Thymic CD19+CD5+CD1dhiIL-10+ B cells play a critical role in the maintenance of immune homeostasis.
Keywords: regulatory T cells, Foxp3, CD72
Abstract
This study tested the hypothesis that besides the spleen, LNs, peripheral blood, and thymus contain a regulatory IL-10-producing CD19+CD5+CD1dhigh B cell subset that may play a critical role in the maintenance of immune homeostasis. Indeed, this population was identified in the murine thymus, and furthermore, when cocultured with CD4+ T cells, this population of B cells supported the maintenance of CD4+Foxp3+ Tregs in vitro, in part, via the CD5–CD72 interaction. Mice homozygous for Cd19Cre (CD19−/−) express B cells with impaired signaling and humoral responses. Strikingly, CD19−/− mice produce fewer CD4+Foxp3+ Tregs and a greater percentage of CD4+CD8− and CD4−CD8+ T cells. Consistent with these results, transfer of thymic CD19+CD5+CD1dhi B cells into CD19−/− mice resulted in significantly up-regulated numbers of CD4+Foxp3+ Tregs with a concomitant reduction in CD4+CD8− and CD4−CD8+ T cell populations in the thymus, spleen, and LNs but not in the BM of recipient mice. In addition, thymic CD19+CD5+CD1dhi B cells significantly suppressed autoimmune responses in lupus-like mice via up-regulation of CD4+Foxp3+ Tregs and IL-10-producing Bregs. This study suggests that thymic CD19+CD5+CD1dhiIL-10+ Bregs play a critical role in the maintenance of immune homeostasis.
Introduction
B Cells are primarily regarded as positive regulators of the pathogenesis of immune-related diseases through antigen-specific autoantibody production [1, 2]. It is also known that B cells negatively regulate immune responses via production of inhibitory cytokines, such as IL-10 and TGF-β [3, 4]. A variety of Breg subsets has been described, and of these, the IL-10-producing Breg subset is the most widely studied [2, 5–7].
Splenic IL-10-producing Bregs are predominantly found within the minor CD5+CD1dhi B cell subpopulation [8, 9], and although they are found at a low frequency (1–5%) in naïve mice, IL-10-producing Bregs are expanded in cases of autoimmunity and can play a key role in controlling disease [9]. In this regard, the absence or loss of IL-10-producing Bregs is implicated in the etiology of several autoimmune diseases [10–12], and aberrant elevation of the levels of Bregs can prevent sterilizing immunity to pathogens. Moreover, tumor-induced Bregs have recently been implicated in carcinogenesis [13–15] and have been found to contribute to breast cancer metastasis by promoting the differentiation of resting CD4+ T cells into Tregs [16]. Additional evidence of a role for Bregs in supporting the development of Tregs comes from studies of Schistosoma mansoni worms and allergic airway inflammation, in which IL-10-producing Bregs induce pulmonary infiltration of CD4+CD25+Foxp3+ Tregs [17, 18].
Following the advent of B cell depletion therapy, Bregs have elicited the interest of a broad spectrum of immunologists and clinicians [2]. Although Bregs have been found to modulate immune responses in autoimmunity [3, 4, 7], infection [19, 20], and cancer [15, 16], their physiologic contribution to overall immune homeostasis and their development and function remain unclear.
Many publications have shown that a small population of B cells comprises approximately 0.1–0.5% of thymocytes in humans and mice [21–25]. In this regard, B cells have been proposed to play a critical function in T cell-negative selection [22, 23]. Thymic B cells preferentially reside at the junction of the thymic cortex and the medulla, an area thought to be where negative selection occurs. In addition, it has been shown that thymic B cells mediate negative selection of T cells in superantigen and self-antigen overexpression models [26, 27]. However, the mechanisms by which thymic B cells mediate T cell-negative selection remain unclear.
We propose the existence of a population of Bregs that mediates negative selection of T cells in the thymus. We identified a population of CD3−CD4−B220+CD19+CD5+CD1dhiIL-10+ B cells in murine thymus. This population of B cells expanded/maintained CD4+Foxp3+ Tregs in vitro and in vivo. In addition, thymic B220+CD19+CD5+CD1dhi Bregs reduced populations of thymic CD4+CD8− and CD4−CD8+ T cells. Finally, thymic B220+CD19+CD5+CD1dhi Bregs significantly suppressed autoimmune responses in lupus-like mice. Together, these findings suggest that thymic B220+CD19+CD5+CD1dhiIL-10+ Bregs play a critical role in maintaining immune homeostasis. CD5+ cells
MATERIALS AND METHODS
Ethics Committee approval
Care, use, and treatment of mice in this study were in strict agreement with international guidelines for the care and use of laboratory animals. This study was approved by the Animal Ethics Committee of the Beijing Institute of Basic Medical Sciences.
Mice
Seven- to 9-week-old C57BL/6, CD19-Cre mice and lupus-like NZB/NZW F1 mice (Chinese Academy of Medical Sciences, Beijing, China) were bred in our animal facilities under specific pathogen-free conditions.
Cytometric analysis and intracellular cytokine staining
All cell experiments were strictly prepared on ice, unless stated otherwise in other specific procedures. Cells (1 × 106 cells/sample) were washed with FACS staining buffer (PBS, 2% FBS or 1% BSA, 0.1% sodium azide). All samples were incubated with anti-FcR antibody (clone 2.4G2; BD Biosciences, San Jose, CA, USA) before incubation with other antibodies diluted in FACS buffer, supplemented with 2% anti-FcR antibody. For intracellular cytokine staining, 50 ng/ml PMA and 1 mg/ml ionomycin (Sigma-Aldrich, St. Louis, MO, USA) were added, and then, 1 mg/ml brefeldin A and 2 mM monensin were added 3 h later. After 3 h, cells were collected and fixed for 20 min with 1 ml fixation buffer (Intracellular Fixation & Permeabilization Buffer Kit; eBioscience, San Diego, CA, USA). After washing, the fixed cells were stained. The samples were filtered immediately before analysis or cell sorting to remove any clumps. The following antibodies were purchased from eBioscience: anti-mouse CD3 (clone 145-2C11), CD4 (clone GK1.5), B220 (clone RA3-6B2), CD19 (clone MB19-1), CD5 (clone 53-7.3), CD1d (clone 1B1), IL-10 (clone JES5-16E3), and Foxp3 (clone NRRF-30). Anti-mouse CD72 antibody (clone K10.6) were purchased from BD PharMingen (San Diego, CA, USA). Data collection and analyses were performed on a FACSCalibur flow cytometer by use of CellQuest software (BD Biosciences).
