Review on the functions of miRNAs in CD8+T cell biology in the settings of cell development, infection, and oncogenesis.
Keywords: development, activation, differentiation
Abstract
During an immune response, CD8+T cells can differentiate into multiple types of effector and memory cells that are important components of immune surveillance. However, their dysregulation has been implicated in infection with viruses or intracellular bacteria and tumorigenesis. miRNAs have been identified as crucial regulators of gene expression, and they perform this function by repressing specific target genes at the post-transcriptional level. Most miRNAs expressed in a given cell type serve the function to impede broadly cell-type-inappropriate gene expression and potently deepen a pre-existing differentiation program. It is increasingly recognized that miRNAs directly modulate the concentration of many regulatory proteins that are required for the development of immune cells in the thymus and their responses in the periphery. This review outlines our current understanding of the function of miRNAs in CD8+T cell biology as it impacts expression of protein-coding genes in the context of proper development, infection, as well as oncogenesis. In addition, we conclude with a perspective on future challenges and the clinical relevance of miRNA biology.
Introduction
miRNAs have been identified over the last decade as key modulators of post-transcriptional gene silencing in mammals. Recently, numerous studies regarding the role of miRNAs in development and function of immune cells have emerged, and several excellent reviews suggest that miRNAs represent a novel layer of control over cellular physiology [1–3]. T cells that express the CD8 coreceptor and recognize peptide–MHC class I complexes have a key role in the control of numerous viral or intracellular bacterial infections [4]. After an acute infection, CD8+T cells rapidly mount a strong defense against pathogens and directly lyse infected cells [5]. In this review, we summarize the recent advances on the functions and mechanisms of action of miRNAs in CD8+T cell biology in the settings of cell development, infection, and oncogenesis.
miRNAs
In this review, we comment only briefly on the basic miRNA biology, as this topic has been reviewed by Baumjohann and Ansel [3], Mendell and Olson [6], Fabian et al. [7], Krol et al. [8], Ceribelli et al. [9], Hammell [10], and Jul Hausser and Zavolan [11]. Specifically, in animals, miRNA genes are transcribed into pri-miRNA transcripts that are processed in the nucleus by the RNase III enzyme complex Drosha and the microprocessor complex subunit DGCR8. The resulting pre-miRNAs are subsequently exported to the cytoplasm by exportin-5. Pre-miRNAs undergo further processing by the RNase III enzyme Dicer and incorporate into an Argonaute-containing, miRNA-induced silencing complex that targets mRNAs for regulation through a variety of mechanisms that involve mRNA deadenylation and translational repression. This ultimately results in mRNA decapping and degradation and thereby, silences protein expression of targeted genes. Interestingly, regulation in the miRNA pathway is a 2-way street. It has been shown in human cells that abundant base-pairing interactions limit miRNAs recycling [12], and this is caused by the addition of nontemplated uridine to the 3′ end of miRNAs [13, 14]. However, studies in Caenorhabditis elegans revealed a model of target-dependent miRNA protection, in which pairing with a partially complementary target mRNA stabilizes the mature miRNAs [15, 16]. The explanation for this discrepancy is still unclear. Nevertheless, these data point to an association between the degree of complementarity and the effect of the target on miRNA stability.
The miRNA provides specificity through complementary base pairing with target mRNAs [17]. Genetic, computational, and biochemical approaches are applied recently to identify miRNA targets [18, 19]. Genetic approaches are based on the finding deletion, or conditional ablation of a gene leads to a partial or complete rescue of the mutant phenotype that caused by the loss of specific miRNA [20]. Based on algorithms, computational approaches, such as PicTar [21], miRanda [22], and TargetScan [23], identify miRNA targets by requiring conserved Watson-Crick pairing to the 5′ region of the miRNA. This criterion is designed to reduce the false-positive rates and promote the sensitivity and the overall accuracy. One disadvantage of these methods is that they are sometimes unable to identify the most biologically key miRNA targets. Biochemical methods, such as high-throughput sequencing of RNA, isolated by cross-linking immunoprecipitation [24] and photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation [25], have been developed recently to identify precise sequences for targeting clinically relevant miRNA–mRNA interactions. Further work is needed to confirm whether the predicted target mRNAs are actually being regulated.
miRNAs IN THYMOCYTE DEVELOPMENT AND MATURATION
Analysis of miRNA expression profiles in thymocytes has identified a wide range of expressed miRNA species and found that specific miRNAs are enriched at distinct stages of development [26, 27]. In addition to this complexity, a trend toward up-regulation of miRNA expression is detected after the DP stage [27]. Furthermore, miRNAs in T cells exhibit an extensive degree of polymorphism at the ends, with the mature miRNAs varying in length at the 3′ end or containing mutated sequences that affect their stability and subcellular localization [28]. These data indicate that expression of miRNAs is dynamically regulated during T cell maturation that could help to preserve the developmental fitness of the CD8+T cell precursors.
Not surprising, an absence of the key factors of the miRNAs biogenesis pathway in immature lymphocytes, such as Dicer, ribonuclease III enzymes Drosha, or the microprocessor complex subunit DGCR8, results in decreased numbers of mature T cells, particularly in the CD8+T compartments, in the periphery [29–32]. Perhaps the best-characterized miRNA during this stage of T cell development is miR-181a, which is the miRNA that is highly expressed in DP thymocytes. During thymic development, miR-181a can function as a rheostat-governing T cell sensitivity [33]. Mechanistically, miR-181a targets several inhibitory phosphatases, including DUSP5, DUSP6, SHP2, and PTPN22, which in turn, leads to an elevated steady state of phosphorylated intermediates, such as ERK1/2 and lymphocyte-specific protein tyrosine kinase, thereby reducing the TCR signaling threshold. In this regard, it is worth pointing out that the repression of individual phosphatase is unable to reproduce fully this phenotype, indicating that the fine-tuned function of miR-181a has not been a result of the dysregulation of a single target gene but results from the synergistic effects of many groups of modestly dysregulated genes [33].
miR-181a comprises a family of 6 miRNAs, which are organized in 3 clusters, 1 of which—miR-181a1b1—has been described recently as essential for thymocyte development. miR-181a1b1 is shown in DP lymphocytes to target directly the 3′ UTRs of Pten, an important inhibitor of PI3K signaling. As a consequence, Pten expression in miR-181a1b1-deficient DP cells is increased, thus explaining the decrease in PI3K signaling, such as activated AKT, repressed forkhead box O protein, and impaired anabolic metabolism. This results in an absence of thymocyte in the thymus, as the development of thymocyte is a life-long process that requires high proliferation rates and elevated biosynthetic demands, and PI3K signaling is a key anabolic determinant required to support these proliferative developmental stages [34].
In addition, Nrarp has been shown to suppress transcriptional activation of Notch-responsive genes in thymocyte development. However, miR-181a1b1 can target Nrarp directly and decrease the threshold of Notch signaling that is required for thymocyte development [35].
