if medication for heart failure was initiated after a myocardial infarct, the need for medication is re-evaluated 6 months later. We do not have access to these individual case statements, and we are therefore unable to determine the aetiology of heart failure or assign our cases to New York Heart Association classes. The Ethics Committee at the National Public Health Institute, Finland approved our study.
Hospital admissions and deaths
We used the personal identification number to trace all the subjects who had been admitted to hospital or had died from 1971 onwards. All hospital discharges in Finland are recorded in the national hospital discharge register; all deaths are recorded in the national mortality register.
Statistical analysis
Because the date when reimbursement for medication began does not coincide with the clinical onset of chronic heart failure, we assessed associations with the prevalence of chronic heart failure using multiple logistic regression always adjusted for the year of birth and sex. The measurements of body size, placental and maternal size were analysed as continuous variables although presented in the tables as groups. Tests for interaction used the product of the variables being studied. We examined height, weight, and body mass index for every child at each birthday until 11 years of age.19 We converted each measurement to a Z-score. A Z-score represents the difference from the mean value for the whole cohort and is expressed in standard deviations. We used a Cox proportional hazards model to calculate the hazard ratios for death in people with chronic heart failure.
Results
We identified 187 people, 138 men and 49 women, who were taking medication for chronic heart failure. One hundred and seventy-six had been admitted to hospital on one or more occasion. The three most common discharge diagnoses were cardiac dysrhythmia, chronic heart failure, and myocardial infarction.
Size at birth
Table 1 shows odds ratios for chronic heart failure according to birth size. Chronic heart failure was not related to birth weight, length, body mass index (birth weight/length2), or the duration of gestation. Odds ratios tended to be higher in people with smaller head circumferences at birth (P = 0.09). Chronic heart failure was not related to the weight of the placenta or its maximal diameter (Table 1). Odds ratios increased, however, as the lesser diameter and area of the placenta decreased. There were no trends with placental thickness or with the ratio of the two diameters. The trends with placental size were similar in men and women.
Table 1.
Odds ratios for taking medication for chronic heart failure according to body size and placental size at birth
| Measurement | No. of cases/no. of subjects | Odds ratio (95% CI) |
|---|---|---|
| Birth weight (kg) | ||
| –2.5 | 6/452 | 1.0 (0.4–2.5) |
| –3.0 | 32/2083 | 1.2 (0.7–2.2) |
| –3.5 | 77/5336 | 1.1 (0.7–1.8) |
| –4.0 | 53/4203 | 0.9 (0.5–1.6) |
| >4.0 | 19/1271 | 1.0 (baseline) |
| P for trend | 0.3 | |
| Head circumference (cm) | ||
| –33 | 25/1789 | 1.4 (0.8–2.4) |
| –34 | 40/2781 | 1.4 (0.8–2.2) |
| –35 | 55/3495 | 1.4 (0.9–2.2) |
| –36 | 38/3019 | 1.0 (0.6–1.6) |
| >36 | 29/2123 | 1.0 (baseline) |
| P for trend | 0.09 | |
| Placental weight (g) | ||
| –550 | 39/3140 | 1.3 (0.8–2.1) |
| –650 | 74/4506 | 1.7 (1.0–2.6) |
| –750 | 53/4203 | 1.4 (0.8–2.2) |
| >750 | 24/2163 | 1.0 (baseline) |
| P for trend | 0.2 | |
| Maximal placental diameter (cm) | ||
| –18 | 68/4478 | 1.1 (0.7–1.6) |
| –19 | 26/2022 | 0.9 (0.5–1.5) |
| –20 | 50/3726 | 1.0 (0.6–1.4) |
| >20 | 43/3045 | 1.0 (baseline) |
| P for trend | 0.1 | |
| Lesser placental diameter (cm) | ||
| –15 | 58/3182 | 1.7 (1.0–2.6) |
| –17 | 58/4482 | 1.2 (0.7–1.8) |
| –18 | 43/3050 | 1.3 (0.8–2.1) |
| >18 | 28/2566 | 1.0 (baseline) |
| P for trend | 0.04 | |
| Placental area (cm2) | ||
| –225 | 60/3122 | 1.7 (1.1–2.5) |
| –255 | 42/3647 | 1.0 (0.6–1.5) |
| –295 | 48/3267 | 1.3 (0.8–1.9) |
| >295 | 37/3229 | 1.0 (baseline) |
| P for trend | 0.05 | |
CI, confidence interval.