Cell sorting
Approximately 6 × 106 lymphocytes were separated by Ficoll solution from thymus of a 7- to 9-week-old female or male C57BL/6 mouse and used to sort 5 × 104 CD3−CD4−B220+CD19+CD5+CD1dhi Bregs and 2 × 105 CD3−CD4−B220+CD19+ CD5−CD1dlo B cells. Multicolor flow cytometry was performed by gating on CD3-CD4−B220+CD19+ cells that were CD5+CD1dhi or CD5−CD1dlo. The lymphocytes from the spleen and mesenteric and inguinal LNs of 7-week-old female or male C57BL/6 mice were used to sort-purify CD4+CD25+ Tregs and CD4+CD25− T cells. Multicolor flow cytometry was performed by gating on CD3+CD4+B220− cells that were CD25+CD62L+ (CD4+CD25+ Tregs) or CD25−CD62L+ (CD4+CD25− T cells). CD19+ B cells and CD4+ T cells were separated by CD19 microbeads and CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively, from the spleen and mesenteric and inguinal LNs of a 7- to 9-week-old female or male C57BL/6 mice. All flow cytometry data were acquired with FACSCanto, FACSCanto II, or FACSAria (BD Biosciences), gated on live lymphocyte-sized cells on the basis of forward- and side-scatter and analyzed by use of FlowJo software (Tree Star, Ashland, OR, USA).
In vitro B–T cell coculture
CD4+ T cells were labeled with CFSE (Molecular Probes, Eugene, OR, USA). CFSE was added to a final concentration of 5 μmol/L and the cells incubated for 10 min at 37°C. The T cells were washed 3 times through FCS and resuspended in complete medium. CD4+ T cells, at a concentration of 1 × 106 cells/ml, were cocultured for 3 d in 96-well plates with 1 × 106 cells/ml CD5−CD1dlo or CD5+CD1dhi cells from thymus in RPMI-1640 medium containing 10% FBS, 2 mM glutamine, penicillin (100 IU/ml), streptomycin (100 µg/ml), and 50 mM 2-ME. CD19+ B cells (1 × 106 cells/ml) were cocultured for 3 d in 96-well plates with 1 × 106 cells/ml CD4+CD25+ Tregs or CD4+CD25− T cells in cultured in RPMI-1640 medium containing 10% FBS, 2 mM glutamine, penicillin (100 IU/ml), streptomycin (100 µg/ml), and 50 mM 2-ME with 10 µg/ml LPS (Sigma-Aldrich). In some conditions, cocultured cells were treated with 10 µg/ml isotype control antibody or neutralizing anti-mouse CD72 antibody (ab25320; Abcam, Cambridge, MA, USA).
Thymic B220+CD19+CD5+CD1dhi Bregs were transferred into CD19-Cre or lupus-like mice
CD3−CD4−B220+CD19+CD5+CD1dhiB (CD5+CD1dhi) cells were sorted by FACS from the thymus of 7- to 9-week-old female or male C57BL/6 mice. Each age- and sex-matched CD19Cre C57BL/6 mouse (6 mice/group) was injected in the tail veil (i.v.) with 1 × 105 thymic CD5+CD1dhi B cells. CD19+ B cells were sorted by CD19 microbeads (Miltenyi Biotec) from the thymus of 7- to 9-week-old female or male C57BL/6 mice. Each age- and sex-matched, lupus-like NZB/NZW F1 mouse (6 mice/group) was injected in the tail veil (i.v.) with 1 × 105 CD5+CD1dhiBregs or CD19+ B cells.
Cytokine analysis by ELISA
Anti-dsDNA IgG and anti-dsDNA IgG1 antibody titers were measured by ELISA kits (Shibayaji, Gunma, Japan). In brief, diluted sera were added in triplicate to the plate (dsDNA precoated; Shibayaji) for 1 h at 37°C. To detect nonspecific IgG and IgG1 titers, diluted sera were coated to the plate (no antigen precoated) overnight at 4°C and then blocked with 4% BSA for 1 h at 37°C. Then, after washing, biotin rat anti-mouse IgG, IgG1 antibody (4 μg/ml) antibodies were added to the plate and were incubated for another hour at 37°C. Thereafter, unbinding antibodies were washed off, followed by addition of avidin-HRP (1/1000 diluted). Plates were incubated for 1 h at 37°C. Finally, the color was developed by incubation with o-phenylenediamine. The OD was read at 450 nm with an ELISA reader (Bio-Rad Laboratories, Hercules, CA, USA). Standard curves were established to quantitate the amounts of the respective cytokines.
Statistics
Statistics were generated by use of t-test in GraphPad Prism (version 5.0; GraphPad Software, La Jolla, CA, USA), and values are represented as mean ± sem. Results were considered statistically significant at P < 0.05.
