Abstract
This narrative clinical review in two parts discusses the prevention of clinical acute kidney injury (AKI). The first part focuses on general prevention measures, including identification of individuals at high risk for AKI, and on the role of volume expansion and fluid therapy. The latter discusses the timing, the goals, the selection of the fluids and the haemodynamic management of the patient receiving parenteral fluids for the prevention of AKI. In addition, this part summarizes the interaction of intensivist-nephrologist in the ICU with attention to tight glycaemia control and the use of low doses of corticoids in the septic shock patients. Finally, the avoidance of drug- and nephrotoxin-induced AKI is discussed. The second part of this review will summarize the possible pharmacological interventions in the patient at risk.
Keywords: acute kidney injury, fluid therapy, prevention, tight glycaemia control, volume expansion
Introduction
The literature on epidemiology, prevention and treatment of acute kidney injury (AKI) is blurred by the use of multiple operational definitions, and its occurrence in different clinical settings, such as community, hospital or intensive care units (ICU).
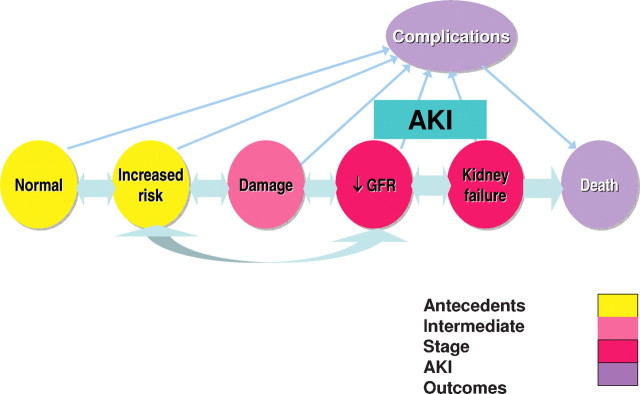
The need for a consensus definition and classification system for AKI has resulted in the development of the RIFLE criteria (Risk, Injury, Failure, Loss of kidney function and End-stage disease) [1]. These criteria were further refined by the Acute Kidney Injury Network (AKIN) [2]. The AKIN group also formulated a conceptual framework of AKI and prioritization of a clinical research agenda [3] (Figure 1).
Fig. 1.
A conceptual framework of acute kidney injury. The process of AKI can be divided into various reversible stages depending on the severity of insult, starting from the increased risk to damage followed by a decrease in glomerular filtration rate (GFR) further progressing to kidney failure and death (modified from [3]).
This editorial review focuses on the primary prevention of clinical AKI, i.e. on the measures that can be used to prevent the decline in GFR in the patient at risk. However, since the diagnosis of AKI in a patient is in general dependent on the findings of the decline in GFR [increase in serum creatinine (SCr), cystatin C or other parameters of GFR], many of the preventative measures that are employed are in fact secondary interventions with the aim to either stabilizing renal function or improving it towards normal.
It should be clear that the efficiency of the preventative measures will depend on the recognition of the population at risk and of the means to diagnose ‘damage’ to the kidney before the fall in GFR occurs (Figure 1). It is in this stage of ‘damage’, probably reflecting tubular injury only, that biomarkers may be useful in the future. The last year has seen an explosion of papers describing the basic molecular biological research and the beginning of the clinical application of a great number of biomarkers in the field of AKI [4].
The possibility that injury biomarkers may be superior to SCr or other clearance-based markers for the identification of AKI will require investigators to test the creatinine-independent associations between biomarker levels and exposures [e.g. cardiopulmonary bypass (CPB) time, dose of nephrotoxin administration] and ‘hard’ outcomes (e.g. mortality, complications, need for dialysis and length of ICU or hospital stay).
Innumerable AKI prevention studies have been conducted over the past three decades, the vast majority of which have targeted persons anticipating a well-defined ischaemic or toxic insult to the kidney.
Basic research has led to substantial advances in our understanding of the pathogenetic mechanisms of AKI and this has led to an exciting array of potentially novel targets for its treatment. However, most of the ‘successful’ interventions that have been tried in animal or in vitro experiments have failed in the clinical setting. One of the many reasons of this failure is that the intervention was started too late in patients with already established AKI [5].
The first part of this review treats the general prevention measures, the fluid therapy, the interaction phase between nephrology and intensive care specialists and the screening for patients at risk for AKI.
The second part will discuss the role of vasopressors, loop diuretics, mannitol, renal vasodilators, acetylcysteine, statins, prophylactic dialysis in contrast-induced nephropathy (CIN), anti-tumour lysis drugs and the possible role of erythropoietin.
General prevention measures
Identification of high-risk individuals
Knowledge of the most common risk factors for renal insult by the nephrologist is recommended in order to give a meaningful advice to non-nephrologists responsible for a particular patient. Only a careful and systematic approach can define these risk factors, as described in many recent clinical textbooks [6–8].
General risk factors for AKI that are consistent across multiple causes include age; hypovolaemia; hypotension; sepsis; pre-existing renal, hepatic or cardiac dysfunction; diabetes mellitus; and exposure to nephrotoxins [e.g. aminoglycosides, amphotericin, immunosuppressive agents, non-steroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs), parenteral contrast media (CM)].
To this list, more specific factors should be added depending on the particular patient category (see Table 1) for a list of major risk factors in five common clinical situations, adapted from [7]). For example, in AKI post-cardiac surgery, procedure-related risk factors include cross-clamp time, the duration of CPB (especially if longer than 70 min), pulsatile versus non-pulsatile bypass flow, normothermic versus hypothermic bypass, haemodilution and on-pump versus off-pump coronary artery bypass (OPCAB) surgery. Recently, a retrospective cohort study of 1358 adult patients who underwent cardiac surgery found that besides the above-mentioned risk factors, there existed an independent and significant association of AKI and preoperative use of ACEI/ARB [9]. Preoperative use of ACEI/ARB was associated with a 27.6% higher risk for AKI postoperatively. Stopping ACEI or ARB before cardiac surgery may reduce the incidence of AKI.
Table 1.