Mother's body size
The mean age of the mothers at delivery was 28(5) years. Forty-nine percent of them were primiparous. Their median height was 160 cm and their median body mass index in late pregnancy was 26 kg/m2. The heights and weights of all except 16 mothers of people with chronic heart failure were recorded. Chronic heart failure was not related to the mother's age, parity, height, weight, or body mass index. As in previous analyses of the effects of placental size, we divided the mothers into two groups around their median height.18 Table 2 shows that the trends in chronic heart failure with the lesser placental diameter and area were confined to people whose mothers' heights were below the median. Among these people, odds ratios tended to rise with increasing mother's body mass index, though the trend was not statistically significant (P = 0.06).
Table 2.
Odds ratios for taking medication for chronic heart failure according to placental size and maternal height
| Placental measurement | Mother's height (cm) |
|
|---|---|---|
| ≤160, odds ratio (95% CI) | >160, odds ratio (95% CI) | |
| Weight (g) | ||
| –550 | 1.2 (0.6–2.7) | 1.3 (0.5–2.9) |
| –650 | 1.7 (0.9–3.4) | 2.0 (1.0–4.2) |
| –750 | 1.6 (0.8–3.3) | 0.9 (0.4–2.0) |
| >750 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.4 | 0.1 |
| Maximal diameter (cm) | ||
| –18 | 1.3 (0.8–2.3) | 0.9 (0.5–1.6) |
| –19 | 1.0 (0.5–1.9) | 0.9 (0.4–1.9) |
| –20 | 1.3 (0.7–2.2) | 0.6 (0.3–1.1) |
| >20 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.1 | 0.7 |
| Lesser diameter (cm) | ||
| –15 | 2.5 (1.3–4.9) | 1.0 (0.5–2.2) |
| –17 | 1.3 (0.7–2.7) | 1.2 (0.6–2.4) |
| –18 | 2.0 (1.0–4.1) | 1.0 (0.5–2.1) |
| >18 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.05 | 0.4 |
| Area (cm2) | ||
| –225 | 2.3 (1.2–4.2) | 1.2 (0.6–2.4) |
| –255 | 1.1 (0.6–2.2) | 0.9 (0.5–1.9) |
| –295 | 1.8 (0.9–3.3) | 1.1 (0.6–2.1) |
| >295 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.05 | 0.5 |
CI, confidence interval.
Father's occupation
The fathers of 41 of the people with chronic heart failure were in middle class occupations and 135 were manual workers. The occupations of 11 fathers were not recorded. Table 3 shows that the associations between chronic heart failure and the lesser placental diameter and area were confined to people whose fathers were manual workers. In these people, chronic heart failure was also associated with low placental weight and a small maximal diameter. The trend with the maximal diameter became statistically non-significant (P= 0.4) in a simultaneous regression with the lesser placental diameter. Father's occupation and mother's height had separate effects so that the strongest trend with the lesser placental diameter was in the offspring of short mother's married to manual workers (odds ratio per cm decrease in lesser diameter = 1.13, 95% CI 1.02–1.26, P = 0.02).
Table 3.