RESULTS
B220+CD19+CD5+CD1dhiIL-10+ Bregs are present in the thymus
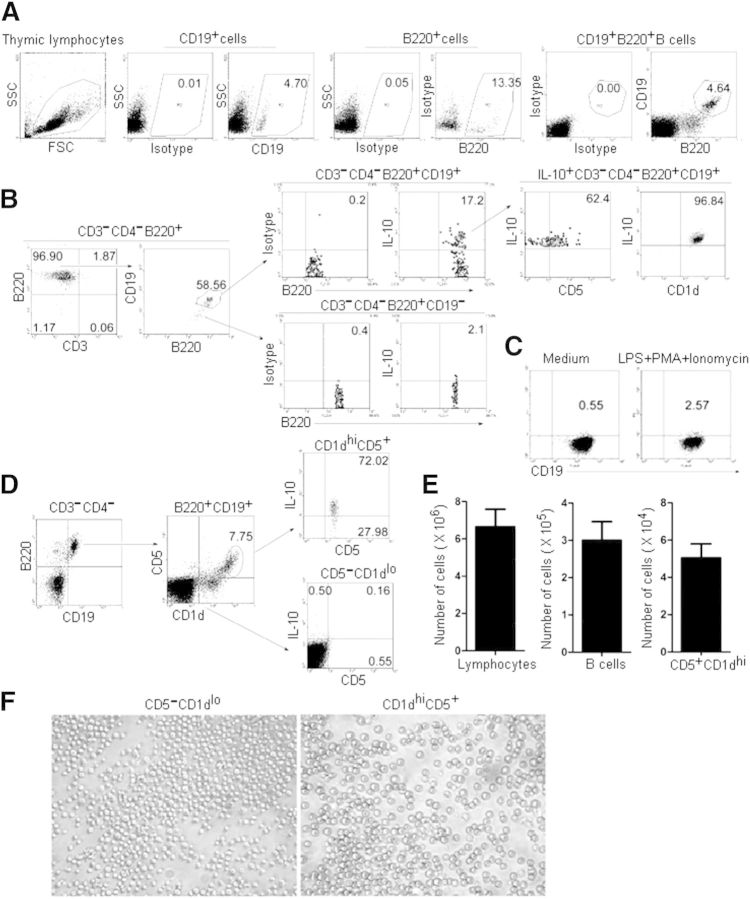
Thymic B cells have been proposed to play a critical role in T cell-negative selection [22, 23]. To this end, we determined whether there exists a population of Bregs in murine thymus. Flow cytometric (FACS) analysis of thymocytes isolated from 7- to 9-week-old female or male C57BL/6 mice revealed that CD19+ B cells represent ∼0.11% of total thymocytes (Supplemental Fig. 1). To enrich CD19+ B cells, lymphocytes were purified further by Ficoll separation solution from thymocytes. In these purified thymic lymphocytes, ∼5% cells were CD3−CD4−CD19+B220+ B cells (Fig. 1A). Furthermore, ∼17% of thymic B220+CD19+ B cells could be induced to express IL-10, and many more B220+CD19+ B cells could be induced to express IL-10 than could CD19−B220+ B cells (Fig. 1B). Notably, the percentage of IL-10-expressing Bregs among thymic B cells is much higher than that among splenic Bregs, which are found at low frequencies (1–5%) in naïve mice [9]. We also found that only 2.57% of splenic CD19+ B cells could be induced to express IL-10 (Fig. 1C). In addition, thymic B220+CD19+IL-10+ Bregs express CD5 and CD1dhi (Fig. 1B). We also found that the percentage of IL-10-expressing B cells was much higher in the thymic B220+CD19+CD1dhiCD5+ B cells than that in B220+CD19+CD1dloCD5− B cells (Fig. 1D). Together, these results demonstrate that thymic IL-10-expressing Bregs are found predominantly within the CD1dhiCD5+ B cell subpopulation and are phenotypically similar to splenic IL-10-producing Bregs [6, 8, 9]. In addition, there are ∼6 × 106 lymphocytes, 3 × 105 B cells, and 5 × 104 CD5+CD1dhiB cells in the thymi of 7- to 9-week-old female or male C57BL/6 mice (Fig. 1E). Furthermore, images of CD1dlowCD5− B cells and CD1dhiCD5+ Bregs under a microscope were shown (Fig. 1F).
Figure 1. Identification of IL-10+CD5+CD1dhiB220+ CD19+ Bregs in the thymus of mice. (A) A population of thymic B220+CD19+ B cells. Thymocytes were isolated from 7- to 9-week-old female or male C57BL/6 mice. To enrich CD19+ B cells, lymphocytes were purified further by Ficoll separation solution from thymocytes. Thymic lymphocytes were stained with anti-mouse B220 and anti-mouse CD19 and analyzed by flow cytometry (FACS). The percentages of CD19+, B220+, and CD19+B220+ B cells are shown. Data represent at least 6 independent experiments. SSC, Side-scatter; FSC, forward-scatter. (B) CD5 and CD1dhi are expressed on IL-10-producing CD3−CD4−B220+CD19+ B cells. Flow cytometric analysis of thymic CD3−CD4−B220+ B cells with anti-mouse CD19, IL-10, CD5, and CD1d. To visualize IL-10-competent B cells, PMA, ionomycin, brefeldin A, and monensin were added to the cultures, 5 h before the cells were stained for cell-surface markers. The percentages of CD3−CD4−B220+ CD19+ B cells among B220+ B cells, IL-10+ B cells among CD3−CD4−B220+CD19+ or CD3−CD4−B220+CD19− B cells, and CD5+ or CD1d+ cells among CD3−CD4−B220+CD19+IL-10+ B cells are shown. The data represent at least 5 independent experiments. (C) IL-10-producing splenic CD19+ B cells. Splenic B cells were separated by CD19 microbeads from 7- to 9-week-old female or male C57BL/6 mice. To visualize IL-10-competent B cells, LPS, PMA, ionomycin, brefeldin A, and monensin were added to the cultures, 5 h before the cells were stained for cell-surface markers. The percentages of IL-10-expressing cells among CD19+ B cells are shown. The data represent at least 3 independent experiments. (D) CD3−CD4−B220+ CD19+CD5+CD1dhi B cells express high levels of IL-10. Flow cytometric analysis of B220+CD19+ B cells stained with surface markers, as described in B. The percentages of CD5- and CD1d-expressing cells after gating on B220+CD19+ B cells are shown (left and middle). To visualize IL-10-competent B cells, PMA, ionomycin, brefeldin A, and monensin were added to the cultures, 5 h before the cells were stained for cell surface markers. The percentages of IL-10-expressing CD3−CD4−B220+CD19+CD5+CD1dhi B cells or CD3−CD4−B220+CD19+CD5−CD1dlo B cells are also shown. The data represent at least 5 independent experiments. (E) Lymphocytes, B cells, and CD5+CD1dhi B cells were separated by Ficoll solution, CD19 microbeads, and FACS, respectively, from individual thymi of 7- to 9-week-old female or male C57BL/6 mice. (F) Light microscope images of CD1dlowCD5− B cells and CD1dhiCD5+ Bregs (400× original magnification).
B220+CD19+CD5+CD1dhi Bregs suppress anti-CD3/CD28 antibody-stimulated CD4+ T cell proliferation in vitro and maintained Foxp3+CD4+ Tregs
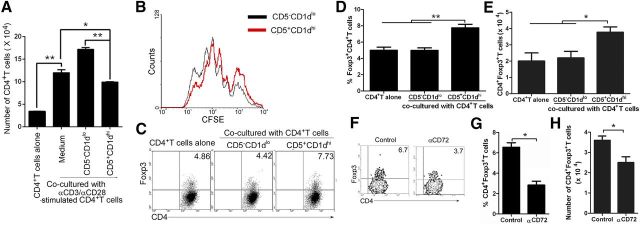
To detect the suppressive function of thymic B220+CD19+CD5+CD1dhi Bregs on CD4+ T cell proliferation, coculture experiments were conducted by use of CD3−CD4−B220+CD19+CD5+CD1dhi Bregs (CD5+CD1dhi) and CD3−CD4−B220+CD19+CD5−CD1dlo B cells (CD5−CD1dlo), sorted from mouse thymus by FACS (purification >95%), and CD4+ T cells, sorted from mouse splenocytes by CD4 microbeads. CD5+CD1dhi or CD5−CD1dlo cells were cocultured for 3 d with CD4+ T cells in the presence of anti-CD3 antibody (precoated on the plate) and anti-CD28 antibody. The results suggest that thymic B220+CD19+CD5+CD1dhi Bregs significantly suppress CD4+ T cell proliferation induced by anti-CD3 and anti-CD28 antibody stimulation (Fig. 2A and B).