Risk factors for the development of ARF in common clinical situations
| Post-operative (general) | Cardiac surgery | Critically ill | Sepsis | Contrast nephropathy | Nephrotoxic antibiotics |
|---|---|---|---|---|---|
| Cardiac | Female gender | Active cancer | S bilirubin >1.5 mg/dL | Systolic BP <80 mmHg for >1 hr | Amphotericin |
| Haemodynamic | ACE inhibitor therapy | Low-serum | Age | and need for inotropic support | Volume depletion |
| instability | Heart failure | albumin | SCr >1.3 mg/dL | or IABP 24 h after procedure | Concurrent other |
| Congestive heart failure | LV ejection fraction < | A-a gradient* | Elevated CVP >8 cm | Use of IABP | nephropathy |
| Aortic cross clamping | 35% | H2O under fluid | Heart failure (NYHA class 3–4), | Aminoglycosides | |
| Major vascular surgery | Preoperative IABP | substitution for | history of pulmonary oedema, or | Duration of >7 d | |
| Hypertension | COPD | haemodynamic | both | Volume depletion | |
| Infection | Insulin-requiring diabetes | instability | Age >75 yrs | Divided dose | |
| Sepsis | Previous cardiac surgery | Hct: <39% for ?; <36% for ? | regimens | ||
| Multi-organ failure | Emergency surgery | Diabetes mellitus | Sepsis | ||
| Gastrointestinal and | Valve surgery only | Volume of contrast >100 mL | Liver disease | ||
| endocrine | Combination of CABG | SCr >1.5 mg/dL or eGFR < | Old age | ||
| Cirrhosis | +valve surgery | 60 mL/min/1.73 m2 | Pre-existing CKD | ||
| Biliary surgery | Other cardiac surgery | Intra-arterial injection | |||
| Obstructive jaundice | Preoperative SCr > | ||||
| Diabetes mellitus | 2.1 mg/dL | ||||
| Renal Transplantation | |||||
| Oliguria < 400 mL/day | |||||
| SCr > 2 mg/dL | |||||
| Miscellaneous | |||||
| Age > 70 years | |||||
| Trauma | |||||
| Massive blood | |||||
| transfusion |
CABG—coronary arterial bypass graft.
CVP—central venous pressure.
IABP: intra-aortic balloon pump.
COPD: chronic obstructive pulmonary disease.
Hct: haematocrit.
*A- a gradient: alveolar-arterial oxygen gradient calculated using the sea level standard formula [(713 × (FiO2)−(PCO2/0.8)−PaO2], where FiO2: fractional inspired oxygen concentration, PaO2: arterial partial oxygen pressure, PCO2: partial CO2 pressure. The normal A-a gradient varies with age and ranges from 7 to 14 mmHg when breathing room air.
Modified from Modified from ref. [7].
In patients at increased risk, or in the phase of incipient AKI, emphasis should be put on non-pharmacologic interventions, such as ensuring adequate renal perfusion pressure by optimizing volume status and maintaining adequate haemodynamic status by the use of vasopressors, and avoidance of further injury by removing or decreasing the effect of any nephrotoxic substances.
Volume expansion and/or fluid therapy
Timing, goals of fluid therapy and haemodynamic management
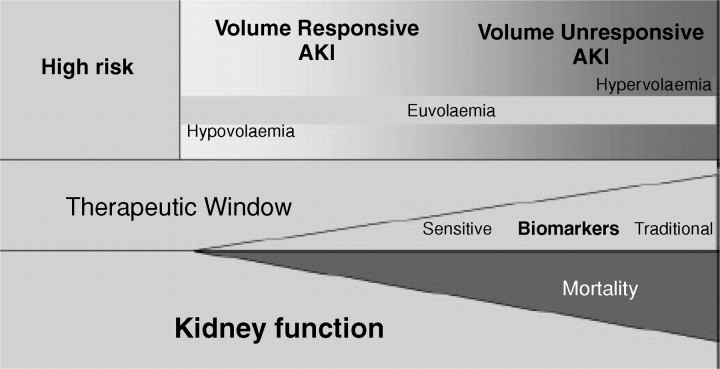
It is accepted that optimization of the haemodynamic status and correction of any volume deficit will have a salutary effect on kidney function, will help minimize further extension of the kidney injury and will potentially facilitate recovery from AKI with minimization of any residual functional impairment. AKI is characterized by a continuum of volume responsiveness and/or unresponsiveness (Figure 2) [10]. Given the theoretical and practical difficulties in the usage of the historical terms ‘prerenal azotaemia’ and ‘acute tubular necrosis’, it is proposed that these terms should be discarded and replaced with ‘volume-responsive AKI’ and ‘volume-unresponsive AKI’. Volume-responsive AKI was previously called pre-renal acute renal failure (ARF). Real hypovolaemia is the most important cause of volume-responsive AKI, particularly in patients outside the ICU. In these patients, organ perfusion and renal function will improve with volume loading. In other circumstances, renal perfusion is suboptimal despite adequate circulating volume, or even volume overload. This is the case in patients with serious congestive heart failure or diastolic dysfunction. In these patients fluid loading will not result in improved kidney perfusion, and might even lead to pulmonary oedema and further worsening of cardiac function and thus organ perfusion. In still other situations, the patient may already be fluid overloaded, but the intravascular circulating volume is reduced, such as in sepsis or other diseases causing third spacing or in ascites in liver cirrhosis. The term ‘volume-responsive AKI’ should thus be reserved for patients in whom volume administration results in improvement of kidney function. Unfortunately, the effect of volume repletion on the general haemodynamic status and renal function is always a ‘retrospective diagnosis’ and can very often only be evaluated by trial and error.
Fig. 2.
Continuum of volume responsiveness and non-responsiveness in AKI (from [9]).
Many of the patients with volume-responsive AKI and overall lesser severity of illness may be managed in a standard clinical setting and may not require an intensive care setting with invasive haemodynamic monitoring to optimize fluid status. A practical tool to predict the impact of volume loading is the leg tilt test, which should be a routine clinical evaluation of volume status in AKI patients [11]. It is now well established that the use of pulmonary artery catheters does not result in improved patient survival or in lesser need of renal replacement therapy (RRT) [12].
The required precision for assessment of volume therapy in AKI increases as the severity of illness increases. The ARDS Network has described a number of trials assessing the effects of goal-directed therapy in the setting of acute lung injury. A conservative approach to fluid administration (net positive fluid balance over 7 days of an average of only 136 mL) compared to a more liberal approach (net positive fluid balance over 7 days of 6992 mL) allowed more rapid weaning from mechanical ventilation with a decrease in the number of ICU days and no proven adverse effect on kidney function or on kidney outcomes [13].
The Surviving Sepsis Campaign recommends that extracellular volume and cardiac output be assessed and supported with adequate and early goal-directed therapy [14]. This includes volume and pressor support to achieve a mean arterial pressure ≥65 mmHg and a central venous pressure of 8–12 mmHg (or 12–15 mmHg in patients who receive positive pressure ventilation) [14,15]. Rivers et al. [16,17] demonstrated in a single-centre study in septic patients that early versus delayed administration of fluid, vasopressors, blood products and inotropes to maintain central venous oxygen saturation of >70% had important benefits in terms of mortality and multi-organ failure. A later, slightly modified goal-directed study randomized patients with septic shock to therapy with or without a written protocol using central venous pressure, mean arterial pressure and urine output as therapeutic goals to their therapy [18]. Implementation of goal-directed therapy caused a more rapid reversal of persistent shock, reduced mortality rates, incidence of renal failure and central nervous system complications compared with non-goal-directed therapy. These benefits may arise from adequate fluid resuscitation, earlier vasopressor administration, rapid shock reversal and protection of major organ function. Most studies with early interventions (defined as before the occurrence of organ failure, within 24 h of trauma or within 12 h after surgery) showed lower mortality rates [19], but targeting supra-normal cardiac index and oxygen delivery later conferred no benefit [20]. The point to emphasize is thus that what is beneficial early is not necessarily beneficial later in the course of critical illness.