miRNA-MEDIATED REGULATION OF CD8+T CELL PROLIFERATION, SURVIVAL, AND MIGRATION
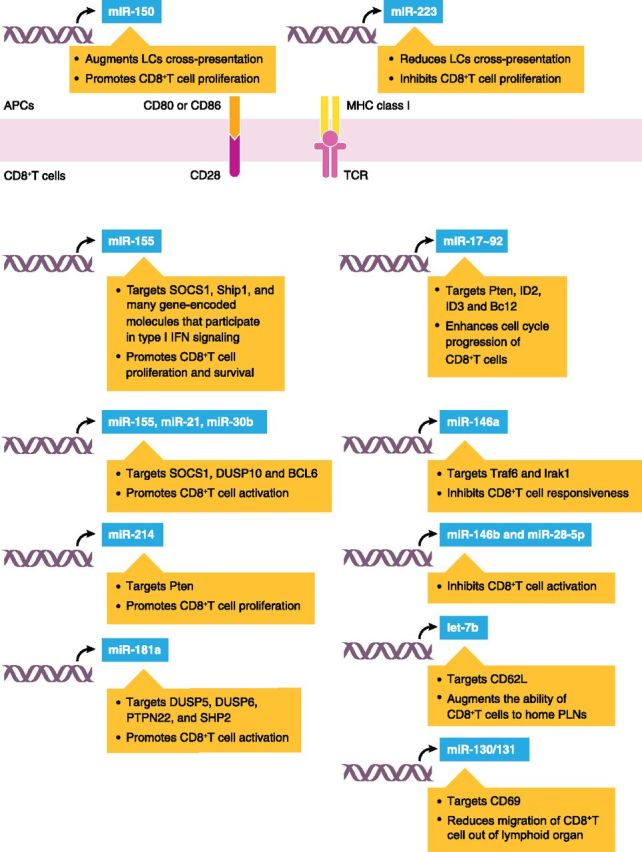
After the identification of the important role of miRNAs in directing thymocyte maturation, the next challenge is to establish whether miRNAs are important for mature CD8+T cell development in the periphery and to discern how they drive CD8+T cell immune responses mechanistically. To circumvent any complications from defective thymocyte development in the absence of miRNAs, several groups have generated Dicer-deficient, mature CD8+T cells, and their results showed that deficiency of Dicer leads to a decrease in the frequency and numbers of CD8+T cells in vivo following virus infections [36, 37]. Intriguingly, Dicer seems to impair initial CD8+T cell activation and proliferation, as suggested in in vitro experiments showing that Dicer-deficient CD8+T cells revealed a surprising increase in cell=cycle entry upon TCR stimulation. However, over time, the percentage of Dicer-deficient cells in vitro is reduced, probably owing to a cell-survival defect. Further work is needed to determine whether this survival defect could explain the in vivo defects. Current data suggest that several specific miRNAs have been implicated in this process and probably account for some of the phenotype of Dicer deficiency in CD8+T cells. For example, miR-155 is up-regulated in in vitro and ex vivo CD8+T cells after activation, and its expression is proportional to the strength of TCR signaling [38]. A recent study found that a lack of miR-155 results in an intrinsic defect of CD8+T cells that affects their proliferation. As such, mice lacking miR-155 in CD8+T cells are impaired in their ability to clear influenza virus. Conversely, the overexpression of miR-155 in CD8+T cells augments protective antiviral responses in vivo that are associated with reductions in viral loads [39]. Another study found a similar role for miR-155 in human CD8+T cells, as patients with T cell-mediated chronic skin disorders, such as vulvar lichen sclerosus and lichen planus, who express high levels of miR-155, have increased numbers of CD8+T cells in the dermis [40]. Among its many identified targets in CD8+T cells, miR-155 inhibits SOCS1 [38], a negative regulator of γc cytokine signaling that plays crucial roles in CD8+T cell expansion [41]. Moreover, following Listeria monocytogenes infection, miR-155-deficient CD8+T cells have a severe block in long-term survival [42]. This block is thought to relate to repressed. proapoptotic protein Akt activation [42], probably as a result of the absence of miR-155-mediated. negative regulation of Ship1 [43, 44]. Lastly, during influenza virus infection, a deficiency of miR-155 in CD8+T cells enhances expression of a battery of genes encoding molecules that promote activation of the type I IFN signaling [39], which has inhibitory function in cell expansion [45, 46]. Together, these data indicate that attenuated common γc cytokine signaling, decreased Akt activation, and aberrant type I IFN signaling likely cooperate to impair the proliferative and survival fitness of miR-155-deficient CD8+T cells. Additionally, a decreased level of expression of a combination of miR-155, miR-21, and miR-30b in CD8+T cells is associated with increased expression of SOCS1, DUSP10, and BCL6, all of which are known inhibitors of TCR signaling, which results in impaired CD8+T cell activation [47, 48]. Like miR-155, miR-17 ∼92 also seems to be particularly important during the expansion phase, owing to the fact that following LCMV infection, miR-17. ∼92, are more expressed in rapidly proliferating effector CD8+T cells than in naïve or nonproliferating effector cells [37]. Furthermore, CD8+T cells, transduced with miR-17 ∼92, enhance their cell-cycle progression, whereas inhibition of miR-17 ∼92 impairs their responses. This function is attributed to the ability of miR-17 ∼92 to target Pten and antiapoptotic molecules, including ID2, ID3, and BCL2 [49]. Furthermore, through the direct targeting of Pten, miR-214, which is the miRNA that is induced in a CD28 costimulation-dependent manner, enhances CD8+T cell proliferation [50]. In contrast to the clear up-regulation observed for miR-155, miR-17 ∼92, and miR-214, expression of the miR-181a tends to decrease along with the mature of CD8+T cells. Indeed, the overexpression of miR-181a in mature cells not only quantitatively enhances TCR sensitivity to antigens but also leads to a qualitative switch and enables them to respond to antagonists [33, 51].
Rather than promote cell development, some miRNAs may function to repress CD8+T cell activation. For example, miR-146a controls the intensity and the duration of NF-κB signaling downstream of TCR activation. Resting CD8+T cells express low constitutive levels of miR-146a that is highly induced upon activation in an NF-κB family member, NF-κB1 (p50)-dependent manner [52, 53]. The sustained expression of miR-146a, in turn, compromises NF-κB signaling by targeting Traf6 and Irak1, both of which are NF-κB signaling transducers [54, 55]. A deficiency of this feedback loop leads to autoimmune-like symptoms and premature death in vivo, as a result of the hyper-response of the miR-146a-deficient CD8+T cells, manifested by increased proliferation, decreased apoptosis, an exaggerated effector phenotype, and aberrant production of effector cytokines [55]. Furthermore, miR-146a, miR-146b, and miR-28-5p have been shown recently to be down-expressed in CD8+T cells from patient with severe asthma syndrome, which results in accelerated activation of circulating CD8+T cells and contributes to the development of disease [56].
Resting CD8+T cells constantly circulate among the blood, lymphoid tissues, and lymphatic vessel. By contrast, during immune responses, activated CD8+T cells suspend migration and reside in lymphoid tissue, where they clonally expand and differentiate into effector cells. It is then vital that CD8+T effector cells regain their motility, exit the lymphoid tissue, and migrate to efferent lymphatic vessel, blood, and the site of inflammation to express immunosuppression [57]. The importance of miRNAs in CD8+T cell trafficking behavior has been confirmed recently in certain well-characterized model systems of infection. Specifically, following Mycobacterium ulcerans infection, CD8+T cells fail to produce let-7b, and consequently, let-7b-deficient CD8+T cells show an impaired capacity to home to PLNs, a process essential for cell proliferation [58]. This defect is caused by the absence of let-7b-mediated, positive regulation of CD62-L, which is involved in initial steps of tethering and rolling of circulating lymphocytes in the high endothelial venules of PLNs [58, 59]. In addition, a deficiency of Dicer results in a significant decrease in the number of CD8+T cells in blood and liver during L. monocytogenes infection, and this is shown to stem from impaired down-regulation of CD69 expression on the surface of Dicer-deficient CD8+T cells that has been linked to the defective migration of activated effector cells out of lymphoid tissues. At least 1 specific miRNA family—miRNA-130/301—has been found to be functionally important in this process, as the overexpression of miR-130/301 in Dicer-deficient CD8+T cells reverses the CD69 retention phenotype to a certain degree [36].
CD8+T cell responses can be influenced indirectly by miRNAs in immune cells other than T cells. Two independent studies have reported that the ability of LCs to mediate CD8+T cell proliferation through cross-presentation is increased in miR-223-deficient mice [60], and that is decreased in miR-150-deficient mice [61]. Further studies are needed to identify the molecular targets of miR-150 and miR-223 in the regulation of LC activity.