Figure 2. In vitro suppression of anti-CD3/CD28 antibody-activated CD4+ T cell proliferation and expansion of Foxp3+CD4+ Tregs by thymic B220+CD19+CD5+CD1dhi Bregs. (A and B) Thymic B220+CD19+CD5+CD1dhi Bregs suppressed CD4+ T cell proliferation induced by stimulation with anti-CD3 and anti-CD28 antibody. CD3−CD4−B220+CD19+CD5+ CD1dhi Bregs (CD5+CD1dhi) and CD3−CD4−B220+CD19+CD5−CD1dlo B cells (CD5−CD1dlo) were sorted from mouse thymus by FACS. CD4+ T cells were sorted from mesenteric and inguinal LNs by CD4 microbeads and labeled with 5 μM CFSE. CD5+CD1dhi or CD5−CD1dlo cells were cocultured for 3 d with CD4+ T cells in the presence of anti-CD3 antibody (precoated in the plate) and anti-CD28 antibody. On day 3, cells were counted, stained with PerCP-conjugated anti-mouse CD4 antibody, and analyzed by FACS. (A) The number of CD4+ T cells and (B) CFSE/CD4+ T cell proliferation response is shown. Data represent at least 3 independent experiments (*P < 0.05, and **P < 0.01). (C–E) Percentages of Foxp3+CD4+ Tregs are increased when cocultured with thymic B220+CD19+CD5+CD1dhi Bregs. Flow cytometric analysis of purified CD4+ T cells cocultured for 3 d with CD3−CD4−B220+CD19+CD5+CD1dhi Bregs (CD5+CD1dhi) or with CD3−CD4−B220+CD19+ CD5−CD1dlo B cells (CD5−CD1dlo) and stained with anti-mouse CD4 and Foxp3 (C). The percentages of CD4+Foxp3+ T cells and (D) statistical analysis of the percentages of CD4+Foxp3+ T cells and (E) the number of CD4+Foxp3+ T cells are shown. Data represent at least 5 independent experiments (*P < 0.05, and **P < 0.01). (F–H) CD5−CD72 interaction is involved in the expansion of Foxp3+CD4+ Tregs induced by thymic B220+CD19+CD5+ CD1dhi Bregs. Flow cytometric analysis of CD4+ T cells cocultured with sorted CD3−CD4−B220+CD19+CD5+CD1dhi B cells (CD5+CD1dhi) in the presence of isotype control antibody or with neutralizing anti-mouse CD72 antibody for 3 d and then stained with anti-mouse CD4 and Foxp3. (F) The percentage of CD4+Foxp3+ T cells, (G) statistical analysis of the percentage of CD4+Foxp3+ T cells and (H) the number of CD4+Foxp3+ T cells are shown. CD4+ cells were used for analysis. The data represent at least 4 independent experiments (*P < 0.05). FACS data were analyzed by Student’s t-test (2-tailed).
Only transfer of splenic, IL-10-sufficient MZ and T2-MZP B cells, but not MZ or follicular B cells, can restore Treg numbers through maintenance of Foxp3 expression in B cell-deficient (μMT) mice [12]. To examine the regulatory function of thymic CD19+B220+CD1dhiCD5+ B cells in maintenance of CD4+Foxp3+ T cells, CD3−CD4−B220+CD19+CD5+CD1dhi Bregs (CD5+CD1dhi) and CD3−CD4−B220+CD19+ CD5−CD1dlo B (CD5−CD1dlo) cells were sort purified from the thymi of 7- to 9-week-old female or male C57BL/6 mice and then cocultured for 3 d with CD4+ T cells. Compared with freshly isolated Foxp3+CD4+ T cells, the percentage of Foxp3+CD4+ T cells decreased in a time-dependent manner during culture (data not shown). As compared with thymic CD5−CD1dlo B cells, coculture of CD4+ T cells with CD5+CD1dhi B cells maintained a greater percentage and number of CD4+Foxp3+ Tregs (Fig. 2C–E). A previous study demonstrated an IL-10-independent regulatory role for B cells in suppressing autoimmunity through maintenance of Tregs via glucocorticoid-induced TNFR-related protein ligand [28]. Our previous study demonstrated that interaction of CD5 and CD72 is involved in Treg and Breg homeostasis [29]. To determine whether the interaction between CD5 and CD72 was also involved in thymic Breg-mediated maintenance of Tregs, neutralizing anti-mouse CD72 antibodies were used to block the CD5–CD72 interaction. First, we test the functional impact of blocking CD72 with neutralizing anti-mouse CD72 antibodies on B cell proliferation induced by LPS. The data demonstrated that LPS-induced proliferation of B cells was suppressed significantly by neutralizing anti-mouse CD72 antibodies (Supplemental Fig. 2). Furthermore, the blockage of the CD5–CD72 interaction with neutralizing anti-mouse CD72 antibodies during coculture of thymic Bregs and Tregs demonstrated that the CD5–CD72 interaction is involved in the maintenance of Foxp3+CD4+ Tregs induced by thymic B220+CD19+CD5+CD1dhi Bregs (Fig. 2F–H).