Fluid administration should stop when patients are no longer fluid responsive—assessed either by direct measures of cardiac output or by pulse-pressure variation [21]. Pressure preload variables (central venous pressure and pulmonary capillary wedge pressure), which continue to be used, often fail to provide reliable information regarding cardiac preload [22] and are incapable of predicting cardiac response to fluid therapy [23].
As an alternative to these static variables, dynamic functional haemodynamic markers, such as pulse pressure or stroke volume variation during positive pressure breathing or mean flow changes with passive leg raising, are highly predictive of volume responsiveness [24,25].
It is clear that during fluid therapy, haemodynamic monitoring particularly in the critically ill patient whether ventilated or not needs the close cooperation between the ICU specialist and the nephrologist. A detailed description of this monitoring is beyond the scope of this paper but can be found in the recent paper by Pinsky et al. [26].
Fluid therapy in the prevention of AKI
Useful reviews on the role of fluid therapy in the prevention of AKI are available [27–29]. Despite the recognition of volume depletion as an important risk factor for AKI, there are no randomized controlled trials (RCTs) that have directly evaluated the role of fluid hydration versus placebo in the prevention of AKI. However, RCTs have compared different fluids and have combined fluid hydration with other interventions [30]. Furthermore, comparisons between outcomes seen in these trials [30] and historical untreated control subjects [31] suggest a large benefit from fluids. One small RCT [32] of 53 patients who underwent non-emergent cardiac catheterization found that IV hydration with saline was more effective than unrestricted oral fluid intake.
Type of fluid therapy
Colloids (albumin, starch) versus crystalloids. Results of the Saline versus Albumin Fluid Evaluation (SAFE) study, a randomized comparison of human albumin (HA) with crystalloid in the ICU, seem to indicate that albumin is safe, albeit not more effective than saline, for fluid resuscitation [33]. SAFE demonstrated further no difference in renal outcomes (including incidence of AKI, need for RRT or duration of RRT).
Hydroxyethylstarch (HES) is a widely used, cheap alternative to HA for correcting hypovolaemia. Different HES preparations are available that vary with regard to concentration, mean molecular weight (Mw), molar substitution and substitution of hydroxyethyl for hydroxyl groups.
Aside from negative effects on coagulation, the development of renal dysfunction is one of the major concerns associated with the use of HES. It has even be recommended that ‘HES should be avoided in intensive care units and during the perioperative period’ (for a summary of this controversy see [34,35]). However, a recent trial comparing a ‘modern’ HES preparation with a low Mw and low molar substitution and a HA solution, given in cardiac surgery patients with preoperative compromised kidney function, showed that this type of HES solution had no negative influence on kidney integrity [36].
In the VISEP study [37] in severe sepsis, patients were randomly given 10% pentastarch solution, a low Mw hydroxyethyl starch (HES 200/0.5) or modified Ringer's lactate for fluid resuscitation. Patients in the HES group received a median cumulative dose of 70.4 mL/kg of body weight (interquartile range, 33.4–144.2). Although the mortality was not significantly different, the HES group had a significantly higher rate of AKI (34.9 versus 22.8%,) and more days on which RRT was required.
Overall, a comprehensive Cochrane review [38] concluded that there is no evidence from RCTs that resuscitation with colloids, instead of crystalloids, reduces the risk of death in patients with trauma, burns or following surgery. There is even some evidence that colloids maybe associated with a higher incidence of AKI. As colloids are considerably more expensive than crystalloids, it is hard to see how their continued use outside the context of RCTs in subsets of patients of particular concern, can be justified.
Tonicity of the fluids and anionic composition of the crystalloids
As pointed out in a recent comprehensive review [27] large-volume crystalloid resuscitation can result in significant alterations in the electrolyte and acid–base balance in critically ill and injured patients. Isotonic saline is well known to induce hyperchloraemic acidosis, and hyperchloraemia has experimentally been shown to induce renal vasoconstriction [39]. An intraoperative comparison of 0.9% saline and lactated Ringer's solution for abdominal aortic reconstruction demonstrated that hyperchloraemic metabolic acidosis indeed occurred with saline not only much more often than with lactated Ringer, but these saline resuscitated patients also required greater amounts of blood component therapy [40]. However, no differences in renal function were identified. A second small study randomized patients undergoing renal transplantation to receive either isotonic saline or lactated Ringer with the SCr as the primary outcome at Day 3 post-transplant [41]. A markedly increased incidence of hyperkalaemia (>6 mEq/L) was found in the saline group, presumably due to the higher incidence of acidosis. Due to these significant differences between the groups, the trial was halted early for safety reasons.
We concur, however, with the previously mentioned review of Kellum et al. [27] that at present there is no evidence that the anionic composition of currently available crystalloids increases the risk of AKI in humans. However, it should be remembered that large volume administration of saline leads to hyperchloraemic acidosis that may be associated with hyperkalaemia and blood coagulation disturbances [42]. Recent in vitro studies [43] showed that hypothermia produced coagulation changes that were worsened by acidosis, whereas acidosis without hypothermia has no significant effect on coagulation. This effect was mediated by the inhibition of coagulation factors and platelet function. Whether these metabolic side effects of large volumes of saline have dramatic clinical impact is not known at this time.
The different tonicity and anionic composition of IV fluids on the prevention of AKI have been explored mainly in the setting of prevention of CIN and of (traumatic) rhabdomyolysis.
Fluid choice in prevention of CIN
Although volume expansion and treatment of dehydration is well established in the prevention of CIN, a recent propensity analysis noted that these strategies to prevent CIN are implemented rather non-uniformly [44].
The fluids that have been tested in this setting are hypotonic saline (0.45%), isotonic saline (0.9%) and isotonic bicarbonate. The interpretation of these studies is hampered by the fact that not in every study all other risk factors for CIN have been excluded or considered, i.e. age of the patient, presence of pre-contrast CKD and/or diabetes, type and dose of contrast agent, associated therapy with N-acetylcysteine (NAC) and other risk factors (for reviews and consensus guidelines on the prevention of CIN, see [45–51]).
Mueller et al. [52] found that IV hydration using a 0.9% saline solution compared with a 0.45% saline solution in dextrose in 1620 patients undergoing coronary angiography significantly reduced CIN. The sustained administration of isotonic saline before and after radiocontrast injection thus seems to be more protective than equivalent volumes of hypotonic saline and saline [53].