In summary, it is increasingly recognized that miRNAs modulate directly the concentration of many regulatory proteins that are required for the development of CD8+T cells in the thymus and their responses in the periphery (Fig. 1). Global deficiency of miRNAs, based on Dicer deficiency, results in poor proliferation and survival, as well as restricted migration of CD8+T cells during infection; these effects likely prevent CD8+T cells from accessing signals necessary to promote expansion in vivo, and these effects are the result of direct and/or indirect action of miRNA on expression of specific signaling pathways and transcription factors. All of these mechanisms are crucial for host defense and are instrumental in regulating multiple differentiation and functional states in CD8+T cells.
Figure 1. miRNA-mediated regulation of CD8+T cell development. miRNAs have been shown to regulate multiple steps in the development of CD8+T cells. miR-155 targets SOCS1, Ship1, and many gene-encoded molecules that participate in type I IFN signaling and promotes CD8+T cell proliferation and survival. In addition, miR-155, miR-21, and miR-30b cooperate to promote CD8+T cell activation by repressing SOCS1, DUSP10, and BCL6, respectively. miR-181a also promotes CD8+T cell activation through targeting phosphatases, including DUSP5, DUSP6, PTPN22, and SHP2. In contrast, miR-146a inhibits CD8+T cell responsiveness through a negative-feedback loop involving the miR-146a target genes Traf6 and Irak1. Like miR-146a, miR-146b and miR-28-5p also suppress CD8+T cell activation, although miRNA-targeting genes in this process have yet to be identified. miR-17 ∼92 targets Pten, ID2, ID3, and BCL2 and enhances cell-cycle progression of CD8+T cells. miR-214 also targets Pten and promotes CD8+T cell proliferation. let-7b and the miR-130/301 cluster influence CD8+T cell migration through regulating expression of CD62-L and CD69, respectively. miR-150 and miR-223 indirectly affect CD8+T cell proliferation by regulating LC cross-presentation.
miRNA-MEDIATED CONTROL OF CD8+T CELL DIFFERENTIATION
After antigen exposure, naive CD8+T cell expansion leads to at least 2 subsets: SLECs, which have poor longevity, exhibit a terminally differentiated phenotype, and lack the ability for antigen-independent homeostatic proliferation, and MPECs, which efficiently survive the contraction phase, acquire memory properties, and constitute the majority population of cells in the memory pool [62–64]. Memory CD8+T cells are also considered to be heterogeneous, consisting of TCM that have better homeostatic turnover and gradually dominate in the lymphoid organs and TEM that preferentially reside in the peripheral organs [65]. The transcriptional programs that determine whether naive CD8+T cells differentiate into TEM or TCM are controlled by an orchestra of antigen receptors, coreceptors, and cytokines that are implicated as key regulatory factors [1, 66]. Emerging evidences are now accumulating to support the involvement of miRNAs in the regulation of CD8+T cell fate (Fig. 2). For example, a lack of Dicer causes a skewing of activated CD8+T cells toward the development of SLECs following LCMV infection [67]. More importantly, the loss of Dicer in these cells and the ensuing terminal effector cell differentiation are indistinguishable from that caused by the combination of IL-2 and inflammatory signaling exposure [67], which indicates the existence of a regulatory pathway downstream of extracellular signaling that promotes the generation of SLECs through miRNAs. Two specific miRNAs—miR-139 and miR-342—have been identified as components of a miRNA network that controls terminal effector cell differentiation in this scenario and as exemplified by, of all of the down-regulated miRNAs, only miR-139 and miR-342, are decreased significantly in SLECs compared with MPECs. The formation of SLECs in the absence of miR-139 and miR-342 is a result of the derepression of their target Eomes. Intriguingly, over time, this regulatory pathway becomes invalid, and expression of Eomes declines progressively; this is in keeping with the notion that Eomes is a contributor to the formation of effector cells during early phases of CD8+T cell differentiation, but it confers competitive fitness to TCM during the TEM to TCM transition [67, 68]. These findings have identified an attractive model of a dynamic interplay between lineage-specific transcription factors and miRNAs.
Figure 2. miRNA-mediated regulation of CD8+T cell differentiation. In the periphery, mature CD8+T cell differentiation is modulated by miRNAs, including miR-17 ∼92 (targets Pten to promote expression and activation of mTOR), miR-155, and miR-21 (targets PDCD4), which promote SLECs, and miRNAs, including miR-139 [targets Eomes (early stage)], miR-342 [targets Eomes (early stage)], miR-16, miR-142-3p, miR-142-5p, miR-150 (targets CD25), miR-15b (targets DEDD), and let-7f, which promotes skewing toward memory cells (MPECs).
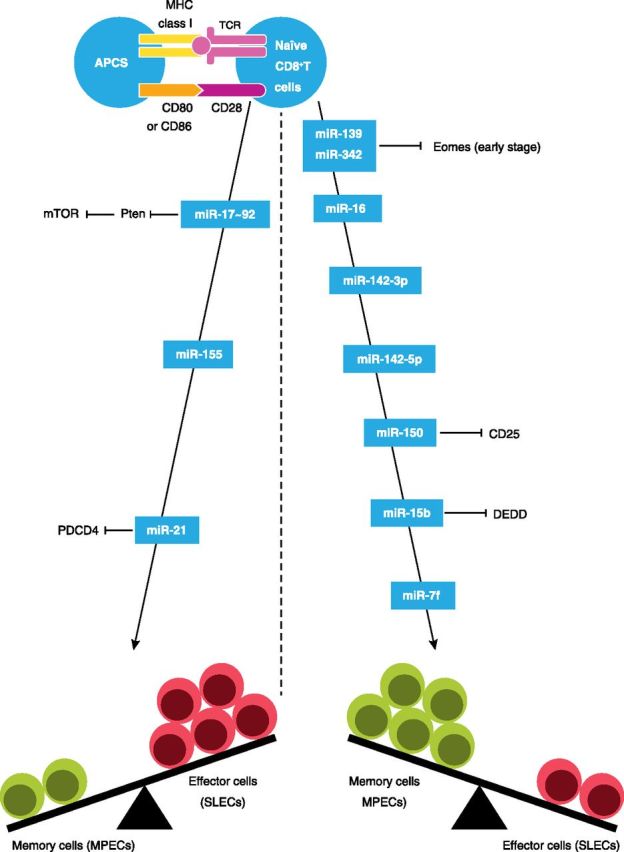
Early systematic miRNA profiling studies have identified a striking global down-regulation of miRNAs, including miR-16, miR-142-3p, miR-142-5p, miR-150, miR-15b, and let-7f, in effector CD8+T cells compared with naive cells and revealed that the miRNA expression levels tend to increase back in memory CD8+T cells [28, 69]. The results indicate that most miRNAs have crucial roles in the promotion of memory T cell potential, which are consistent with the view that major changes in gene expression characterize CD8+T cell differentiation states, with levels of gene expression getting enormously up-regulated in effector T cells but decreasing in memory T cells. One of these miRNAs, miR-150, is shown in CD8+T cells to target directly the 3′ UTR of CD25, the high-affinity IL-2Rα chain [67]. Given that the duration or relative amount of IL-2 signaling is a critical regulator of effector and memory lineages, it seems probable that the transition from prolonged IL-2 signaling regulated by miR-150low to attenuated IL-2 signaling regulated by miR-150high is responsible for effector CD8+T cells to survive and develop into memory cells [70, 71]. Additionally, overexpression of miR-15b in effector CD8+T cells enables them to acquire memory phenotype in tumor microenvironment; however, through regulating the translation of DEDD, a contributor to activation-induced cell death, the effect of miR-15b may have more to do with its selective effect on the apoptosis of CD8+T cells than their differentiation [72].