Defective CD19+CD5+ B cells reduced Foxp3+CD4+ Tregs and up-regulated CD8−CD4+ and CD8+CD4− T cells in Cd19Cre mice
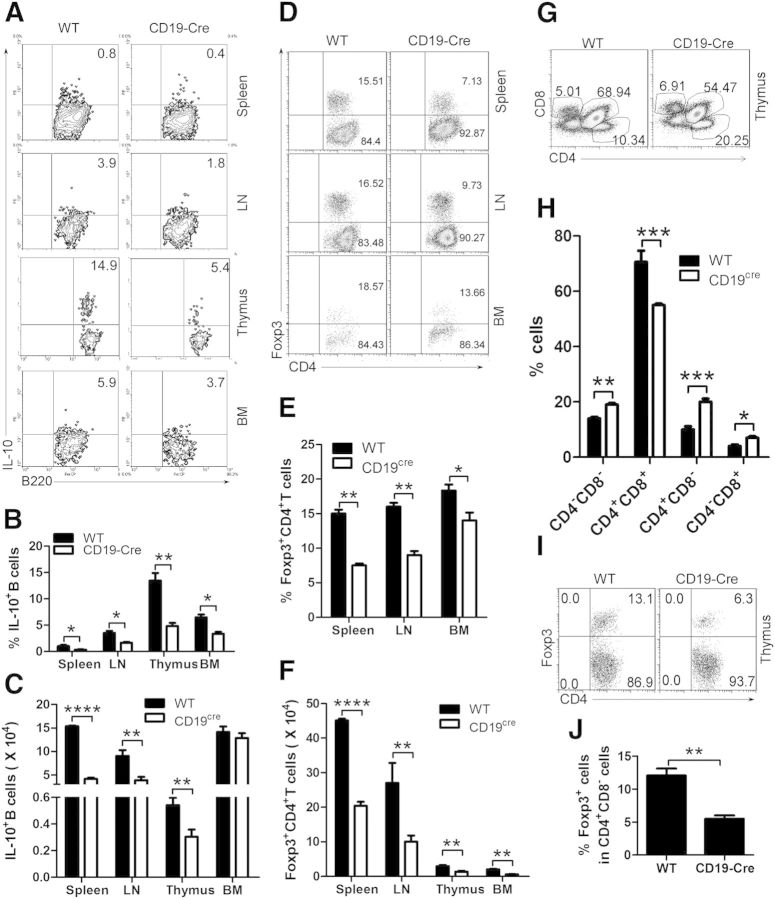
To examine the physiologic function of thymic Bregs, we used B cells isolated from homozygous Cd19Cre-expressing C57BL/6 CD19−/− [30]. Previous studies demonstrated that deletion of CD19 had no deleterious effects on the generation of B cells in the BM, but there was a significant reduction in the number of B cells in peripheral lymphoid tissues, such as blood and spleen, and in the peritoneal cavity [31, 32]. In addition, CD19−/− mice are essentially devoid of IL-10-expressing B220+ Bregs, which leads to exacerbated inflammation and disease symptoms during contact hypersensitivity and in the experimental autoimmune encephalomyelitis model of multiple sclerosis [6, 33]. Furthermore, our data showed that deletion of CD19 resulted in a significant reduction in the number of mature B cells (Supplemental Fig. 1A–C) and IL-10-producing Bregs (Fig. 3A–C) in the thymus.
Figure 3. IL-10-expressing Bregs and Foxp3+CD4+ Tregs are decreased in CD19Cre mice. (A–C) IL-10-expressing Bregs are reduced in CD19Cre mice. Flow cytometric analysis of lymphocytes from the spleen, LN, thymus, and BM of CD19Cre and WT mice stained with anti-mouse B220 and IL-10. To visualize IL-10-competent B cells, LPS, PMA, ionomycin, brefeldin A, and monensin were added to the cultures, 5 h before the cells were stained for cell-surface B220 and cytoplasmic IL-10 and analyzed by FACS. The percentages of IL-10-expressing B cells and statistical analysis of the percentage of IL-10-expressing B cells and IL-10-expressing B cell number/spleen, LN, thymus, and BM are shown in A–C, respectively. The data represent at least 3 independent experiments (*P < 0.05; **P < 0.01; ****P < 0.0001). (D–F) Foxp3+CD4+ Tregs are decreased in the spleen, LN, and BM of CD19Cre mice. Flow cytometric analysis of lymphocytes from the spleen, LN, and BM of CD19Cre and WT mice stained with anti-mouse CD4 and Foxp3. The percentages of CD4 and/or Foxp3-expressing cells and statistical analysis of the percentages of CD4+ Foxp3+ cells and CD4+Foxp3+ cell number/spleen, LN, and BM are shown in D–F, respectively. (G and H) CD8−CD4+ and CD8+CD4− T cells are increased in the thymi of CD19Cre mice. Flow cytometric analysis of lymphocytes from the thymi of CD19Cre and WT mice stained with anti-mouse CD4 and CD8. The percentages of CD4- and/or CD8-expressing cells and statistical analysis of the percentages of CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells in the thymus are shown in G and H, respectively. The data in D–H represent at least 4 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (F, I, and J) Foxp3+CD4+ Tregs are decreased in the thymus of CD19Cre mice. Flow cytometric analysis of lymphocytes from the thymus of CD19Cre and WT mice stained with anti-mouse CD4, CD8, and Foxp3. Gated on CD4+CD8− T cells, the percentage of Foxp3-expressing cells and statistical analysis of the percentages of Foxp3+ cells and Foxp3+ cell number/thymus are shown in F, I, and J, respectively. The data represent at least 3 independent experiments (**P < 0.01). FACS data were analyzed by Student’s t-test (2-tailed).
To examine the ability of Bregs to induce the conversion of CD4+ T cells into Tregs, we analyzed CD4+Foxp3+ Tregs by flow cytometry. We found that Foxp3+CD4+ Tregs are decreased in spleen, LN, and BM of CD19−/− mice (Fig. 3D–F). Our data also demonstrated that the reduced frequency of Foxp3+CD4+ Tregs was associated with an increased frequency of total CD4+ T cells (Fig. 3D), consistent with studies in which Tregs were shown to suppress the proliferation of autologous CD4+CD25− T cells [34]. By enumerating CD8−CD4−, CD8+CD4+, CD8−CD4+, and CD8+CD4− T cells, we found that greater CD8−CD4+ and CD8+CD4− T cell differentiation is associated with reduced thymic Tregs in CD19−/− mice (Fig. 3G and H). Furthermore, we found that Foxp3+CD4+CD8− Tregs are decreased in the thymus of CD19−/− mice (Fig. 3F, I, and J). Our data also demonstrated that the reduced frequency of Foxp3+CD4+ Tregs is associated with an increased frequency of CD8-CD4+ and CD8+CD4− T cells (Fig. 3G–J). Taken together, these results suggest that by reducing the population of thymic IL-10-expressing CD19+CD5+ Bregs, Foxp3+CD4+ Tregs are also reduced, resulting in an expansion of CD8−CD4+ and CD8+CD4− T cells in the thymus.