A small single-centre RCT [54] enrolling 119 patients with stable SCr of at least 1.1 mg/dL, randomized to either infusion of isotonic saline or isotonic sodium bicarbonate before and after CM administration. CIN (defined as an increase of 25% in SCr from baseline within 48 h) developed in 1.7% in the bicarbonate group, compared to 13.6% in the saline solution group. The hypothesis that IV sodium bicarbonate might decrease the incidence of CIN compared with saline was subsequently tested in two more recent trials [55,56]. While these two studies also suggest that isotonic bicarbonate may provide greater benefit than isotonic saline, either in association with NAC or not, neither study can be considered to be conclusive. Two recent studies prospectively directly compared the efficacy of sodium bicarbonate versus isotonic saline in the prevention of CIN. Maioli et al. [57] investigated the two solutions in addition to NAC in a reasonably large population of 502 patients with an estimated creatinine clearance <60 mL/min and undergoing coronary angiography or intervention. Patients in both groups received oral NAC 600 mg twice a day and CIN was defined as an absolute increase of SCr ≥0.5 mg/dL measured within 5 days. CIN occurred in 10.8%; 10% were treated with sodium bicarbonate and 11.5% with saline. In patients with CIN, the mean increase in creatinine was not significantly different in the two study groups.
The smaller Renal Insufficiency Following Radiocontrast Exposure (REINFORCE) study [58] is an RCT including 145 patients (age 72.6 ± 6.7 years) with slightly elevated baseline SCr levels (mean 132.6 ± 29.3 μmol/L) and randomized to either an isotonic infusion of sodium bicarbonate or sodium chloride 0.9% solution for volume expansion. The primary endpoint was an elevation of SCr beyond 25% or 44 μmol/L on the first or second day following exposure to the CM. An overall low incidence of CIN (3.4%) was observed but with equal distribution among the groups (4.2% in sodium bicarbonate versus 2.7% in sodium chloride group). Parameters of renal function demonstrated no differences between the two hydration regimens on Day 1 after angiography; even on Day 2 most parameters were similar in both groups.
Based on these two last prospective studies, bicarbonate does not seem to be more efficient than saline. Furthermore, a retrospective cohort study at the Mayo Clinic assessed the risk of CIN associated with the use of sodium bicarbonate, NAC or the combination. Surprisingly, IV sodium bicarbonate was associated with an increased incidence of CIN [59]. At present, it should be concluded that the use of sodium bicarbonate to prevent contrast nephropathy should be evaluated further rather than adopted into clinical practice
There is also no clear evidence to guide the choice of the optimal rate and duration of infusion in CIN prevention. However, good urine output (>150 mL/h) in the 6 h after the radiological procedure has been associated with reduced rates of AKI in one study [60]. Since not all of intravenously administered isotonic crystalloid remains in the vascular space, in order to achieve a urine flow rate of at least 150 mL/h, ≥1.0–1.5 mL/kg/min of IV fluid has to be administered for 3–12 h before and 6–12 h after contrast exposure. Oral volume expansion may have some benefit, but there is not enough evidence to show that it is as effective as IV volume expansion [61].
Fluid selection in traumatic and non-traumatic rhabdomyolysis
Impaired kidney perfusion and intratubular obstruction by myoglobin and uric acid contribute to the pathogenesis of AKI in rhabdomyolysis. Prevention of AKI involves vigorous plasma volume expansion to maintain renal perfusion pressure and dilute myoglobin and other toxins. The traditional approach to prevention and treatment of pigment-induced ARF is to use isotonic saline solution resuscitation followed by a forced mannitol–alkaline diuresis to maintain the urine pH >6.5 [62]. Theoretically, urine alkalinization helps prevent tubular pigment cast formation and may also reduce the conversion of haemoglobin to methaemoglobin, and release of iron from myoglobin. There is, however, no clinical evidence that mannitol and bicarbonate are more effective than saline solution alone. Furthermore, there are potential risks to bicarbonate therapy, including precipitation of calcium phosphate and inducing or exacerbating hypocalcaemia [63]. Knottenbelt has demonstrated that large-volume infusion of crystalloid alone creates a solute diuresis sufficient to alkalinize the urine [64].
In certain settings, such as traumatic rhabdomyolysis in disaster circumstances, early and aggressive fluid resuscitation has clearly been shown to be beneficial [31,65,66]. Early fluid resuscitation (within the first 6 h, preferably before the victim is extricated) is essential (for review see [66]. The preferred fluid is isotonic saline, given at a rate of 1 L/h (10–15 mL/kg of body weight), while the victim is under the rubble, followed by hypotonic saline soon after rescue. Despite the reservations made above, adding 50 mEq of sodium bicarbonate to each second or third litre of hypotonic saline (usually a total of 200–300 mEq the first day) will maintain urinary pH >6.5 and should help prevention of intratubular deposition of myoglobin and uric acid. If urinary flow exceeds 20 mL/h, 50 mL of 20% mannitol [1–2 g/kg/day (total, 120 g) [67] given at a rate of 5 g/h] may be added to each litre of infusate. The addition of mannitol also decreases compartmental pressure [68]. Once a patient with the crush syndrome has been hospitalized, urinary output should ideally exceed 300 mL/h. Such a goal may require the IV infusion of up to 12 L of fluid per day (4–6 L of which will contain bicarbonate). The volume administered is generally much greater than the urinary output; the difference between intake and output is due to the accumulation of fluid in the damaged muscles, which may exceed 4 L. This protocol should be continued until clinical or biochemical evidence of myoglobinuria disappears (usually by Day 3). However, the urinary response may differ from patient to patient, and fluid administration should be individualized according to the patient's clinical course or haemodynamic monitoring. If the patient cannot be monitored closely because of chaotic disaster conditions, <6 L of a mannitol–alkaline solution should be infused per day to avoid volume overload [69]. Patients with insufficient urinary output should be monitored closely, so that hypervolaemia can be prevented or, if necessary, dialysis initiated.
Interaction intensivist-nephrologist in the ICU
In the case of critically ill ICU patients, the nephrologist should be familiar with some important general measures that may have a potential beneficial impact on the kidney and that at present are applied by intensivists.
In sepsis, some interventions have led to a reduction in total mortality but none has clearly reduced the incidence of septic AKI, except maybe the administration of activated protein C, as was shown in an animal model [70].
Early fluid resuscitation of critically ill patients with sepsis, initiated already in the emergency room, may reduce not only the mortality but also AKI in these patients [16].
Nevertheless, it is important to realize that already on the first day of sepsis, important ‘cross-talk’ between the kidney and other organs (heart, lungs, brain) takes place [71–73] and fluid loading can further enhance pulmonary congestion [74].
Tight glycaemia control
Many adverse effects of hyperglycaemia in critically ill patients [75] and of glycaemia variability in sepsis patients [76] have been described, and controlled trials in a single ICU centre at the University of Leuven showed a reduction in AKI incidence and mortality with strict control of blood glucose concentration by continuous insulin administration [77]. A follow-up study in patients in the medical ICU did not significantly reduce in-hospital mortality in patients who stayed in the ICU for <3 days. However, in-hospital mortality was lower, in part by the prevention of newly acquired kidney injury in patients who stayed in the ICU for 3 or more days [78]. Analysis of the pooled data showed a clear reno-protective effect of strict glycaemia control in the overall critically ill patient population [79].