Although most miRNAs are highly expressed in memory cells but are only present in effector cells at very low levels, miR-17 ∼92, miR-21, and miR-155 are the exception, showing reverse kinetics with up-regulation in effector cells but progressively decline as memory cells form [28, 37, 49, 73]. Therefore, these miRNAs may be predicted to drive terminal effector cell differentiation. Consistent with this hypothesis, CD8+T cells lacking miR-17 ∼92 are impaired in forming SLECs during LCMV infection, and when overexpressed, miR-17 ∼92 can induce the formation of these effector cells. miR-17 ∼92 also suppresses certain aspects of the memory cell transcriptional program, as a deficiency of miR-17 ∼92 promotes the formation of TCM cells and their precursors, MPECs [49]. In this regard, it is worth noting that phenotypic analysis of miR-17 ∼92-overexpressing effector cells has identified a logical link between the enhanced clonal expansion and increased contraction, which supports a model whereby increased cell-cycle progression of effector CD8+T cells governed by miR-17 ∼92 overexpression drives their terminal differentiation [31, 43]. This may be attributed to the ability of miR-17 ∼92 to repress Pten, thereby relieving the inhibition on signaling downstream of PI3K-Akt and promoting the expression and activation of mTOR [37, 49], a factor that promotes metabolism and terminal differentiation of effector T cells [74, 75]. Similar to miR-17 ∼92-deficient cells, miR-155-deficient CD8+T cells have a decreased potential to differentiate to SLECs in response to murid herpesvirus 68 infection in vivo, and this defect correlates with a substantial increase in viral titers in mice lacking miR-155 in CD8+T cells. Moreover, at the memory stage, CD8+T cells that lack miR-155 preferentially differentiate into TCM cells [73]. Additional studies would be necessary to define the mechanisms through which miR-155 regulates effector and memory T cell fates and maintains their identity. Lastly, 2 studies in mice have confirmed an importance of miR-21 in autoimmune T cell responses. The overexpression of miR-21 in T cells causes autoimmune cholangitis in vivo, whereas inhibition of miR-21 in T cells reverses cardinal manifestations of autoimmunity in lupus-prone mice [76, 77]. These activities are linked to the ability of miR-21 to repress PDCD4, a selective protein translation inhibitor of genes involved in effector T cell survival and function [78, 79].
Collectively, miRNA expression patterns in CD8+T cells undergo dramatic changes in the course of cell differentiation. These effects have important consequences for T cell-effector functions, and they are complemented by the miRNA-mediated regulation of particular cell-differentiation pathways and effector mechanisms. These findings are conceptually relevant for the design of vaccination strategies that target to enhance potently effector functions or select effectively for the induction of protective memory cells.
miRNAs IN CD8+T CELL-EFFECTOR RESPONSES
Concomitant with effector cell transcriptomes in CD8+T cells is the genetic remodeling that leads to the expression of signature genes central to effector functions, including genes that encode cytokine IFN-γ and cytotoxic molecules perforin and GZMB [80]. In their absence, effector CD8+T cells are limited in their ability to kill infected or tumorigenic cells, and this defect results in susceptibility to infection and tumorigenesis in vivo [81, 82]. The important evidences for the miRNA-mediated regulation of effector functions come from studies with Dicer-deficient mouse and human CD8+T cells, which show increased differentiation into effector cells expressing perforin and GZMB, respectively [67]. This indicates that attention needs to be given to species-specific differences, as all of the miRNA expressed in cells or organisms of CD8+T cells and their target mRNAs can vary between mice and humans, even though the control of terminal effector cell differentiation by miRNAs is partially conserved.
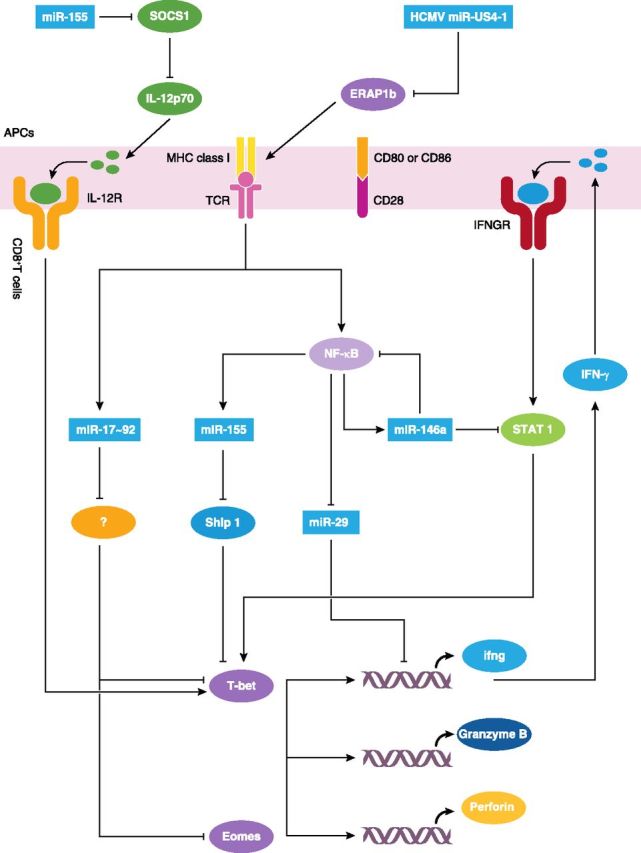
Beyond global perturbation of miRNA levels, studies on individual miRNAs have further refined our understanding of how specific miRNAs regulate effector functions of CD8+T cells. Several groups have identified unique miRNAs that regulate CD8+T cell production of IFN-γ. miR-29, for example, directly targets the mRNA that encodes IFN-γ, and consequently, miR-29-sufficient CD8+T cells produce less IFN-γ in vitro and in vivo than miR-29-deficient CD8+T cells. However, following activation, NF-κB signaling suppresses the expression of miR-29 and thus, relieves the transcriptional suppression of miR-29 in IFN-γ-producing CD8+T cells [83]. Additionally, as already suggested, miR-146a inhibits CD8+T cell responses by targeting Traf6 and Irak1. However, it has been shown in CD8+T cells from patients with chronic hepatitis B that it specifically regulates effector functions by targeting STAT1 [84]. In contrast, miR-155 substantially enhances effector functions through repression of Ship1, a potent, negative regulator of expression of T-bet and its transcriptional target, IFN-γ [85–87]. As a result, miR-146a-deficient mice restrict tumor growth, whereas miR-155-deficient mice permit enhanced tumor activity. Of note, a lack of miR-155 plays a dominant role compared with a lack of miR-146a in this context, as in the absence of miR-155 and miR-146a, CD8+T cells have defects in IFN-γ expression and antitumor immunity, which is similar to the phenotype observed in CD8+T cells that only have miR-155 deficiency [87]. Additionally, miR-155 modulates DC activity and cytokine production and exerts indirect effects on CD8+T cell functions. Through the direct targeting of SOCS1, miR-155 augments production of IL-12p70 by DCs [88], which results in increased effector CD8+T cell-mediated IFN-γ expression and promotes antiviral and antitumor responses [89, 90]. Furthermore, CD8+T cell-effector functions can be inhibited indirectly by miR-US4-1 during infection with HCMV [91]. This activity is attributed to the ability of miR-US4-1 to repress aminopeptidase ERAP1b, a factor that is required for the production of antigenic peptides, and thereby interfere with the presentation of antigenic peptides to CD8+T cells [91, 92].
Except for IFN-γ production, miRNAs also mediate other CD8+T cell-effector responses, such as cytotoxicity and secretion of cytotoxic molecules. Early activated miR-17 ∼92-deficient CD8+T cells fail to up-regulate T-bet and Eomes, which ultimately results in a decrease in the production of GZMB and perforin [49, 93], although miR-17 ∼92-targeting genes in this process have yet to be identified. In addition, these cells exhibit enhanced, multicytokine polyfunctionality, a hallmark trait of lymphoid memory cells [49, 94]. Given that miR-17 ∼92-deficient CD8+T cells show a bias toward memory differentiation, it is likely that this miRNA regulates effector functions through its effect on cell differentiation.