Transferred thymic B220+CD19+CD5+CD1dhi Bregs promote conversion of Foxp3+CD4+ Tregs and suppress CD4+ and CD8+ T cell differentiation
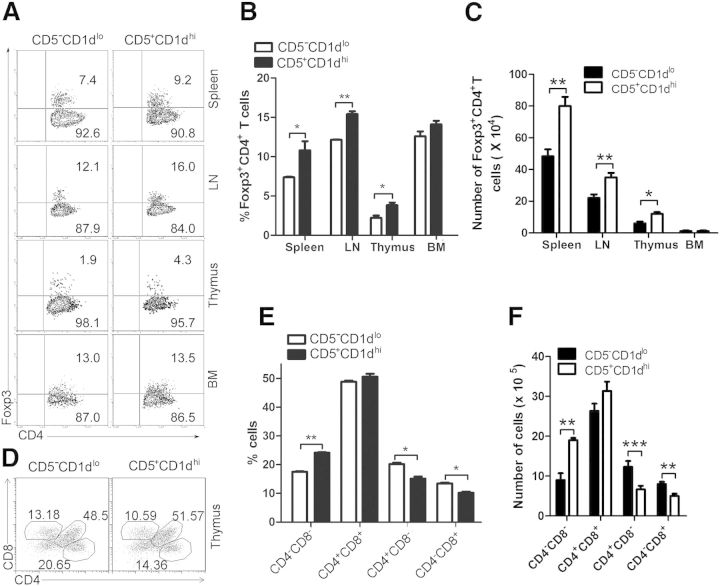
To elucidate further the role of thymic B220+CD19+CD5+CD1dhi Bregs in inducing Tregs and suppressing CD8−CD4+ and CD8+CD4− T cell expansion/differentiation, CD3−CD4−B220+CD19+CD5+CD1dhi B (CD5+CD1dhi) and CD3−CD4−B220+CD19+ CD5−CD1dlo B (CD5−CD1dlo) cells were sort purified from the thymi of 7- to 9-week-old female or male C57BL/6 mice and transferred i.v. into CD19−/− mice. The results demonstrate that compared with CD19+CD5−CD1dlo B cells, transferred thymic CD5+CD1dhi Bregs promote the development of Foxp3+CD4+ Tregs in the thymus, spleen, and LN but not in BM of recipient mice (Fig. 4A and B). We found reduced CD8−CD4+ and CD8+CD4− T cell differentiation in CD19−/− recipients receiving thymic CD5+CD1dhi Bregs compared with CD5−CD1dlo-transferred control mice (Fig. 4C and D). We also observed fewer CD4+ and CD8+ T cells in the spleens and LNs of CD19−/− mice that received thymic CD5+ CD1dhi Bregs compared with controls (Supplemental Fig. 2). These results are consistent with the idea that thymic CD19+ CD5+ Bregs suppress CD8−CD4+ and CD8+CD4− T cell expansion/differentiation by up-regulating Foxp3+CD4+ Tregs in the thymus and concur with prior studies showing that thymic Tregs are actively involved in maintaining the peripheral tolerance of autoreactive T cells that have escaped thymic selection [35–38].
Figure 4. Thymic B220+CD19+CD5+CD1dhi Bregs up-regulate Foxp3+CD4+ Tregs and suppress CD4+ and CD8+ T cell differentiation in CD19Cre mice. (A and B) Foxp3+CD4+ Tregs are increased in CD19Cre mice receiving thymic B220+CD19+CD5+CD1dhi Bregs. Sort-purified thymic CD3−CD4−B220+CD19+CD5+CD1dhi B (CD5+CD1dhi) and CD19+ CD5−CD1dlo B (CD5−CD1dlo) cells from 7- to 9-week-old female or male C57BL/6 mice (1 × 105 cells/mouse) were transferred into CD19Cre recipients. On day 14 after cell transfer, lymphocytes were collected and analyzed. (A) The percentages of CD4+Foxp3+ Tregs and statistical analysis of (B) the percentages or (C) the number of CD4+Foxp3+ Tregs are shown in each spleen, LN, thymus, and BM from control animals versus CD19Cre mice that received Bregs. The data represent at least 4 independent experiments (*P < 0.05; **P < 0.01). (D–F) Thymic B220+CD19+CD5+CD1dhiBregs suppress CD8−CD4+ and CD8+CD4− T cell differentiation in the thymus of CD19Cre mice. Flow cytometric analysis of lymphocytes collected from the thymi of control CD19Cre mice and CD19Cre mice that received Bregs, as described in A, and stained with anti-mouse CD4 and CD8. (D) The percentages of CD4- and/or CD8-expressing thymocytes and statistical analysis of (E) the percentages or (F) the number of CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells in each spleen, LN, thymus, and BM from control animals versus CD19Cre mice that received Bregs are shown. The data represent at least 4 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001). FACS data were analyzed by Student’s t-test (2-tailed).
Transferred thymic B220+CD19+CD5+CD1dhi Bregs suppress autoimmune disease by promoting Foxp3+CD4+ Treg differentiation in lupus-like mice
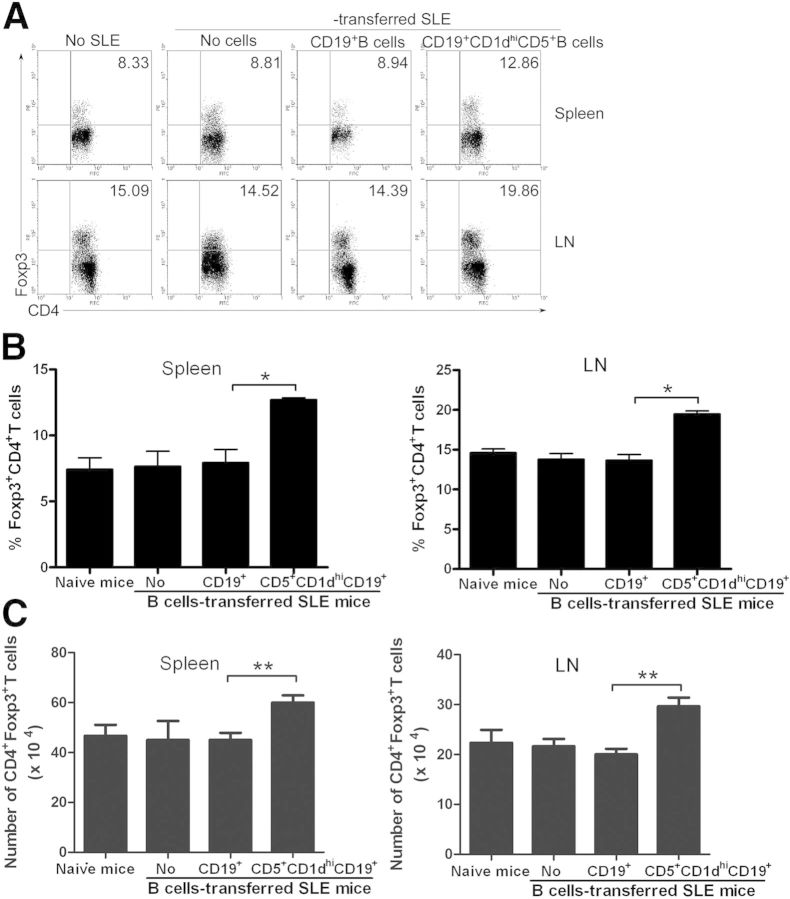
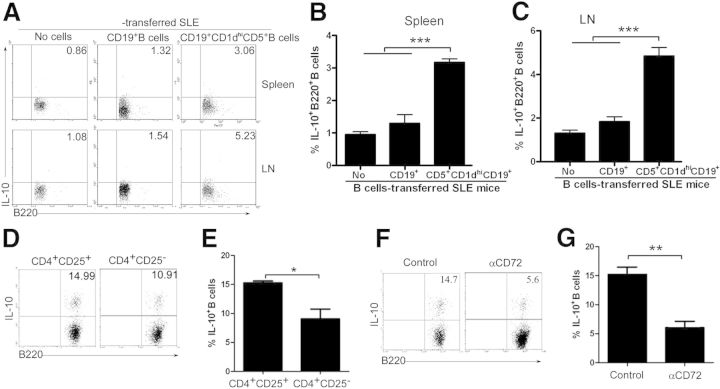
To determine whether thymic B220+CD19+CD5+CD1dhi Bregs suppress autoimmune disease progression, CD3−CD4−B220+CD19+CD5+CD1dhi B (CD5+CD1dhi) cells were sort purified, and CD19+ B cells were enriched by CD19 microbeads from the thymi of 7- to 9-week-old female or male C57BL/6 mice. Thymic B220 +CD19+CD5+CD1dhi B cells or CD19+ B cells were then transferred into lupus-prone NZB/NZW F1 mice. Flow cytometric analyses revealed that transfer of thymic B220+CD19+CD5+CD1dhi Bregs resulted in an up-regulation of CD4+Foxp3+ Treg differentiation (Fig. 5) as well as IL-10+ Breg expansion (Fig. 6A–C) in recipient NZB/NZW F1 mice.