Odds ratios for taking medication for chronic heart failure according to placental size and father's occupational status
| Placental measurement | Father's occupational status |
|
|---|---|---|
| Manual worker, odds ratio (95% CI) | Middle class, odds ratio (95% CI) | |
| Weight (g) | ||
| –550 | 1.7 (0.9–3.2) | 0.6 (0.2–1.6) |
| –650 | 1.9 (1.1–3.4) | 1.1 (0.5–2.5) |
| –750 | 1.6 (0.9–3.0) | 0.9 (0.3–2.2) |
| >750 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.04 | 0.3 |
| Maximal diameter (cm) | ||
| –18 | 1.3 (0.8–2.1) | 0.5 (0.2–1.1) |
| –19 | 1.2 (0.7–2.1) | 0.5 (0.2–1.5) |
| –20 | 1.1 (0.7–1.8) | 0.8 (0.4–1.6) |
| >20 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.02 | 0.1 |
| Lesser diameter (cm) | ||
| –15 | 2.8 (1.5–5.0) | 0.4 (0.2–1.1) |
| –17 | 1.5 (0.8–2.7) | 0.9 (0.4–1.9) |
| –18 | 2.2 (1.2–4.1) | 0.4 (0.1–1.1) |
| >18 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.004 | 0.5 |
| Area (cm2) | ||
| –225 | 2.5 (1.5–4.1) | 0.5 (0.2–1.2) |
| –255 | 1.1 (0.6–1.9) | 0.8 (0.4–1.7) |
| –295 | 1.8 (1.0–3.0) | 0.6 (0.3–1.5) |
| >295 | 1.1 (baseline) | 1.0 (baseline) |
| P for trend | 0.004 | 0.2 |
CI, confidence interval.
Post-natal growth
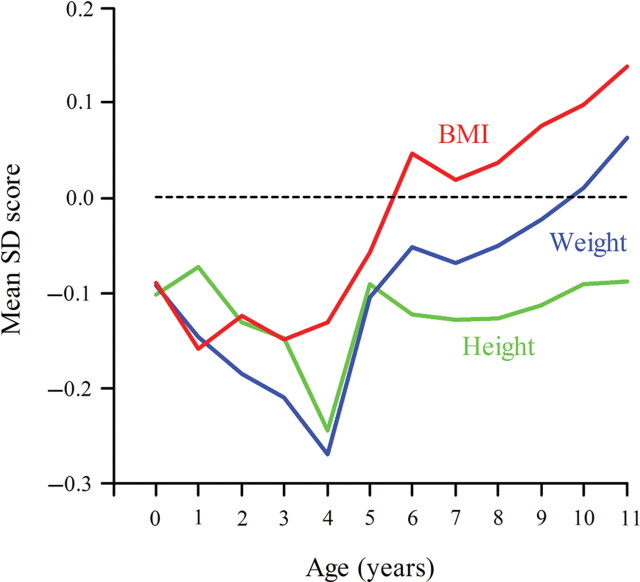
Figure 1 shows the growth of children who as adults had chronic heart failure. At birth their mean height, weight, and body mass index was below the average. Thereafter, it fell further below the average. After around 2 years of age, however, their mean weight and body mass index increased rapidly so that by 11 years both measures were above the average. At no age from birth to 11 years did weight or body mass index predict chronic heart failure in later life. Rather it was the change between 2 and 11 years that predicted the disease. In a simultaneous regression, chronic heart failure was associated with a low body mass index at 2 years and a high body mass index at 11 years (P = 0.008 and 0.001, respectively). These trends were similar for people with and without type 2 diabetes. Low body mass index at age 2 was strongly correlated with a small lesser placental diameter and area (P < 0.001 for both). We examined the combined effects of the lesser placental diameter and body mass index at 2 and 11 years. In a simultaneous regression, each was statistically significant (P = 0.02, 0.01, and 0.001).
Figure 1.
Mean standard deviation scores for height, weight, and body mass index (BMI) in the first 11 years after birth among children who had chronic heart failure as adults.