A 2007 systematic review on five studies that evaluated the effect of insulin therapy on outcomes among critically ill patients [80] showed a reduced incidence of AKI by 38%. Not unexpectedly, intensive insulin therapy was associated with a >4-fold increase in the risk of hypoglycaemia.
However, these positive results have recently been contradicted by three other studies. The first of these studies, the VISEP trial, used a two-by-two factorial design in which 537 critically ill patients with sepsis in 18 academic hospitals were randomized to receive either intensive insulin treatment (IIT) or conventional glucose control, together with either pentastarch or Ringer's lactate for fluid resuscitation [37]. The blood glucose levels were equivalent to those used in the Leuven studies. The VISEP study was terminated early for safety reasons because 17% of the IIT group developed severe hypoglycaemia compared with 4.1% of the conventional therapy group. In addition, this study found no significant evidence, or even an indication, of a trend towards a renal benefit from IIT.
These negative findings were confirmed by the Glucontrol study [81], which was designed to randomize 3500 critically ill patients from 21 surgical and medical units IIT or to conventional glucose control. Similar to VISEP, however, the Glucontrol trial was terminated because of safety concerns with IIT (hypoglycaemia), after 1101 patients had been randomized. Additionally, this study showed no difference in mortality between patients randomized to IIT and those randomized to conventional glucose control and found no difference in renal outcomes between the two groups. Finally, a recent cohort study [82] including 10 456 patients admitted to ICUs in a single centre and comparing three consecutive time periods before and after implementation of an IIT protocol showed that this policy was not associated with decreased in-hospital mortality.
Also, a recent meta-analysis [83] showed that tight glycaemic control does not significantly reduce in-hospital mortality in critically ill adult patients, and was not associated with significantly decreased risk for new need for dialysis; there was a beneficial effect on risk of septicaemia, but a higher risk of hypoglycaemia. Further data on the benefits and harms of tight glycaemic control in critically ill patients will be available once the NICE SUGAR study, a pragmatic study of tight glycaemic control being conducted in 41 hospitals in Australia, New Zealand and Canada and at the Mayo Clinic in the United States, is completed. At present, the most recent results on this important issue should give pause to those who have adopted or are considering adopting stringent blood glucose control protocols in the ICU. Such protocols should be reserved to ICUs that can apply quality standards for tight glycaemic control and dispose of affordable methods of frequent and highly accurate measurement of blood glucose on the bedside [84].
This does not mean that a better attention to the blood glucose level control in these patients should not be recommended but with careful attention to avoiding hypoglycaemia.
Low doses of corticoids in septic shock patients
Since many years the use of low-dose hydrocortisone in patients with septic shock has been recommended, and recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill patients have been published [85].
These recommendations are based primarily on a study of patients with septic shock who remained hypotensive after at least 1 h of resuscitation with fluids and vasopressors [86]. In this study, a benefit was seen in patients with no response to corticotrophin who received hydrocortisone and fludrocortisone. A Cochrane review in 2004 concluded, however, that low doses of corticosteroids did not change 28-day mortality and hospital mortality in severe sepsis and septic shock [87]. A long course of low-dose corticosteroids reduced 28-day all-cause mortality and ICU and hospital mortality. The recently published CORTICUS study [88] concluded that hydrocortisone did not improve survival or reversal of shock in patients with septic shock, either overall or in patients who did not have a response to corticotrophin; however, hydrocortisone hastened the reversal of shock in patients in whom shock was reversed. Whether the more rapid correction of shock results in less AKI is unclear from this study; it should be noted that there were more episodes of superinfection, including new sepsis and septic shock in the hydrocortisone treated patients.
Avoidance of drug- and nephrotoxin-induced AKI
Before some selected drugs are discussed, an important remark should be made: for all medications that are largely dependent on the renal function for their elimination, dose adaptations in patients with either acute or chronic reduction of renal function should be applied. These adaptations should be based on knowledge of the altered pharmacokinetics, including changes in distribution volume and/or protein binding of these drugs and/or on frequent plasma drug monitoring.
ACEIs, ARBs and NSAIDs (for review, see [89])
These drugs interfere with the autoregulation of renal blood flow (RBF) and GFR, and can provoke acute haemodynamically mediated renal dysfunction.
In particular, ACEIs and ARBs may be associated more commonly with renal dysfunction because any decline in intraglomerular pressure due to blood pressure lowering will be exaggerated by concomitant vasodilatation of the efferent arteriole. In patients in whom the increase in SCr is >30% after initiation of ACEI and ARB treatment or in whom repeated measurements show a progressive increase, these drugs should be discontinued and bilateral renal-artery stenosis, stenosis of the renal artery in a solitary kidney, intrarenal diffuse nephrosclerosis, polycystic kidney disease with the renal arteries being extrinsically compressed by large cysts, decreased absolute or effective arterial blood volume, use of NSAIDs, calcineurin inhibitors and sepsis should be excluded. The frequency of AKI induced by these drugs varies between 6% and 23% in patients with bilateral renal-artery stenosis and increases to 38% in patients with unilateral stenosis in a single kidney. Patients chronically treated with ACEIs have an increased risk of post-operative renal dysfunction [90], most probably as a consequence of intraoperative hypotensive episodes or of post-operative hypovolaemia.
A common scenario in which ACEIs or ARBs may be associated with acute renal dysfunction is the presence of decompensated heart failure, the so-called cardio-renal syndrome. However, the typical patient with worsening renal function during an acute episode of decompensation has been treated with these agents for many years, and initiation of ACEIs or ARBs is not commonly the explanation for worsening renal function [91].
Several recent studies show that 20–25% of patients hospitalized for congestive heart failure will develop renal dysfunction, irrespective of treatment with ACEIs and/or ARBs [92,93]. Reasons for this type of progressive renal failure include overdiuresis resulting in intravascular volume depletion, critical renal-artery stenosis or patients with acute decompensation who have a precipitous drop in cardiac output. This acute deterioration of renal function during therapy should be distinguished from the two other forms of ‘cardio-renal syndrome’ like heart failure with concomitant and significant renal disease (cardio-renal failure) and diuretic resistance [94].
Acute inhibition of cyclo-oxygenase (type I or II) by NSAIDs can reduce GFR and RBF in particular clinical situations, such as atherosclerotic cardiovascular disease in a patient older than 60 years, pre-existing chronic renal insufficiency and states of renal hypoperfusion such as sodium depletion, diuretic use, hypotension and sodium-avid states such as cirrhosis, nephrotic syndrome and congestive heart failure or in the presence of other potentially nephrotoxic medications, such as aminoglycosides, ACEIs and ARBs. Renal hypoperfusion due to decreased effective circulating volume is relatively common in critically ill patients and inhibition of prostaglandin-induced vasodilation may further compromise RBF and exacerbate ischaemic injury. There is little evidence that NSAIDs impair renal function in otherwise healthy individuals.
