Introduction
Several circumstances can cause a metabolic acidosis. Some cases pose diagnostic problems. Mindful clinical evaluation and logical laboratory work-up are crucial to find the correct diagnosis. We report the case of a patient with a severe metabolic acidosis. Further investigations led to an unexspected diagnosis in a timely fashion contributing to an uneventful recovery.
Case
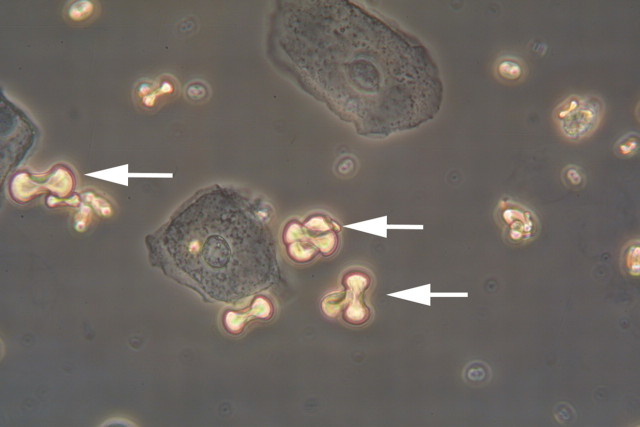
A 49-year-old man consulted the emergency department with a brief history of moderate headache, anomia and ataxia. There was no fever, and no evidence of infection, trauma or alcohol intake. His medications included mirtazepin and escitalopram for a history of depression. On first examination, the patient was confused and incoherent without focal neurologic signs. Electrocardiogram, head CT-scan, chest X-ray and lumbar puncture showed no abnormalities. Initial laboratory findings (haematology, serum electrolytes, creatinine, urea, glucose, C-reactive protein, ethanol) were within normal limits. A moderate leucocytosis of 13.3 g/l was noted. Could this be basilar-type migraine or something else? In the course of the following hours, the patient developed hyperventilation. Why is he hyperventilating? Could it be a psychogenic cause? Blood gas analysis showed a pronounced metabolic acidosis (pH 7.2, base excess −18.4 mmol/l, serum bicarbonate 6.2 mmol/l). What would be your next step? Giving a bicarbonate infusion? Measure the anion and the osmolar gap. An elevated anion gap of 24 mmol/l and an osmolar gap of 47 mmol/l were detected. The finding of a high osmolar gap in the absence of ethanol ingestion, ketoacidaemia and uraemia suggested an intoxication with methanol or ethylene glycol (EG). To further differentiate between different toxins, a urinary analysis was performed. Dumbbell-shaped monohydrate crystals were detected in urine sediment (Figure 1). What is your suspicion and according to these results what treatment should be prescribed?
Fig. 1.
Dumbbell-shaped calcium oxalate monohydrate crystals (arrow) were detected in urine sediment. Aggrandisement 400×
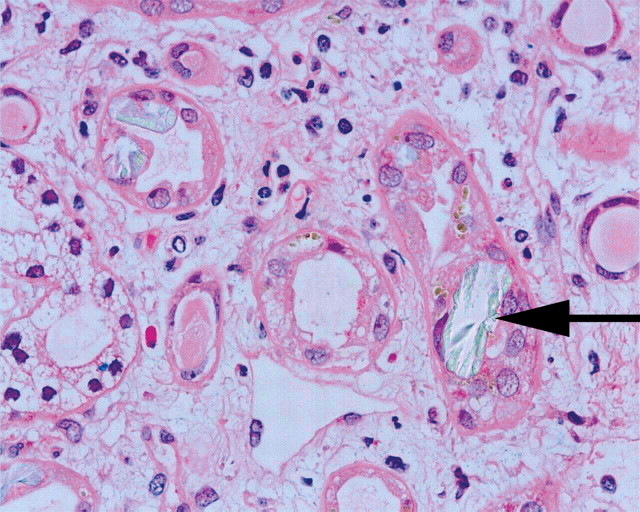
With a high suspicion of EG intoxication, therapy with intravenous ethanol was initiated. Thiamin 100 mg IV and pyridoxine 50 mg IV were given additionally. Because of respiratory failure, the patient was intubated and transferred to a tertiary care hospital where ICU and fomepizole were available. On admission to the intensive care unit, initial arterial pH was 6.97 with serum bicarbonate of 6.4 mmol/l and lactate of 11.1 mmol/l peaking at 21.0 mmol/l 40 min later. Bicarbonate infusion as well as therapy with fomepizole was initiated immediately. Two concomitant high-flux haemodialysis treatments were performed (every 3.5 h). The first measured serum glycolic acid concentration was 878 mg/l (toxic level >60 mg/l); it decreased to 27 mg/l after initiation of fomepizole and haemodialysis therapy. Over the next 24 h, the patient developed non-oliguric acute renal failure needing renal replacement therapy over a period of 5 days followed by a polyuric phase. Two days after admission, the patient suffered from severe bilateral flank pain without macrohaematuria. Neither hydronephrosis nor crystal aggregation could be seen in the CT scan. The renal biopsy performed 8 days after admission was consistent with oxalate nephropathy (Figure 2). The patient had an uneventful recovery within the next weeks. Clarification of the circumstances leading to the EG intoxication suggested an accidental intake without suicidal ideation.