Altogether, it is evident that specific miRNAs carry out important roles in regulation of responses of CD8+T cells against infection and tumorigenesis. Key features of this control are a dose-dependent regulation of target-protein concentrations over a mostly modest range and the targeting of multiple functionally related proteins. Subtle changes of intracellular protein concentrations can have profound physiologic effects, as documented by the many pathologies arising from haploinsufficiency, and the targeting of several components of a functional network may further enhance the functional impact of miRNA control. (Fig. 3). Undoubtedly, the availability of genetic tools that ablate miRNAs in vivo will hasten the pace of the identification of additional miRNAs with crucial function in CD8+T cell responses to viral or malignant attacks and allow the exploitation of miRNAs as therapeutic entities for the treatment of disease.
Figure 3. miRNA-mediated regulation of CD8+T cell-effector function. During immune responses, CD8+T cell precursors undergo genetic remodeling that results in the expression of signature genes central to effector functions, including genes that encode T-box transcription factors T-bet and Eomes and genes associated with cytolysis. These processes are regulated by several miRNAs at distinct levels. Specifically, NF-κB signaling induces the expression of miR-155, which promotes IFN-γ production by targeting Ship1. miR-155 also indirectly promotes CD8+T cell production of IFN-γ by a mechanism whereby miR-155 enhances IL-12p70 production by DCs through targeting SOCS1. miR-146a, the miRNA that is up-regulated in response to NF-κB signaling, targets STAT1 and inhibits CD8+T cell-effector functions. miR-29 can directly target IFN-γ mRNA and decrease IFN-γ production, and its expression is inhibited by NF-κB signaling following activation. miR-17 ∼92 promotes expression of T-bet and Eomes through mechanisms that are not clear to us, which ultimately results in an increase in the production of GZMB and perforin. miR-US4-1 targets ERAP1b and thereby, interferes with the presentation of antigenic peptides to CD8+T cells. IFNGR, IFN-γR; ifng, IFN-γ.
miRNAs IN CD8+T CELL-RELATED DISEASES AND miRNA-TARGETING THERAPEUTICS
As discussed above, miRNAs directly modulate the concentration of many regulatory proteins that are crucial for development and function of the CD8+T cells. Dysregulation of these proteins has been linked to CD8+T cell-related immunologic diseases, in which the miRNAs are found to be mutated, or their expression levels are dysregulated. Recent work in animals lacking or overexpressing individual miRNAs has allowed investigators to explore the role of miRNAs, including miR-155 [38–40, 42, 73, 85, 87, 88], miR-17 ∼92 [37, 49], miR-150 [28, 67], miR-21 [28], miR-146a [87], miR-29 [83], miR-15b [72], miR-139/342 [67], and miR-US4-1 [85] in these diseases. Unsurprisingly, severe physiologic consequences, such as viral or malignant attacks, were observed frequently in such experimental models, accompanied by damaged development and function of the CD8+T cells. Additional evidence that miRNA-specific loss of CD8+T cell function could lead to diminished infection immunosurveillance comes from human experimental data showing that up-regulation of miR-146a in the patients of chronic hepatitis B causes impaired T cell function, which may contribute to persistent viral infection and immunopathogenesis [84]. The roles of miRNAs are only beginning to be explored in CD8+T cell-related autoimmune diseases, in which they may be involved in regulating immune responses against self-tissues. Studies in mice showed that the overexpression of miR-21 in T cells potentiates disease severity in the context of experimental autoimmune cholangitis [77], whereas inhibition of miR-21 in T cells attenuates disease severity in the setting of experimental autoimmune lupus [76]. Table 1 indicates the role of candidate miRNAs in various CD8+T cell-related diseases.
TABLE 1.
The role of candidate miRNAs in CD8+T cell biology and various CD8+T cell-related diseases
| miRNAs | Model/cell type | Regulation of CD8+T cell biology | Biologic role | Targets | Ref. |
|---|---|---|---|---|---|
| miR-155 | Mouse model (LCMV; melanoma) | Promote cell proliferation and survival | Anti-infection; anti-tumor | SOCS1 | [38] |
| Mouse model (influenza virus WSN-OVA) | Promote cell proliferation | Anti-infection | Type 1 IFN signaling | [39] | |
| Mouse model (L. monocytogenes) | Promote cell survival | Anti-infection | AKT | [42] | |
| Mouse model (murid herpesvirus 68) | Promote effector cell (SLEC) generation | Anti-infection | ? | [73] | |
| Mouse model (herpes simplex encephalitis) | Increase IFN-γ production | Anti-infection | ? | [85] | |
| Mouse model (lymphoma; melanoma) | Increase IFN-γ production | Anti-tumor | Ship1 | [87] | |
| Human DCs | Increase IFN-γ production | Anti-infection; anti-tumor | IL-12p70 | [88] | |
| Patients with vulvar lichen sclerosus and lichen planus | Promote cell proliferation in the dermis | Proautoimmunity | ? | [40] | |
| miRNA-17 ∼92 | Mouse model (LCMV) | Promote effector cell (SLECs) generation Inhibit memory cell (MPEC) generation Increase GZMB and perforin production | Anti-infection | Pten | [37, 49] |
| miRNA-150 | Mouse model (LCMV) | Promote memory cell (MPEC) generation | Proinfection | CD25 | [67] |
| Mouse CD8+T cells (LCMV) | Promote memory cell (MPEC) generation | ? | ? | [28] | |
| miR-21 | Mouse model (autoimmune lupus) | Promote effector cell function and survival | Proautoimmunity | PDCD4 | [76] |
| Mouse model (autoimmune cholangitis) | Promote effector cell function | Proautoimmunity | ? | [77] | |
| Mouse CD8+T cells (LCMV) | Promote effector cell (SLEC) generation | ? | ? | [28] | |
| miRNA-146a | Patients with chronic hepatitis B | Decrease IFN-γ production | Proinfection | STAT1 | [84] |
| Mouse model (lymphoma; melanoma) | Decrease IFN-γ production | Pro-tumor | ? | [87] | |
| miRNA-146a/b; miRNA-28-5p | Patients with severe asthma | Inhibit cell activation | Antiasthma | ? | [56] |
| miRNA-29 | Mouse model (L. monocytogenes) | Decrease IFN-γ production | Proinfection | IFN-γ | [83] |
| miR-15b | Mouse model (Lewis lung carcinoma) | Promote memory cell (MPEC) generation Inhibit cell activation and apoptosis | Pro-tumor | DEDD | [72] |
| miR-139/342 | Mouse model (LCMV) | Promote memory cell (MPEC) generation | Pro-tumor; proinfection | Eomes | [73] |
| miR-US4-1 | Mouse model (HCMV) | Inhibit effector cell function | Proinfection | ERAP1b | [91] |
| let-7f; miR-142-3f; miR-142-5p; miR-16 | Mouse CD8+T cells (LCMV) | Promote memory cell (MPEC) generation | ? | ? | [28] |
The realization that many miRNAs have crucial roles in CD8+T cell biology and that dysregulation of miRNAs is common in CD8+T cells from patients has led to considerable interest in the therapeutic targeting of miRNAs for CD8+T cell-related diseases. To date, three main approaches have been taken: expression vectors (miRNA ‘‘sponges’’), small-molecule inhibitors, and ASOs. The use of miRNA sponges that are produced from transgenes within cells facilitates the blocking of a whole family of related miRNAs, as the sponge’s binding sites can be designed to be specific to the miRNA seed region. Other genetic methods, such as those with use of recombinant virus vectors, have also proven to be effective to inhibit specific miRNAs in vivo. As alternative approaches to these transgenetic techniques, the use of chemically synthesized ASOs also serves well as a specific silencer of endogenous miRNAs [95]. In addition to modulation of the expression of specific miRNAs, application of small-molecule inhibitors that target the secondary structures of pri- and pre-miRNAs or miRNA processing might represent potential leads in drug development [96]. Recent years have seen considerable progress in the field of miRNA-based therapies; however, relatively little is known about methods for the delivery of these modalities specifically to CD8+T cells in patients. Given that 1 advantage of the immune system is that many of its effector cells recirculate between sites of inflammation and secondary lymphoid organs through the blood and the lymph fluid, it is entirely possible that the systemic delivery of agents that target CD8+T cells is more easily accomplished than if the agents are delivered to specific organs. Whereas challenges remain in this regard, the pace of this field suggests that new discoveries are forthcoming.