Aminoglycosides (for extensive review, see [95]. Aminoglycoside nephrotoxicity develops in ∼10–15% of patients treated with aminoglycosides. Since aminoglycosides are excreted entirely by glomerular filtration, dosing of these drugs appears to be a critical factor in the development of AKI, particularly in patients with already compromised renal function, e.g. because of sepsis or hypovolaemia. With multiple daily dosing schedules, elevated peak levels appear to correlate with toxicity. Since aminoglycoside uptake by proximal tubular cells is saturable, once-daily dosing has been postulated to decrease tubular cell toxicity by reducing the fraction of the cumulative dose of drug taken up by proximal tubular cells [95]. The administration of large single doses may not result in increased renal uptake, and in fact is associated with decreased uptake because the drug is given less often [89,95].
Extended interval dosing has been shown to attain an adequate anti-microbial target in the general patient population, while decreasing the risk of nephrotoxicity compared with multiple daily dosing. However, critically ill ICU patients have pharmacokinetic differences compared with patients who are less ill, including an increased volume of distribution and variable clearance, which may make attainment of these targets difficult [96]. When extended-interval aminoglycoside dosing is applied, antibiotic maximal serum concentration (Cmax) monitoring and determination of the minimal inhibitory concentration (MIC) of the pathogen may be needed to optimally treat serious infections in this type of patient.
Once-daily dosing of aminoglycosides is the only clinical approach that is commonly used, but a clear reduction in nephrotoxicity has not been demonstrated by this regimen [89]. It should be remembered that clinical evidence of aminoglycoside-induced AKI (increase in SCr) is seen only 5–10 days after initiation of the treatment, is generally non-oliguric and may be associated with decreased urine-concentrating ability and urinary magnesium wasting. It is generally reversible after discontinuation of the drug; however, supportive RRT may be required.
Amphotericin B
AKI, defined as a 50% increase in baseline SCr with a peak ≥2.0 mg/dL, and associated with amphotericin B occurred in as many as one-third of treated patients, with progressive increase in the risk of AKI with increases in cumulative dose [97]. Lipid formulations of amphotericin B seem to cause less nephrotoxicity compared with standard formulations [98], and are thus an important strategy to preserve renal function and improve survival in critically ill patients with systemic fungal infections. However, they are considerably more expensive and the recent introduction of alternative antifungal agents such as itraconazole, voriconazole, and caspofungin has largely supplanted the use of amphotericin B in high-risk patients with renal impairment. Amphotericin B continues to be used widely in patients with normal renal function because of its relatively low cost and broad spectrum of activity [89].
Contrast media (CM)
Iodinated CM can be categorized according to osmolality, high-osmolal CM (HOCM ∼ 2000 mOsm/kg), low-osmolal CM (LOCM, 600–800 mOsm/kg) and iso-osmolal CM (IOCM, 290 mOsm/kg). Evidence to date suggests that compared to low- and high-osmolal formulations, the iso-osmolal, non-ionic CM are the least nephrotoxic, particularly after intravascular administration [99–101]. Clinically significant CIN following nonemergent computed tomography with IV CM is uncommon among outpatients even with mild baseline kidney disease [102].
In a pooled analysis of 16 trials of intra-arterial CM, the incidence of CIN was significantly lower with the iso-osmolal iodixanol than with the comparator LOCM [101]. A meta-analysis of the renal tolerability of another IOCM, iotrolan 280, provides further evidence that IOCM is associated with a lower risk of post-procedure renal impairment [103]. The recent RECOVER trial also showed a significantly lower rate of CIN with iodixanol compared with ioxaglate in high-risk patients undergoing coronary angiography [104]. However, in trials in low-risk patients, the rates of CIN were similar with iodixanol and iopamidol (LOCM) after IV administration for computerized tomography (IMPACT trial) [105] or intra-coronary administration (CARE trial) [106]. On the basis of these results, in all patients at risk for CIN (mainly pre-existing CKD and diabetes mellitus), non-ionic iso-osmolar CM are a reasonable choice for intravascular procedures.
Also, the volume of the CM is a crucial risk factor and independent predictor of contrast-induced AKI [50]. As a general rule, the volume of contrast received should not exceed twice the baseline level of eGFR in millilitres [107]. Recent evidence suggests that in patients at risk for CIN, even with the use of an IOCM like iodixanol, the use of ultra-low doses (<50 mL) was effective in reducing CIN [108]. In general, administration of a volume <100 mL should be attempted and repetitive, closely spaced studies (e.g. <48 h apart) should be avoided.
Another approach to prevent CIN is to use an alternative, less nephrotoxic contrast agent, i.e. gadolinium salts. Results from several case series and isolated case reports suggest improved renal safety in patients with pre-existing CKD. A recent study [109] showed that compared to iodinated contrast, gadolinium contrast is associated with a significantly lower incidence of CIN and early progression to ESRD in patients with pre-existing CKD. In the case of relatively mild renal dysfunction, and in contrast with CIN following iodinated CM, a direct correlation between the volume of gadolinium contrast and post-procedure nephropathy could not be established [109]. More concerning is the strong association of gadolinium with nephrogenic systemic fibrosis (NSF), a devastating fibrosing disorder of the skin and other systemic organs. Although cause and effect have not been proven for the NSF–gadolinium link, the impaired renal elimination of gadolinium in patients with kidney disease and the instability of gadolinium-chelate binding may expose tissues to toxic free Gd (3+) and promote this fibrosing disorder [110,111].
A great number of other potential nephrotoxins exist, and the discussion of the prevention of each individual nephrotoxin is beyond the scope of this paper. A comprehensive overview can be found in a textbook [112] and recent reviews [89,90]. The potential nephrotoxic effects of specific anti-tumoural drugs have also recently been detailed [113].
Conflict of interest statement. None declared.