Fig. 2.
Renal biopsy of our patient performed in the subacute phase (8 days after EG ingestion) showing a tubulo-interstitial nephritis with intratubular deposition of oxalate crystals (arrow). Arrow; H&E, 400×
Discussion
EG is a colourless and sweet-tasting alcohol compound, commonly used as an antifreeze and de-icing solution in windshield wiper fluid, solvents, fuels and other chemical products. The most severe intoxications occur secondary to ingestion, while inhalation and dermal exposure only rarely cause toxic effects.
Without treatment, an intake of 1–1.5 g/kg body weight of EG is considered lethal. EG is absorbed rapidly following oral ingestion with serum concentrations peaking within 1–2 h. The estimated serum half-time without treatment lies between 3 and 8 h [1]. Although not toxic itself, EG is rapidly metabolized by alcohol dehydrogenase (ADH) to glycolaldehyde (rate determining step) that is in turn oxidized by aldehyde dehydrogenase to glycolic acid (physiologically present in its anionic form, glycolate). The latter is further converted by a metabolically slow step to glyoxalate and ultimately oxalate. Both first steps require as co-factors reduced nicotinamide adenine dinucleotide (NADH) [2]. Metabolic acidosis observed in EG poisoning is almost entirely due to glycolic acid, although increased lactic acid production may play a contributory role in certain cases. In contrast to EG, which is an alcohol and therefore leads to an osmolar gap, glycolic acid is physiologically dissociate and does not per se account for this finding. The level of EG in mg/dl can be estimated by multiplying the osmolar gap by 6.2 [3]. Since EG is rapidly metabolized to glycolic acid, the typical finding of an osmolar gap may be lacking at presentation and thus misleading. For this reason, when available, diagnostic confirmation by colorimetric or gas chromatographic quantification of glycolid acid should be performed [4]. Glycolic acid concentration and serum bicarbonate at admission correlate better with the severity of the poisoning and the outcome than the EG level itself [3]. High serum lactate observed in this case is not a typical feature encountered in EG poisoning, but may be present if reduced NADH is highly depleted. This finding is often encountered in patients treated primarily with ethanol, since its proper metabolism consumes NADH, a co-factor needed to further metabolize lactic acid [5].
The clinical course can be divided into three phases. Shortly after ingestion, mild effects like inebriation and sedation are present (similar to alcohol intoxication). Coma, seizures, hyperventilation (Kussmaul respirations) and hypotension follow. In a second phase (12–24 h), cardiopulmonary symptoms including tachycardia, hypotension, heart failure and pulmonary oedema develop due to the toxic effects of glycolic acid; most deaths happen in this stage [5]. The final phase is reached 24–72 h after ingestion and is characterized by the development of acute renal failure consistent with acute tubular necrosis, which may be accompanied by flank pain caused by hydronephrosis secondary to obstruction with calcium oxalate crystals. The pathophysiology of renal injury is not entirely clear. Deposition of calcium oxalate monohydrate crystals exhibit direct cytotoxic, cytolytic and apoptotic effects in animal and human cellular models of proximal tubules involving cell membrane injury, activation of enzyme activity and production of free radicals and lipid peroxidation [6]. In cell-culture studies, direct toxicity of glycolic acid has not been observed, whereas glycolaldehyde and glyoxylate induced severe toxicity in cell cultures of proximal tubules. Urine can contain characteristic dumbbell-shaped monohydrate crystals or octahedral dihydrate crystals. Dihydrate crystals require higher oxalate concentrations and are indicative of an intoxication with EG. These crystals following EG ingestion can help in the diagnosis when present but are unreliable and may be absent even with high-level exposure [7]. The differential diagnoses of an increased anion gap include lactic acidosis, uraemia, ketoacidosis and intoxication with methanol, salicylate or EG.
Because of rapid absorption, there is little role for gastrointestinal decontamination. Bicarbonate is indicated for patients with a pH <7.3 [8]. ADH should be inhibited preferentially with fomepizole or ethanol. Fomepizole is preferred to ethanol because of its better pharmacokinetic predictability, simple use, superior metabolic properties, higher affinity to ADH and better tolerance [7,9]. After an intravenous loading dose of 15 mg/kg every 12 h, 10 mg/kg every 12 h for maximum 48 h is administered until the EG level falls to <20 mg/dl. The dose should be adjusted in the case of haemodialysis (4 h interval or maintance dose increased to 1.5 mg/kg/h). Thiamin (100 mg IV) and pyridoxin (50 mg IV every 6 h) may enhance the conversion of some toxic metabolites to non-toxic products as co-factors of the intermediary metabolism [7,10], but human data are lacking. Haemodialysis is effective in clearing EG and its metabolites, thus leading to rapid correction of metabolic acidosis.