Type 2 diabetes, coronary heart disease, and hypertension
Ninety-two of the people taking medication for chronic heart failure were also taking medications for coronary heart disease; 55 were taking medications for type 2 diabetes mellitus; 49 were taking medications for hypertension. Table 4 shows the trends in odds ratios for chronic heart failure with placental size in people with and without type 2 diabetes. The trends with the lesser placental diameter and area were confined to people with diabetes (P for interaction = 0.05 for both). There were no similar interactions for coronary heart disease or hypertension. We examined the combined effects of type 2 diabetes and father's occupation. Among people with type 2 diabetes who were born into the families of manual workers, a 1 cm decrease in the lesser placental diameter was associated with a odds ratio for chronic heart failure of 1.28 (95% CI 1.11–1.48, P < 0.001). The association between rapid gain in body mass index after the age of 2 years and later chronic heart failure was similar in people with and without type 2 diabetes.
Table 4.
Odds ratios for taking medication for chronic heart failure according to placental size and type 2 diabetes in the offspring
| Placental measurement | Type 2 diabetes |
|
|---|---|---|
| Present, odds ratio (95% CI) | Absent, odds ratio (95% CI) | |
| Weight (g) | ||
| –550 | 2.5 (0.8–7.7) | 0.9 (0.5–1.7) |
| –650 | 2.7 (0.9–8.1) | 1.4 (0.9–2.1) |
| –750 | 2.1 (0.7–6.5) | 1.2 (0.7–2.1) |
| >750 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.08 | 1.0 |
| Maximal diameter (cm) | ||
| –18 | 1.8 (0.8–3.9) | 0.9 (0.6–1.4) |
| –19 | 1.1 (0.4–3.2) | 0.8 (0.5–1.4) |
| –20 | 1.7 (0.7–3.9) | 0.9 (0.5–1.4) |
| >20 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.1 | 0.7 |
| Lesser diameter (cm) | ||
| –15 | 2.9 (1.1–8.0) | 1.3 (0.7–2.2) |
| –17 | 1.8 (0.6–4.9) | 1.0 (0.6–1.7) |
| –18 | 2.0 (0.7–5.9) | 1.2 (0.7–2.1) |
| >18 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.01 | 0.8 |
| Area (cm2) | ||
| –225 | 2.4 (0.9–5.7) | 1.4 (0.9–2.2) |
| –255 | 2.1 (0.8–5.1) | 0.7 (0.4–1.2) |
| –295 | 1.8 (0.7–4.7) | 1.2 (0.7–1.9) |
| >295 | 1.0 (baseline) | 1.0 (baseline) |
| P for trend | 0.01 | 0.7 |
CI, confidence interval.
Adult socioeconomic status
Although chronic heart failure was associated with low socioeconomic status and income in adult life, the trends with the lesser placental diameter and area were little changed by adjustment for these variables. In a simultaneous regression, there were significant associations between chronic heart failure and low social class (P= 0.02), short lesser placental diameter (P = 0.02), low body mass index at age 2 years (P = 0.01) and high body mass index at 11 years (P < 0.001).
Mortality
Seventy-three of the 187 subjects with chronic heart failure had died. Twenty-five of these subjects also had type 2 diabetes. Compared to people with neither condition the hazard ratios for death were 1.12 (95% CI 0.93–1.36, P = 0.2) in those with type 2 diabetes, 2.45 (1.84–3.27, P < 0.001) in those with chronic heart failure, and 3.53 (2.38–5.26, P < 0.001) in those with both type 2 diabetes and chronic heart failure.
Discussion
We found that people taking medication for chronic heart failure were born with a small placental surface area and gained weight rapidly after 2 years of age (Table 1, Figure 1). The association with a small placental surface area was the result of reduced growth along the lesser axis of the surface. The effects of a reduced surface area were confined to people whose mother's were below the median height and whose father's were manual workers (Tables 2 and 3). They occurred in people who were also taking medication for type 2 diabetes, but not in people taking medication for hypertension (Table 4).