Figure 5. Thymic B220+CD19+CD5+CD1dhi Bregs up-regulate Foxp3+CD4+ Tregs in lupus-like mice. Sort-purified WT thymic CD3−CD4−B220+CD19+CD5+CD1dhiB (CD5+CD1dhi) cells or microbead-enriched WT CD19+ B cells (1 × 105 cells/mouse) were transferred into lupus-prone NZB/NZW F1 recipients. (A) The percentages of CD4+Foxp3+ Tregs, (B) statistical analysis of the percentages of CD4+Foxp3+ Tregs, and (C) the number of CD4+Foxp3+ Tregs/mouse in the spleen and LN are shown. The data represent at least 3 independent experiments (*P < 0.05; **P < 0.01). FACS data were analyzed by Student’s t-test (2-tailed).
Figure 6. Thymic B220+CD19+CD5+CD1dhi Bregs up-regulate IL-10+ Bregs via Foxp3+CD4+ Tregs in lupus-prone mice. (A–C) Thymic IL-10+ Bregs are increased in NZB/NZW F1 recipients of B220+CD19+CD5+CD1dhi Bregs and CD3−CD4−B220+CD19+CD5+CD1dhi B (CD5+CD1dhi) cells, or CD19+ B cells (1 × 105 cells/mouse) were sorted and transferred into NZB/NZW F1 mice, as described in Fig. 5. Lymphocytes were collected from the spleen and LN, 2 weeks following transfer. (A) The percentage of B220+IL-10+ Bregs and statistical analysis of the percentage of B220+IL-10+ Bregs in the (B) spleen and (C) LN of recipient mice. The data represent at least 3 independent experiments (***P < 0.001). (D and E) B220+IL-10+ Bregs are increased when cocultured with CD4+CD25+ Tregs, and CD19+ B cells were cocultured for 3 d with CD4+CD25+ Tregs or CD4+CD25− T cells. (D) The percentages of B220+ IL-10+ Bregs and (E) statistical analysis of the percentages of B220+IL-10+ Bregs. Cells in the B220+ gate were used for analysis. The data represent at least 5 independent experiments (*P < 0.05). (F and G) CD5−CD72 interactions are involved in the expansion of B220+IL-10+ Bregs induced by Foxp3+CD4+ Tregs. CD4+CD25+ Tregs and CD19+ B cells were sorted as described in D and E. CD19+ B cells were cocultured in the presence of isotype antibody or neutralizing anti-mouse CD72 antibody for 3 d with CD4+CD25+ Tregs. (F) The percentages of B220+IL-10+ Bregs and (G) statistical analysis of the percentages of B220+IL-10+ Bregs. Cells in the B220+ gate were used for analysis. The data represent at least 4 independent experiments (**P < 0.01). FACS data were analyzed by Student’s t-test (2-tailed).
To determine whether IL-10+ Bregs were maintained/induced by CD4+Foxp3+ Tregs, CD4+CD25+ Tregs or CD4+CD25− T cells, sort purified from the spleens and LNs of 7-week-old female or male C57BL/6 mice, were cocultured for 3 d with CD19+ B cells, followed by flow cytometric analysis. These experiments showed that B220+IL-10+ Bregs increase when cocultured with CD4+CD25+ Tregs (Fig. 6D and E). Furthermore, the CD5–CD72 interaction is involved in the expansion/maintaining of B220+IL-10+ Bregs induced by Foxp3+CD4+ Tregs, as demonstrated by experiments in which CD19+ B cells were cocultured with CD4+CD25+ Tregs in the presence of isotype control antibody or neutralizing anti-mouse CD72 antibody (Fig. 6F and G). These results suggest that Tregs induce/maintain IL-10+ Bregs via the CD5–CD72 interaction.