CONCLUDING REMARKS
A solid basis of data from in vitro and in vivo models is now accumulating to support the critical role of miRNAs as a regulator of development, proliferation, survival, migration, differentiation, and effector functions of CD8+T cells. During these processes, miRNAs and signaling molecules modulate 1 another in regulatory loops, finely tuning the level of translatable mRNAs in the face of a broad range of environmental cues.
Whereas our knowledge about the miRNA regulation of CD8+T cell biology has advanced considerably over the last several years, multiple areas warrant future investigation. First, most of the progress has, so far, been obtained from in vitro and in vivo mouse models, except for a few early studies in human infectious diseases. As a way of overcoming this lack of knowledge, it will be interesting to use systems biology approaches, such as exome sequencing, proteomics, and metabonomics, to discover mutations in CD8+T cells from patients that might correlate with aberrant miRNA expression. Second, the mechanisms controlling miRNA levels and stability as CD8+T cells differentiate or proliferation must be determined, including the processes by which mature miRNAs are degraded or cleared from the cell. These data will provide a rationale for novel approaches to promoting T cell-effector function and generating long-lasting immunity. Third, the ability of multiple miRNAs to target combinatorially a common pathway should be assessed. For instance, miR-17 ∼92, miR-155, and miR-21 may work synergistically to promote effector cell (SLEC) generation by targeting different components of the CD8+T cell polarization pathway. The coordinated regulation of a pathway by multiple miRNAs may lead to much greater activation or repression than that mediated by a single miRNA. Fourth, the ability of several miRNAs to regulate combinatorially a key mRNA and a single miRNA to suppress many target mRNAs simultaneously needs to be elucidated further, and this may reveal new gene networks and gene–gene interactions. Fifth, miRNAs are being considered as a novel type of biomarkers and potential therapeutic targets for various CD8+T cell-related disease. The improvement in sensitivity and reliability, together with lowered cost for miRNA detection, will definitely promote the practical application of miRNAs as important biomarkers. It is obvious that research on miRNAs is an interdisciplinary area in which the cooperation of clinicians and scientists from different fields, such as basic immunology, RNA biology, genetics, protein chemistry, and bioinformatics, is needed.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (30830089).
Glossary
- γc
γ chain
- ASO
antisense oligonucleotide
- BCL6
B cell lymphoma 6
- CD62-L
L-selectin
- DC
dendritic cell
- DEDD
death effector domain-containing DNA-binding protein
- DGCR8
DiGeorge syndrome critical region gene 8
- DP
double-positive
- DUSP5
dual-specificity protein phosphatase 5
- Eomes
eomesodermin
- ERAP
endoplasmic reticulum-associated aminopeptidase
- GZMB
granzyme B
- HCMV
human cytomegalovirus
- ID2
inhibitor of DNA binding 2
- IL-12p70
IL-12 p70 subunit
- Irak1
IL-1R-associated kinase 1
- LC
Langerhans cell
- LCMV
lymphocytic choriomeningitis virus
- miRNA
microRNA
- MPEC
memory precursor effector cell
- mTOR
mammalian target of rapamycin
- Nrarp
Notch-regulated ankyrin repeat protein
- PDCD4
programmed cell death protein 4
- PLN
peripheral lymph node
- pri-miRNA
primary microRNA
- Pten
phosphatase and tensin homologue
- PTPN22
protein tyrosine phosphatase, nonreceptor type 22
- Ship1
Src homology 2-containing inositol-5′-phosphatase 1
- SHP2
Src homology 2 domain-containing protein tyrosine phosphatase 2
- SLEC
short-lived effector cells
- SOCS1
suppressor of cytokine signaling 1
- TCM
central memory T cell
- TEM
effector memory T cell
- Traf6
TNFR-associated factor 6
- UTR
untranslated region
DISCLOSURES
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.O’Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay M. A. (2008) microRNAs and the immune response. Trends Immunol. 29, 343–351. [DOI] [PubMed] [Google Scholar]
- 3.Baumjohann D., Ansel K. M. (2013) MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 13, 666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech S. M., Cui W. (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N., Bevan M. J. (2011) CD8(+) T cells: foot soldiers of the immune system. Immunity 35, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendell J. T., Olson E. N. (2012) MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian M. R., Sonenberg N., Filipowicz W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379. [DOI] [PubMed] [Google Scholar]
- 8.Krol J., Loedige I., Filipowicz W. (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610. [DOI] [PubMed] [Google Scholar]
- 9.Ceribelli A., Satoh M., Chan E. K. (2012) MicroRNAs and autoimmunity. Curr. Opin. Immunol. 24, 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammell M. (2010) Computational methods to identify miRNA targets. Semin. Cell Dev. Biol. 21, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausser J., Zavolan M. (2014) Identification and consequences of miRNA-target interactions—beyond repression of gene expression. Nat. Rev. Genet. 15, 599–612. [DOI] [PubMed] [Google Scholar]
- 12.Ameres S. L., Horwich M. D., Hung J. H., Xu J., Ghildiyal M., Weng Z., Zamore P. D. (2010) Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kai Z. S., Pasquinelli A. E. (2010) MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat. Struct. Mol. Biol. 17, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baccarini A., Chauhan H., Gardner T. J., Jayaprakash A. D., Sachidanandam R., Brown B. D. (2011) Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Curr. Biol. 21, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee S., Fasler M., Büssing I., Grosshans H. (2011) Target-mediated protection of endogenous microRNAs in C. elegans. Dev. Cell 20, 388–396. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S., Grosshans H. (2009) Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature 461, 546–549. [DOI] [PubMed] [Google Scholar]
- 17.Bartel D. P. (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson D. W., Bracken C. P., Goodall G. J. (2011) Experimental strategies for microRNA target identification. Nucleic Acids Res. 39, 6845–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquinelli A. E. (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 13, 271–282. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., Rougvie A. E., Horvitz H. R., Ruvkun G. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906. [DOI] [PubMed] [Google Scholar]
- 21.Krek A., Grün D., Poy M. N., Wolf R., Rosenberg L., Epstein E. J., MacMenamin P., da Piedade I., Gunsalus K. C., Stoffel M., Rajewsky N. (2005) Combinatorial microRNA target predictions. Nat. Genet. 37, 495–500. [DOI] [PubMed] [Google Scholar]
- 22.Betel D., Wilson M., Gabow A., Marks D. S., Sander C. (2008) The microRNA.org resource: targets and expression. Nucleic Acids Res. 36, D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimson A., Farh K. K., Johnston W. K., Garrett-Engele P., Lim L. P., Bartel D. P. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi S. W., Zang J. B., Mele A., Darnell R. B. (2009) Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M. Jr., Jungkamp A. C., Munschauer M., Ulrich A., Wardle G. S., Dewell S., Zavolan M., Tuschl T. (2010) Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilson J. R., Zheng G. X., Burge C. B., Sharp P. A. (2007) Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 21, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghisi M., Corradin A., Basso K., Frasson C., Serafin V., Mukherjee S., Mussolin L., Ruggero K., Bonanno L., Guffanti A., De Bellis G., Gerosa G., Stellin G., D’Agostino D. M., Basso G., Bronte V., Indraccolo S., Amadori A., Zanovello P. (2011) Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood 117, 7053–7062. [DOI] [PubMed] [Google Scholar]