Placental surface area
Since the placental surface is more oval than circular, the birth records of each subject in the Helsinki Birth Cohort included two measures of the placental surface, a maximal diameter, and a lesser diameter at right angles to it.16,17 The length of the two diameters was highly correlated (correlation coefficient = 0.63). Only the length of the lesser diameter predicted chronic heart failure. We have previously examined these two diameters in pregnancies complicated by preeclampsia, a disorder that is initiated by impaired implantation and reduced placental growth.20 We found that preeclampsia was associated with a short lesser diameter but not with the maximal diameter. This finding led to the conclusion that growth of the placental surface is polarized, and that failure of growth along the major and minor axes may have different causes. The present study suggests that they have different functional consequences on the developing heart. We speculate that chronic heart failure may originate through impaired growth along the minor axis of the placental surface and consequent adverse effects on the developing heart.
Mother's height and father's occupation
We found that the effects of a short lesser diameter and small placental surface area on chronic heart failure were confined to people whose mothers' heights were below the median (Table 2). We have found a similar relationship with mother's height and the development of hypertension in the offspring.18 The strong effects of small placental size on chronic heart failure in people born to short mothers suggest a link with the lifetime nutrition of the mother. Short adult stature is a product of poor foetal or childhood nutrition, or recurrent exposure to infections, though there are also genetic influences.21 We speculate that some aspect of poor nutrition among girls results in metabolic incompetence, which amplifies the adverse effects of impaired placentation on the foetus. Women who are short are known to have lower rates of protein synthesis during pregnancy.22
These adverse effects may be exacerbated by poor maternal nutrition during pregnancy. There were food shortages in Helsinki before and during the Second World War, the time when our subjects were born.23 These may have been more severe in families of low socio-economic status. We found that the effects of small placental size on chronic heart failure were stronger in people born into the families of manual workers (Table 3).
Animal studies support the relationship of maternal malnutrition to heart failure in the offspring. When pregnant rats were given a low protein diet, their adult offspring had cardiac dysfunction.24 They also had altered beta-adrenergic responsiveness and attenuated adrenergic and insulin signalling, both of which increase the risk of heart failure.25 When ewes were undernourished during early pregnancy, their lambs had cardiac hypertrophy.26 Also in sheep, pressure loading the foetal heart stimulates premature maturation and loss of generative capacity.15 Increased loading of the heart in foetuses with a small placenta is likely to reduce cardiomyocyte numbers for life and hence make the heart vulnerable to developing chronic failure.
Post-natal growth
Chronic heart failure was not associated with body size at birth or at any age in childhood. Rather, it was associated with a rapid increase in body mass index after the age of 2 (Figure 1). We have previously shown that, in this cohort, this pattern of growth is linked to two markers of insulin resistance at ages around 62 years, raised fasting plasma insulin and pro-insulin concentrations.19 Insulin resistance in a known risk factor for chronic heart failure.3,27,28
In a simultaneous analysis, a short lesser placental diameter and a rapid increase in body mass index after the age of 2 years were both associated with chronic heart failure. The combination predicted chronic heart failure more strongly than either of them alone. We hypothesize that impaired placental growth creates a vulnerable heart and that insulin resistance is a second stressor, which develops postnatally and further increases the likelihood of chronic heart failure. Consistent with this, we found that the association between a short lesser placental diameter and chronic heart failure occurred in people taking medication for type 2 diabetes but not in people taking medication for hypertension (Table 4).
Adult socioeconomic status
Consistent with previous observations, we found that chronic heart failure was associated with low socioeconomic status in adult life.29 In a simultaneous regression, however, the effects of a short lesser placental diameter and rapid gain in body mass index after age 2 years were little changed by adjustment for socioeconomic status. The strength of the effect of the lesser placental diameter on chronic heart failure was similar to that of socioeconomic status.