References
- 1.Kellum JA, Levin N, Bouman C, et al. Developing a classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–514. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008;3:864–868. doi: 10.2215/CJN.04851107. [DOI] [PubMed] [Google Scholar]
- 4.Thurman JM, Parikh CR. Peeking into the black box: new biomarkers for acute kidney injury. Kidney Int. 2008;73:379–381. doi: 10.1038/sj.ki.5002739. [DOI] [PubMed] [Google Scholar]
- 5.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson MR, Friedewald JJ, Eustace JA, et al. Acute kidney injury. In: Brenner BM, editor. Brenner & Rector's the Kidney. Philadelphia: Saunders & Elsevier; 2008. pp. 943–986. [Google Scholar]
- 7.Lameire N, Van Biesen W, Vanholder R. Epidemiology, clinical evaluation, and prevention of acute renal failure. In: Feehally J, Floege J, Johnson RJ, editors. Comprehensive Clinical Nephrology. Philadelphia: Mosby-Elsevier; 2007. pp. 771–785. [Google Scholar]
- 8.Lee VWS, Harris D, Anderson RJ, et al. Acute renal failure. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2007. pp. 986–1034. [Google Scholar]
- 9.Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3:1266–1273. doi: 10.2215/CJN.05271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himmelfarb J, Joannidis M, Molitoris B, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962–967. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 12.De Broe ME, Porter GA, Bennett WM, et al., editors. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 15.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 16.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 17.Rivers EP, McIntyre L, Morro DC, et al. Early and innovative interventions for severe sepsis and septic shock: taking advantage of a window of opportunity. CMAJ. 2005;173:1054–1065. doi: 10.1503/cmaj.050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SM, Huang CD, Lin HC, et al. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26:551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 19.Durairay L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008;133:252–263. doi: 10.1378/chest.07-1496. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients: SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- 21.Michard F. Volume management using dynamic parameters: the good, the bad, and the ugly. Chest. 2005;128:1902–1903. doi: 10.1378/chest.128.4.1902. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 23.Hofer CK, Muller SM, Furrer L, et al. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848–854. doi: 10.1378/chest.128.2.848. [DOI] [PubMed] [Google Scholar]
- 24.Monnet X, Teboul JL. Passive leg raising. Intensive Care Med. 2008;34:659–663. doi: 10.1007/s00134-008-0994-y. [DOI] [PubMed] [Google Scholar]
- 25.Reuter DA, Felbinger TW, Schmidt C, et al. Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med. 2002;28:392–398. doi: 10.1007/s00134-002-1211-z. [DOI] [PubMed] [Google Scholar]
- 26.Pinsky MR, Brophy P, Padilla J, et al. Fluid and volume monitoring. Int J Artif Organs. 2008;31:111–126. doi: 10.1177/039139880803100205. [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA, Cerda J, Kaplan LJ, et al. Fluids for prevention and management of acute kidney injury. Int J Artif Organs. 2008;31:96–110. doi: 10.1177/039139880803100204. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Ronco C, Mehta RL. Fluid management in acute kidney injury. Int J Artif Organs. 2008;31:94–95. doi: 10.1177/039139880803100203. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman R, Kellum JA. Prevention of acute renal failure. Chest. 2007;131:300–308. doi: 10.1378/chest.06-1246. [DOI] [PubMed] [Google Scholar]
- 30.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 31.Better OS, Rubinstein I. Management of shock and acute renal failure in casualties suffering from the crush syndrome. Ren Fail. 1997;19:647–653. doi: 10.3109/08860229709109030. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–C34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 33.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 34.Gallet de Saint-Aurin R, Kloeckner M, Annane D. Crystalloids versus colloids for fluid resuscitation in critically-ill patients. Acta Clin Belg Suppl. 2007;62:412–416. doi: 10.1179/acb.2007.093. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL. Fluid resuscitation: colloids vs crystalloids. Acta Clin Belg Suppl. 2007;62:408–411. [PubMed] [Google Scholar]
- 36.Boldt J, Brosch C, Ducke M, et al. Influence of volume therapy with a modern hydroxyethylstarch preparation on kidney function in cardiac surgery patients with compromised renal function: a comparison with human albumin. Crit Care Med. 2007;35:2740–2746. doi: 10.1097/01.CCM.0000288101.02556.DE. [DOI] [PubMed] [Google Scholar]
- 37.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 38.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients (review) Cochrane Database of Systematic Reviews. 2007;(4):1–393. doi: 10.1002/14651858.CD000567.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters JH, Gottlieb A, Schoenwald P, et al. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 41.O’Malley CM, Frumento RJ, Hardy MA, et al. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–1524. doi: 10.1213/01.ANE.0000150939.28904.81. table. [DOI] [PubMed] [Google Scholar]
- 42.Engstrom M, Schott U, Romner B, et al. Acidosis impairs the coagulation: a thromboelastographic study. J Trauma. 2006;61:624–628. doi: 10.1097/01.ta.0000226739.30655.75. [DOI] [PubMed] [Google Scholar]
- 43.Dirkmann D, Hanke AA, Gorlinger K, et al. Hypothermia and acidosis synergistically impair coagulation in human whole blood. Anesth Analg. 2008;106:1627–1632. doi: 10.1213/ane.0b013e31817340ad. [DOI] [PubMed] [Google Scholar]
- 44.Weisbord SD, Mor MK, Resnick AL, et al. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch Intern Med. 2008;168:1325–1332. doi: 10.1001/archinte.168.12.1325. [DOI] [PubMed] [Google Scholar]
- 45.Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 46.Pannu N, Tonelli M. Strategies to reduce the risk of contrast nephropathy: an evidence-based approach. Curr Opin Nephrol Hypertens. 2006;15:285–290. doi: 10.1097/01.mnh.0000222696.92088.28. [DOI] [PubMed] [Google Scholar]
- 47.Lameire N. Contrast-induced nephropathy in the critically-ill patient: focus on emergency screening and prevention. Acta Clin Belg Suppl. 2007;62:346–352. doi: 10.1179/acb.2007.078. [DOI] [PubMed] [Google Scholar]
- 48.Lameire NH. Contrast-induced nephropathy—prevention and risk reduction. Nephrol Dial Transplant. 2006;21(Suppl 1):i11–i23. doi: 10.1093/ndt/gfl215. [DOI] [PubMed] [Google Scholar]
- 49.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113:1799–1806. doi: 10.1161/CIRCULATIONAHA.105.595090. [DOI] [PubMed] [Google Scholar]
- 50.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 52.Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 53.Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clin J Am Soc Nephrol. 2008;3:273–280. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 54.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 55.Briguori C, Airoldi F, D’Andrea D, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 56.Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007;49:1283–1288. doi: 10.1016/j.jacc.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 58.Adolph E, Holdt-Lehmann B, Chatterjee T, et al. Renal insufficiency following radiocontrast exposure trial (REINFORCE): a randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coron Artery Dis. 2008;19:413–419. doi: 10.1097/MCA.0b013e3283021ac6. [DOI] [PubMed] [Google Scholar]
- 59.From AM, Bartholmai BJ, Williams AW, et al. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol. 2008;3:10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33:403–411. doi: 10.1016/s0735-1097(98)00574-9. [DOI] [PubMed] [Google Scholar]