- 28.Wu H., Neilson J. R., Kumar P., Manocha M., Shankar P., Sharp P. A., Manjunath N. (2007) miRNA profiling of naïve, effector and memory CD8 T cells. PLoS ONE 2, e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muljo S. A., Ansel K. M., Kanellopoulou C., Livingston D. M., Rao A., Rajewsky K. (2005) Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 202, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobb B. S., Hertweck A., Smith J., O’Connor E., Graf D., Cook T., Smale S. T., Sakaguchi S., Livesey F. J., Fisher A. G., Merkenschlager M. (2006) A role for Dicer in immune regulation. J. Exp. Med. 203, 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong M. M., Rasmussen J. P., Rudensky A. Y., Littman D. R. (2008) The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J. Exp. Med. 205, 2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner D. F., Thomas M. F., Hu J. K., Yang Z., Babiarz J. E., Allen C. D., Matloubian M., Blelloch R., Ansel K. M. (2011) MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q. J., Chau J., Ebert P. J., Sylvester G., Min H., Liu G., Braich R., Manoharan M., Soutschek J., Skare P., Klein L. O., Davis M. M., Chen C. Z. (2007) miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161. [DOI] [PubMed] [Google Scholar]
- 34.Henao-Mejia J., Williams A., Goff L. A., Staron M., Licona-Limón P., Kaech S. M., Nakayama M., Rinn J. L., Flavell R. A. (2013) The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity 38, 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fragoso R., Mao T., Wang S., Schaffert S., Gong X., Yue S., Luong R., Min H., Yashiro-Ohtani Y., Davis M., Pear W., Chen C. Z. (2012) Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genet. 8, e1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N., Bevan M. J. (2010) Dicer controls CD8+ T-cell activation, migration, and survival. Proc. Natl. Acad. Sci. USA 107, 21629–21634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu T., Wieland A., Araki K., Davis C. W., Ye L., Hale J. S., Ahmed R. (2012) Temporal expression of microRNA cluster miR-17-92 regulates effector and memory CD8+ T-cell differentiation. Proc. Natl. Acad. Sci. USA 109, 9965–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudda J. C., Salaun B., Ji Y., Palmer D. C., Monnot G. C., Merck E., Boudousquie C., Utzschneider D. T., Escobar T. M., Perret R., Muljo S. A., Hebeisen M., Rufer N., Zehn D., Donda A., Restifo N. P., Held W., Gattinoni L., Romero P. (2013) MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 38, 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gracias D. T., Stelekati E., Hope J. L., Boesteanu A. C., Doering T. A., Norton J., Mueller Y. M., Fraietta J. A., Wherry E. J., Turner M., Katsikis P. D. (2013) The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat. Immunol. 14, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terlou A., Santegoets L. A., van der Meijden W. I., Heijmans-Antonissen C., Swagemakers S. M., van der Spek P. J., Ewing P. C., van Beurden M., Helmerhorst T. J., Blok L. J. (2012) An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA-155. J. Invest. Dermatol. 132, 658–666. [DOI] [PubMed] [Google Scholar]
- 41.Decaluwe H., Taillardet M., Corcuff E., Munitic I., Law H. K., Rocha B., Rivière Y., Di Santo J. P. (2010) Gamma(c) deficiency precludes CD8+ T cell memory despite formation of potent T cell effectors. Proc. Natl. Acad. Sci. USA 107, 9311–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lind E. F., Elford A. R., Ohashi P. S. (2013) Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J. Immunol. 190, 1210–1216. [DOI] [PubMed] [Google Scholar]
- 43.Trotta R., Chen L., Ciarlariello D., Josyula S., Mao C., Costinean S., Yu L., Butchar J. P., Tridandapani S., Croce C. M., Caligiuri M. A. (2012) miR-155 regulates IFN-γ production in natural killer cells. Blood 119, 3478–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr W. G. (2011) Inhibitor and activator: dual functions for SHIP in immunity and cancer. Ann. N. Y. Acad. Sci. 1217, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil M. P., Salomon R., Louten J., Biron C. A. (2006) Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 107, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen K. B., Watford W. T., Salomon R., Hofmann S. R., Pien G. C., Morinobu A., Gadina M., O’Shea J. J., Biron C. A. (2002) Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297, 2063–2066. [DOI] [PubMed] [Google Scholar]
- 47.Chang C. C., Zhang Q. Y., Liu Z., Clynes R. A., Suciu-Foca N., Vlad G. (2012) Downregulation of inflammatory microRNAs by Ig-like transcript 3 is essential for the differentiation of human CD8(+) T suppressor cells. J. Immunol. 188, 3042–3052. [DOI] [PubMed] [Google Scholar]
- 48.Chen L., Xu Z., Chang C., Ho S., Liu Z., Vlad G., Cortesini R., Clynes R. A., Luo Y., Suciu-Foca N. (2014) Allospecific CD8 T suppressor cells induced by multiple MLC stimulation or priming in the presence of ILT3.Fc have similar gene expression profiles. Hum. Immunol. 75, 190–196. [DOI] [PubMed] [Google Scholar]
- 49.Khan A. A., Penny L. A., Yuzefpolskiy Y., Sarkar S., Kalia V. (2013) MicroRNA-17~92 regulates effector and memory CD8 T-cell fates by modulating proliferation in response to infections. Blood 121, 4473–4483. [DOI] [PubMed] [Google Scholar]
- 50.Jindra P. T., Bagley J., Godwin J. G., Iacomini J. (2010) Costimulation-dependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten. J. Immunol. 185, 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schietinger A., Delrow J. J., Basom R. S., Blattman J. N., Greenberg P. D. (2012) Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335, 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rusca N., Dehò L., Montagner S., Zielinski C. E., Sica A., Sallusto F., Monticelli S. (2012) MiR-146a and NF-κB1 regulate mast cell survival and T lymphocyte differentiation. Mol. Cell. Biol. 32, 4432–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curtale G., Citarella F., Carissimi C., Goldoni M., Carucci N., Fulci V., Franceschini D., Meloni F., Barnaba V., Macino G. (2010) An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood 115, 265–273. [DOI] [PubMed] [Google Scholar]
- 54.Boldin M. P., Taganov K. D., Rao D. S., Yang L., Zhao J. L., Kalwani M., Garcia-Flores Y., Luong M., Devrekanli A., Xu J., Sun G., Tay J., Linsley P. S., Baltimore D. (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 208, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L., Boldin M. P., Yu Y., Liu C. S., Ea C. K., Ramakrishnan P., Taganov K. D., Zhao J. L., Baltimore D. (2012) miR-146a controls the resolution of T cell responses in mice. J. Exp. Med. 209, 1655–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsitsiou E., Williams A. E., Moschos S. A., Patel K., Rossios C., Jiang X., Adams O. D., Macedo P., Booton R., Gibeon D., Chung K. F., Lindsay M. A. (2012) Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 129, 95–103. [DOI] [PubMed] [Google Scholar]
- 57.Finlay D., Cantrell D. A. (2011) Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guenin-Macé L., Carrette F., Asperti-Boursin F., Le Bon A., Caleechurn L., Di Bartolo V., Fontanet A., Bismuth G., Demangel C. (2011) Mycolactone impairs T cell homing by suppressing microRNA control of L-selectin expression. Proc. Natl. Acad. Sci. USA 108, 12833–12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlson C. M., Endrizzi B. T., Wu J., Ding X., Weinreich M. A., Walsh E. R., Wani M. A., Lingrel J. B., Hogquist K. A., Jameson S. C. (2006) Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302. [DOI] [PubMed] [Google Scholar]