Limitations
We ascertained chronic heart failure, through the use of medication for the disorder. The prevalence of the disorder ascertained in this way was 1.4% which is slightly below recent estimates of around 2% in Europe and the USA.30,31 Our cohort, however, has only a few people who have reached the age of 75, as they were born during 1934–1944. The prevalence of chronic heart failure rises steeply with increasing age. We found that people taking medication for chronic heart failure were at increased risk of dying, especially if they also had type 2 diabetes. This suggests that chronic heart failure in our study defines a group of people who were seriously ill. Our data, however, do not include detailed clinical measurements or the cardiovascular risk profiles. In addition, our data on the occurrence of type 2 diabetes, hypertension, and coronary heart disease were obtained from the medication register. Although we do not have the detailed clinical history, the case notes of each subject were reviewed by a physician before payment for the medication was approved. The placental measurements in our study were made during routine obstetric practice 70 years ago. The mean placental weight was above the median recorded in a recent series of deliveries in Europe.32 Routine measurements of the placental diameters ceased in Helsinki in the 1970s. We have discussed these simple procedures with people who worked as midwives at that time. There were no routine checks of the quality of the measurements, just as there are no routine checks of the quality of blood pressure and other measurements made in current clinical practice. Measurement errors would tend to diminish the associations between the placental diameters and chronic heart failure in later life. Our study was restricted to people who had visited child welfare clinics. Although the majority of children visited these clinics, which were free, the visits were voluntary. The people in our study may not be representative of all people now living in Helsinki, although at birth their social class distribution was similar to that in the city as a whole.
Conclusion
We found that people taking medication for chronic heart failure were born with a small placental surface area. The reduced surface area resulted from failure of growth along the minor axis of the placental surface. We found that the effects of a short lesser placental diameter on chronic heart failure were confined to people whose mothers were likely to have been the least well-nourished through their lives. Rapid increase in body mass index after the age of 2 further increased the risk of chronic heart failure. This pattern of weight gain is known to be associated with insulin resistance. Consistent with this, we found that the association between a short lesser placental diameter and chronic heart failure occurred in people taking medication for type 2 diabetes but not in people taking medication for hypertension. We therefore postulate that people with a vulnerable heart, as a result of prenatal programming, develop chronic heart failure if they become insulin resistant.
Funding
Funding was received from The British Heart Foundation, the Academy of Finland, the Päivikki and Sakari Sohlberg Foundation, the Finnish Diabetes Research Foundation, the Finnish Foundation for Cardiovascular Research, the Finnish Foundation for Pediatric Research, the Finnish Medical Societies (Duodecim and Finska Läkaresällskapet), the Novo Nordisk Foundation, the Sigrid Jusélius Foundation, the Jalmari and Rauha Ahokas Foundation, the Juho Vainio Foundation, the Yrjö Jahnsson Foundation, and the M. Lowell Edwards Endowment.