Indications for haemodialysis in patients with EG intoxication remain not uniformally defined. They include metabolic acidosis (arterial pH <7.30 despite bicarbonate infusion), EG level >50 mg/dl, alternatively an anion gap >20 mmol/l or a glycolic acid level >8 mmol/l and presence of end-organ damage [8,11]. Haemodialysis should be continued until serum pH is within normal limits, EG concentration is <20 mg/dl and no signs of systemic toxicity persist. Assuming a first-order dialytic elimination rate and one-compartment system, the equation C1/C0 = e−kt/V, where C1 (mg/dl) represents the desired drug level, C0 the current drug level, k the clearence constant (0.2 l/min, available for high-flux haemodialysis) and Vd the volume of distribution (0.6 l/kg), the required dialysis time (t) in minutes can be estimated using the formula t = −Vd/k × ln(C1/C0) [12].
Conversion factors:
glycolic acid 1 mmol/l = 76 mg/l = 7.6 mg/dl.
ethylene glycol: 1 mmol/l = 62 mg/l = 6.2 mg/dl.
Teaching points
In disoriented patients with deteriorating vital signs and metabolic acidosis, intoxication with ethylene glycol (EG) should be considered. Blood gas analysis helps in determining the direction of the diagnostic work-up.
In patients with hyperventilation (Kussmaul respiration) ketoacidosis, uraemia, lactic acidosis and intoxication with methanol, salicylate or EG has to be ruled out.
Anion gap acidosis, high osmolar gap and the presence of oxalate crystals in urine are highly suggestive of EG intoxication.
Cornerstones of EG intoxication treatment are prompt inhibition of alcohol dehydrogenase, preferentially by fomipezole, haemodialysis and supportive treatments.
Conflict of interest statement. Robert Schorn, Robert Kalicki, Niklaus Höfliger and Fabienne Aregger were responsible for conception, design, analysis and interpretation of data; drafting the article; providing intellectual content of critical importance to the work described and final approval of the version to be published. Cornelius Remschmidt and Gunnar Schley were responsible for analysis and interpretation of data; revising the article; providing intellectual content of critical importance to the work described and final approval of the version to be published. The results presented in this paper have not been published previously in whole or part.
References
- 1.Eder AF, McGrath CM, Dowdy YG, et al. Ethylene glycol poisoning: toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem. 1998;44:168–177. [PubMed] [Google Scholar]
- 2.Gabow PA, Clay K, Sullivan JB, et al. Organic acids in ethylene glycol intoxication. Ann Intern Med. 1986;105:16–20. doi: 10.7326/0003-4819-105-1-16. [DOI] [PubMed] [Google Scholar]
- 3.Glaser DS. Utility of the serum osmol gap in the diagnosis of methanol or ethylene glycol ingestion. Ann Emerg Med. 1996;27:343–346. doi: 10.1016/s0196-0644(96)70271-8. [DOI] [PubMed] [Google Scholar]
- 4.Fraser AD. Clinical toxicologic implications of ethylene glycol and glycolic acid poisoning. Ther Drug Monit. 2002;24:232–238. doi: 10.1097/00007691-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Litovitz TL, Klein-Schwartz W, Rodgers GC, Jr, et al. 2001 Annual report of the American association of poison control centers toxic exposure surveillance system. Am J Emerg Med. 2002;20:391–452. doi: 10.1053/ajem.2002.34955. [DOI] [PubMed] [Google Scholar]
- 6.Guo C, McMartin KE. The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology. 2005;208:347–355. doi: 10.1016/j.tox.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Betten DP, Vohra RB, Cook MD, et al. Antidote use in the critically ill poisoned patient. J Intensive Care Med. 2006;21:255–277. doi: 10.1177/0885066606290386. [DOI] [PubMed] [Google Scholar]
- 8.Barceloux DC, Krenzelok EP, Olson K, et al. American academy of clinical toxicology practices guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol. 1999;37:537–560. doi: 10.1081/clt-100102445. [DOI] [PubMed] [Google Scholar]
- 9.Brent J, McMartin K, Phillips S, et al. Fomipezol for the treatment of ethylene glycol poisoning. Methylpyrazole for toxic alcohols study group. N Engl J Med. 1999;340:832–838. doi: 10.1056/NEJM199903183401102. [DOI] [PubMed] [Google Scholar]
- 10.Beasley VR, Buck WB. Acute ethylene glycol toxicosis: a review. Vet Hum Toxicol. 1980;22:255–263. [PubMed] [Google Scholar]
- 11.Porter WH, Rutter PW, Bush BA, et al. Ethylene glycol toxicity: the role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39:607–615. doi: 10.1081/clt-100108493. [DOI] [PubMed] [Google Scholar]
- 12.Leblanc M, Raymond M, Bonnardeaux A, et al. Lithium poisoning treated by high-performance continuous arteriovenous and venovenous hemodiafiltration. Am J Kidney Dis. 1996;27:363–372. doi: 10.1016/s0272-6386(96)90359-5. [DOI] [PubMed] [Google Scholar]