- 60.Mi Q. S., Xu Y. P., Wang H., Qi R. Q., Dong Z., Zhou L. (2013) Deletion of microRNA miR-223 increases Langerhans cell cross-presentation. Int. J. Biochem. Cell Biol. 45, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mi Q. S., Xu Y. P., Qi R. Q., Shi Y. L., Zhou L. (2012) Lack of microRNA miR-150 reduces the capacity of epidermal Langerhans cell cross-presentation. Exp. Dermatol. 21, 876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshi N. S., Cui W., Chandele A., Lee H. K., Urso D. R., Hagman J., Gapin L., Kaech S. M. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaech S. M., Tan J. T., Wherry E. J., Konieczny B. T., Surh C. D., Ahmed R. (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 64.Sarkar S., Kalia V., Haining W. N., Konieczny B. T., Subramaniam S., Ahmed R. (2008) Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712. [DOI] [PubMed] [Google Scholar]
- 66.Zehn D., Lee S. Y., Bevan M. J. (2009) Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trifari S., Pipkin M. E., Bandukwala H. S., Äijö T., Bassein J., Chen R., Martinez G. J., Rao A. (2013) MicroRNA-directed program of cytotoxic CD8+ T-cell differentiation. Proc. Natl. Acad. Sci. USA 110, 18608–18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banerjee A., Gordon S. M., Intlekofer A. M., Paley M. A., Mooney E. C., Lindsten T., Wherry E. J., Reiner S. L. (2010) Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almanza G., Fernandez A., Volinia S., Cortez-Gonzalez X., Croce C. M., Zanetti M. (2010) Selected microRNAs define cell fate determination of murine central memory CD8 T cells. PLoS ONE 5, e11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalia V., Sarkar S., Subramaniam S., Haining W. N., Smith K. A., Ahmed R. (2010) Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103. [DOI] [PubMed] [Google Scholar]
- 71.Pipkin M. E., Sacks J. A., Cruz-Guilloty F., Lichtenheld M. G., Bevan M. J., Rao A. (2010) Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong G., Cheng X., Long H., He L., Qi W., Xiang T., Zhao Z., Zhu B. (2013) Dynamically expressed microRNA-15b modulates the activities of CD8+ T lymphocytes in mice with Lewis lung carcinoma. J. Transl. Med. 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai C. Y., Allie S. R., Zhang W., Usherwood E. J. (2013) MicroRNA miR-155 affects antiviral effector and effector memory CD8 T cell differentiation. J. Virol. 87, 2348–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delgoffe G. M., Kole T. P., Zheng Y., Zarek P. E., Matthews K. L., Xiao B., Worley P. F., Kozma S. C., Powell J. D. (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearce E. L., Walsh M. C., Cejas P. J., Harms G. M., Shen H., Wang L. S., Jones R. G., Choi Y. (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ando Y., Yang G. X., Kenny T. P., Kawata K., Zhang W., Huang W., Leung P. S., Lian Z. X., Okazaki K., Ansari A. A., He X. S., Invernizzi P., Ridgway W. M., Lu Q., Gershwin M. E. (2013) Overexpression of microRNA-21 is associated with elevated pro-inflammatory cytokines in dominant-negative TGF-β receptor type II mouse. J. Autoimmun. 41, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garchow B. G., Bartulos Encinas O., Leung Y. T., Tsao P. Y., Eisenberg R. A., Caricchio R., Obad S., Petri A., Kauppinen S., Kiriakidou M. (2011) Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol. Med. 3, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stagakis E., Bertsias G., Verginis P., Nakou M., Hatziapostolou M., Kritikos H., Iliopoulos D., Boumpas D. T. (2011) Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann. Rheum. Dis. 70, 1496–1506. [DOI] [PubMed] [Google Scholar]
- 79.Iliopoulos D., Kavousanaki M., Ioannou M., Boumpas D., Verginis P. (2011) The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur. J. Immunol. 41, 1754–1763. [DOI] [PubMed] [Google Scholar]
- 80.Glimcher L. H., Townsend M. J., Sullivan B. M., Lord G. M. (2004) Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 4, 900–911. [DOI] [PubMed] [Google Scholar]
- 81.Wilson J. J., Lin E., Pack C. D., Frost E. L., Hadley A., Swimm A. I., Wang J., Dong Y., Breeden C. P., Kalman D., Newell K. A., Lukacher A. E. (2011) Gamma interferon controls mouse polyomavirus infection in vivo. J. Virol. 85, 10126–10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kmieciak M., Payne K. K., Wang X. Y., Manjili M. H. (2013) IFN-γ Rα is a key determinant of CD8+ T cell-mediated tumor elimination or tumor escape and relapse in FVB mouse. PLoS ONE 8, e82544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma F., Xu S., Liu X., Zhang Q., Xu X., Liu M., Hua M., Li N., Yao H., Cao X. (2011) The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat. Immunol. 12, 861–869. [DOI] [PubMed] [Google Scholar]
- 84.Wang S., Zhang X., Ju Y., Zhao B., Yan X., Hu J., Shi L., Yang L., Ma Z., Chen L., Liu Y., Duan Z., Chen X., Meng S. (2013) MicroRNA-146a feedback suppresses T cell immune function by targeting Stat1 in patients with chronic hepatitis B. J. Immunol. 191, 293–301. [DOI] [PubMed] [Google Scholar]
- 85.Bhela S., Mulik S., Reddy P. B., Richardson R. L., Gimenez F., Rajasagi N. K., Veiga-Parga T., Osmand A. P., Rouse B. T. (2014) Critical role of microRNA-155 in herpes simplex encephalitis. J. Immunol. 192, 2734–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tarasenko T., Kole H. K., Chi A. W., Mentink-Kane M. M., Wynn T. A., Bolland S. (2007) T Cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc. Natl. Acad. Sci. USA 104, 11382–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huffaker T. B., Hu R., Runtsch M. C., Bake E., Chen X., Zhao J., Round J. L., Baltimore D., O’Connell R. M. (2012) Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell Reports 2, 1697–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu C., Huang X., Zhang X., Roensch K., Cao Q., Nakayama K. I., Blazar B. R., Zeng Y., Zhou X. (2011) miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood 117, 4293–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carreno B. M., Becker-Hapak M., Huang A., Chan M., Alyasiry A., Lie W. R., Aft R. L., Cornelius L. A., Trinkaus K. M., Linette G. P. (2013) IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J. Clin. Invest. 123, 3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nizzoli G., Krietsch J., Weick A., Steinfelder S., Facciotti F., Gruarin P., Bianco A., Steckel B., Moro M., Crosti M., Romagnani C., Stölzel K., Torretta S., Pignataro L., Scheibenbogen C., Neddermann P., De Francesco R., Abrignani S., Geginat J. (2013) Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122, 932–942. [DOI] [PubMed] [Google Scholar]
- 91.Kim S., Lee S., Shin J., Kim Y., Evnouchidou I., Kim D., Kim Y. K., Kim Y. E., Ahn J. H., Riddell S. R., Stratikos E., Kim V. N., Ahn K. (2011) Human cytomegalovirus microRNA miR-US4-1 inhibits CD8(+) T cell responses by targeting the aminopeptidase ERAP1. Nat. Immunol. 12, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang S. C., Momburg F., Bhutani N., Goldberg A. L. (2005) The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl. Acad. Sci. USA 102, 17107–17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cruz-Guilloty F., Pipkin M. E., Djuretic I. M., Levanon D., Lotem J., Lichtenheld M. G., Groner Y., Rao A. (2009) Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lelic A., Verschoor C. P., Ventresca M., Parsons R., Evelegh C., Bowdish D., Betts M. R., Loeb M. B., Bramson J. L. (2012) The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog. 8, e1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi E., Cha M. J., Hwang K. C. (2014) Roles of calcium regulating microRNAs in cardiac ischemia-reperfusion injury. Cells 3, 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreno-Moya J. M., Vilella F., Simón C. (2014) MicroRNA: key gene expression regulators. Fertil. Steril. 101, 1516–1523. [DOI] [PubMed] [Google Scholar]