Conflict of interest: none declared.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis, management of chronic heart failure in the adult: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure) J Am Coll Cardiol. 2005;46:1116–1143. doi: 10.1016/j.jacc.2005.08.022. doi:10.1016/j.jacc.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Fang JC, Givertz MM. Diastolic Heart Failure. In: Baughman KL, editor. Treatment of Advanced Heart Disease. New York: Taylor and Francis; 2006. pp. 229–247. [Google Scholar]
- 3.Witteles RM, Fowler MD. Insulin-resistant cardiomyopathy. Clinical evidence, mechanisms and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. doi:10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birthweight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1977;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall CHD, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. doi:10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson JG, Forsen T, Kajantie E, Osmond C, Barker DJP. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. doi:10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- 8.Harding J. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. doi:10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, Sun W, Maas EM. Placental characteristics and birth weight. Paediatr Perinat Epidemiol. 2008;22:229–239. doi: 10.1111/j.1365-3016.2008.00935.x. doi:10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 11.Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJP. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315:837–840. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol. 2006;28:126–136. doi: 10.1002/uog.2832. doi:10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 13.Rosso P, Kava R. Effects of food restriction on cardiac output and blood flow to the uterus and placenta in the pregnant rat. J Nutr. 1980;110:2350–2354. doi: 10.1093/jn/110.12.2350. [DOI] [PubMed] [Google Scholar]
- 14.Mäkikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation. 2002;105:2058–2063. doi: 10.1161/01.cir.0000015505.24187.fa. doi:10.1161/01.CIR.0000015505.24187.FA. [DOI] [PubMed] [Google Scholar]
- 15.Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- 16.Anderson MC. Lessons in Midwifery for Nurses and Midwifes. London: A & C Black; 1930. [Google Scholar]
- 17.Hinselmann H. Biologie und Pathologie des Weibes. Berlin: Urban & Schwarzenberg; 1925. [Google Scholar]
- 18.Barker DJP, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. doi: 10.1387/ijdb.082760db. doi:10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. doi:10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 20.Kajantie E, Thornburg K, Eriksson JG, Osmond C, Barker DJP. In preeclampsia the placenta grows slowly along its minor axis. Int J Dev Biol. 2010;54:469–473. doi: 10.1387/ijdb.082833ek. doi:10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 21.Tanner JM. Fetus into Man. 2nd edn. Castlemead: Ware; 1989. [Google Scholar]
- 22.Duggleby SL, Jackson AA. Relationship of maternal protein turnover and lean body mass during pregnancy and birth length. Clin Sci (Lond) 2001;101:65–72. doi:10.1042/CS20000330. [PubMed] [Google Scholar]
- 23.Pesonen AK, Räikkönen K, Heinonen K, Kajantie, Forsen T, Eriksson JG. Depressive symptoms in adults separated from their parents as children: a natural experiment during World War II. Am J Epidemiol. 2007;166:1126–1133. doi: 10.1093/aje/kwm254. doi:10.1093/aje/kwm254. [DOI] [PubMed] [Google Scholar]
- 24.Cheema KK, Dent MR, Saini HK, Aroutiounova N, Tappia PS. Prenatal exposure to maternal undernutrition induces adult cardiac dysfunction. Br J Nutr. 2005;93:471–477. doi: 10.1079/bjn20041392. doi:10.1079/BJN20041392. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Twinn DS, Ekizogiou S, Wayman A, Petry CJ, Ozanne SE. Maternal low-protein diet programs cardiac beta-adrenergic response and signaling in a 3 month-old male offspring. Am J Physiol Regul Integr Comp Physiol. 2006;291:R429–R436. doi: 10.1152/ajpregu.00608.2005. [DOI] [PubMed] [Google Scholar]
- 26.Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, Hanson MA, Green LR. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA. 2007;1004:9529–9533. doi: 10.1073/pnas.0610373104. doi:10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and the risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. doi:10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 28.Arnlöv J, Lind L, Zethelius B, Andrén B, Hales CN, Vessby B, Lithell H. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J. 2001;142:720–724. doi: 10.1067/mhj.2001.116957. [DOI] [PubMed] [Google Scholar]
- 29.Schaufelberger M, Rosengren A. Heart failure in different occupational classes in Sweden. Eur Heart J. 2007;28:212–218. doi: 10.1093/eurheartj/ehl435. doi:10.1093/eurheartj/ehl435. [DOI] [PubMed] [Google Scholar]
- 30.ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2342. doi: 10.1093/eurheartj/ehn309. doi:10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 31.American College of Cardiology. American Heart Association Task Force for the Diagnosis and Management of Heart Failure in Adults. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. doi:10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt T, Schaffer L, Schneider C, Zimmermann R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight–birth weight ratios. Eur J Obstet Gynecol. 2006;128:248–252. doi: 10.1016/j.ejogrb.2005.10.032. doi:10.1016/j.ejogrb.2005.10.032. [DOI] [PubMed] [Google Scholar]