- 61.Taylor AJ, Hotchkiss D, Morse RW, et al. PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest. 1998;114:1570–1574. doi: 10.1378/chest.114.6.1570. [DOI] [PubMed] [Google Scholar]
- 62.Eneas JF, Schoenfeld PY, Humphreys MH. The effect of infusion of mannitol-sodium bicarbonate on the clinical course of myoglobinuria. Arch Intern Med. 1979;139:801–805. [PubMed] [Google Scholar]
- 63.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 64.Knottenbelt JD. Traumatic rhabdomyolysis from severe beating— experience of volume diuresis in 200 patients. J Trauma. 1994;37:214–219. [PubMed] [Google Scholar]
- 65.Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862–1867. doi: 10.1097/01.asn.0000129336.09976.73. [DOI] [PubMed] [Google Scholar]
- 66.Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med. 2006;354:1052–1063. doi: 10.1056/NEJMra054329. [DOI] [PubMed] [Google Scholar]
- 67.Better OS. Rescue and salvage of casualties suffering from the crush syndrome after mass disasters. Mil Med. 1999;164:366–369. [PubMed] [Google Scholar]
- 68.Better OS, Rubinstein I, Winaver JM, et al. Mannitol therapy revisited (1940–1997) Kidney Int. 1997;52:886–894. doi: 10.1038/ki.1997.409. [DOI] [PubMed] [Google Scholar]
- 69.Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 70.Gupta A, Berg DT, Gerlitz B, et al. Role of protein C in renal dysfunction after polymicrobial sepsis. J Am Soc Nephrol. 2007;18:860–867. doi: 10.1681/ASN.2006101167. [DOI] [PubMed] [Google Scholar]
- 71.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabb H, Wang Z, Nemoto T, et al. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63:600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 73.Vanholder R, De Deyn PP, Van Biesen W, et al. Marconi revisited: from kidney to brain—two organ systems communicating at long distance. J Am Soc Nephrol. 2008;19:1253–1255. doi: 10.1681/ASN.2008040404. [DOI] [PubMed] [Google Scholar]
- 74.Van Biesen W, Yegenaga I, Vanholder R, et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18:54–60. [PubMed] [Google Scholar]
- 75.Berkers J, Gunst J, Vanhorebeek I, et al. Glycaemic control and perioperative organ protection. Best Pract Res Clin Anaesthesiol. 2008;22:135–149. doi: 10.1016/j.bpa.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Ali NA, O’Brien JM, Jr, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 78.Van Den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 79.Schetz M, Vanhorebeek I, Wouters PJ, et al. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol. 2008;19:571–578. doi: 10.1681/ASN.2006101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas G, Rojas MC, Epstein SK, et al. Insulin therapy and acute kidney injury in critically ill patients a systematic review. Nephrol Dial Transplant. 2007;22:2849–2855. doi: 10.1093/ndt/gfm401. [DOI] [PubMed] [Google Scholar]
- 81.Devos P, Preiser J, Mélot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the glucocontrol study. Int Care Med. 2007;33:S189. [Google Scholar]
- 82.Treggiari MM, Karir V, Yanez ND, et al. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008;12:R29. doi: 10.1186/cc6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adult patients: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 84.Finfer S, Delaney A. Tight glycemic control in critically ill adult patients. JAMA. 2008;300:963–965. doi: 10.1001/jama.300.8.963. [DOI] [PubMed] [Google Scholar]
- 85.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 86.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 87.Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. Br Med J. 2004;329:480. doi: 10.1136/bmj.38181.482222.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 89.Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36:S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 90.Evenepoel P. Acute toxic renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:37–52. doi: 10.1016/j.bpa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Chittineni H, Miyawaki N, Gulipelli S, et al. Risk for acute renal failure in patients hospitalized for decompensated congestive heart failure. Am J Nephrol. 2007;27:55–62. doi: 10.1159/000099012. [DOI] [PubMed] [Google Scholar]
- 93.Cruz CS, Cruz LS, Silva GR, et al. Incidence and predictors of development of acute renal failure related to treatment of congestive heart failure with ACE inhibitors. Nephron Clin Pract. 2007;105:c77–c83. doi: 10.1159/000097658. [DOI] [PubMed] [Google Scholar]
- 94.Liang KV, Williams AW, Greene EL, et al. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med. 2008;36:S75–S88. doi: 10.1097/01.CCM.0000296270.41256.5C. [DOI] [PubMed] [Google Scholar]
- 95.Bosmans JL, De Broe ME. Antibiotic- and immunosuppression-related renal failure. In: Schrier RW, editor. Diseases of the Kidney & Urinary Tract. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 1035–1067. [Google Scholar]
- 96.Rea RS, Capitano B. Optimizing use of aminoglycosides in the critically ill. Semin Respir Crit Care Med. 2007;28:596–603. doi: 10.1055/s-2007-996406. [DOI] [PubMed] [Google Scholar]
- 97.Bates DW, Su L, Yu DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001;32:686–693. doi: 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- 98.Saliba F, Dupont B. Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med Mycol. 2008;46:97–112. doi: 10.1080/13693780701730469. [DOI] [PubMed] [Google Scholar]
- 99.Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–499. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 100.Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999;72:701–703. doi: 10.1259/bjr.72.859.10624328. [DOI] [PubMed] [Google Scholar]
- 101.McCullough PA, Bertrand ME, Brinker JA, et al. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006;48:692–699. doi: 10.1016/j.jacc.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 102.Weisbord SD, Mor MK, Resnick AL, et al. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol. 2008;3:1274–1281. doi: 10.2215/CJN.01260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clauss W, Dinger J, Meissner C. Renal tolerance of iotrolan 280: a meta-analysis of 14 double-blind studies. Eur Radiol. 1995;5:S79–S84. [Google Scholar]
- 104.Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006;48:924–930. doi: 10.1016/j.jacc.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 105.Barrett BJ, Katzberg RW, Thomsen HS, et al. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest Radiol. 2006;41:815–821. doi: 10.1097/01.rli.0000242807.01818.24. [DOI] [PubMed] [Google Scholar]
- 106.Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189–3196. doi: 10.1161/CIRCULATIONAHA.106.671644. [DOI] [PubMed] [Google Scholar]
- 107.Laskey WK, Jenkins C, Selzer F, et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584–590. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 108.Kane GC, Doyle BJ, Lerman A, et al. Ultra-low contrast volumes reduce rates of contrast-induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. J Am Coll Cardiol. 2008;51:89–90. doi: 10.1016/j.jacc.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 109.Kane GC, Stanson AW, Kalnicka D, et al. Comparison between gadolinium and iodine contrast for percutaneous intervention in atherosclerotic renal artery stenosis: clinical outcomes. Nephrol Dial Transplant. 2008;23:1233–1240. doi: 10.1093/ndt/gfm725. [DOI] [PubMed] [Google Scholar]
- 110.Nortier JL, del Marmol V. Nephrogenic systemic fibrosis—the need for a multidisciplinary approach. Nephrol Dial Transplant. 2007;22:3097–3101. doi: 10.1093/ndt/gfm430. [DOI] [PubMed] [Google Scholar]
- 111.Perazella MA, Rodby RA. Gadolinium use in patients with kidney disease: a cause for concern. Semin Dial. 2007;20:179–185. doi: 10.1111/j.1525-139X.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 112.Clinical Nephrotoxins. Dordrecht: Kluwer; 2003. p. 3–712. [Google Scholar]
- 113.Lameire NH, Flombaum CD, Moreau D, et al. Acute renal failure in cancer patients. Ann Med. 2005;37:13–25. doi: 10.1080/07853890510007205. [DOI] [PubMed] [Google Scholar]