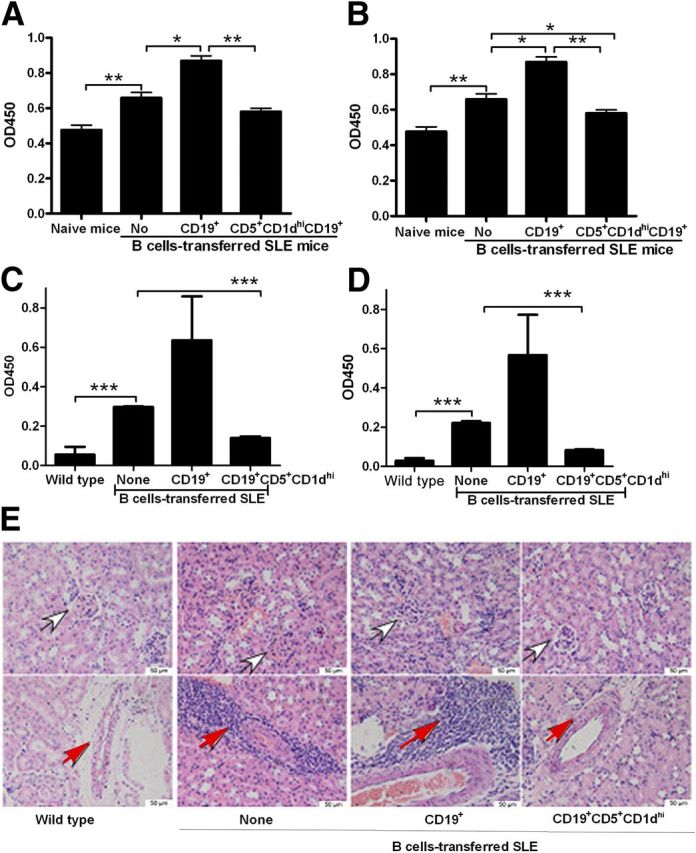
Among the murine lupus models, NZB/NZW F1 mice are the most similar to the human lupus phenotype, including autoantibody production (especially anti-dsDNA antibody) [39]. Therefore, we next assessed the effects Bregs on antibody production in this murine lupus model by examining antigen-specific or nonspecific antibody titers by use of ELISA. Compared with WT mice, unmanipulated, lupus-prone mice had higher levels of nonspecific IgG antibodies (Fig. 7A), nonspecific IgG1 antibodies (Fig. 7B), anti-dsDNA IgG antibodies (Fig. 7C), and anti-dsDNA IgG1 antibodies (Fig. 7D), as expected. Compared with untreated/control mice and mice receiving thymic CD19+ B cells, lupus-prone mice that received thymic B220+CD19+CD5+CD1dhi Bregs had lower titers of nonspecific IgG antibodies (Fig. 7A), nonspecific IgG1 antibodies (Fig. 7B), anti-dsDNA IgG antibodies (Fig. 7C), and anti-dsDNA IgG1 antibodies (Fig. 7D). Histology images of untreated mice with lupus (not treated with CD19+ B cells or CD1dhiCD5+ Bregs) or those of mice that received CD19+ B cells demonstrated shrinkage of the balloon cavity and appearance of excessive inflammatory cells in the surrounding interstitial vasculature. In contrast, CD1dhiCD5+ Breg-treated mice had fewer infiltrating inflammatory cells and an overall normal structure of the glomerular region (Fig. 7E). These results indicate that transferred thymic B220+CD19+CD5+CD1dhi Bregs suppress autoimmune disease in recipient mice. Collectively, our results suggest that thymic Bregs may suppress autoimmunity via up-regulation of Foxp3+CD4+ Tregs and CD5+ CD1dhi Bregs in lupus-prone mice.
Figure 7. Thymic B220+CD19+CD5+CD1dhi Bregs suppress autoantibody production in lupus-prone NZB/NZW F1 mice. CD3−CD4−B220+CD19+CD5+CD1dhi B (CD5+CD1dhi) cells or CD19+ B cells were sorted and (1 × 105 cells/mouse) transferred as described in Fig. 5 and serum collected from recipient mice, 1 month postinjection. (A) Total IgG antibody, (B) IgG1 antibody, (C) anti-ds DNA IgG antibody, (D) and anti-ds DNA IgG1 antibody titers, as determined by ELISA. The data represent at least 4 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001). ELISA data were analyzed by Student’s t-test (2-tailed). (E) H&E-stained sections of the kidney from WT, lupus-prone NZB/NZW F1 mice, CD19+ B cells, or CD1dhiCD5+ Bregs-transferred NZB/NZW F1 mice, enucleated at 30 d after the transfer of cells. Original scale bars, 50 μM. White arrows show glomeruli; red arrows show infiltrated inflammatory cells that appeared in the surrounding interstitial vasculature.
DISCUSSION
Many publications have shown that a small population of B cells comprises 0.1–0.5% of thymocytes in humans and mice [21–25]. Our data showed that CD19+ B cells represent ∼0.11% of total thymocytes (Supplemental Fig. 1). To enrich CD19+ B cells, lymphocytes were purified further by Ficoll separation solution from thymocytes. We demonstrate here that CD3−CD4−CD19+B220+ B cells represent ∼5% of thymic lymphocytes (Fig. 1A).
A subset of Bregs has been functionally defined in humans and mice by their ability to express IL-10 [6, 33, 40]. In fact, an essential feature of Breg function is the ability to inhibit proinflammatory cytokine production and to support Treg differentiation via IL-10 production [2]. Whereas this Breg subset is termed B10 and has a CD1dhiCD5+ phenotype, there are no data supporting the hypothesis that Bregs belong to a unique lineage. Bregs have been detected within the splenic MZ population [41–43] and the less mature, T2-MZP population [44, 45]. Here, we found that ∼17% of thymic B220+CD19+ B cells expressed IL-10 (Fig. 1A and B). The prevalence of thymic IL-10-expressing Bregs is much greater than the prevalence of splenic Bregs, which are found at low frequencies (1–5%) in naïve mice [9]. Furthermore, we found that thymic B220+CD19+IL-10+ Bregs are also CD5+CD1dhi (Fig. 1B and D). Like thymic B cells and splenic Bregs, the origin of thymic Bregs is unknown.
As a result of a lack of specific surface markers, Bregs are a functionally defined cell subset, currently identified only by their ability to secrete IL-10 [4]. One of the important functions of Breg is to suppress CD4+ T cell proliferation and another is to induce the differentiation of Tregs [2]. Our results demonstrate that CD5+CD1dhi Bregs significantly suppress CD4+ T cell proliferation following anti-CD3 and anti-CD28 antibody stimulation and increase the percentage of CD4+Foxp3+ Tregs in an in vitro coculture system (Fig. 2A–D). It also appears that the CD5−CD1dlow B cells enhance the number of CD4+ T cells (Fig. 2A). Thymic B cells express unique phenotypic markers compared with peripheral B cells; particularly, they express high levels of MHC class II [46]. These suggest that thymic CD5−CD1dlow B cells may be efficient APCs. Further experiments showed that CD5–CD72 interactions were involved in the expansion of Foxp3+CD4+ Tregs induced by thymic B220+CD19+CD5+CD1dhi Bregs (Fig. 2E and F). Transferred B220+CD19+CD5+CD1dhi Bregs up-regulated the number of Tregs in CD19−/− mice (Fig. 4) and lupus-prone mice (Fig. 5). On the other hand, CD19−/− mice, which have fewer Bregs, also showed reduced Tregs in the thymus (Fig. 3). These data suggest that thymic B220+CD19+CD5+CD1dhi B cells act functionally as Bregs.
CD4+CD25+Foxp3+ Tregs have been found in the thymus and the peripheral blood of mice and humans [47]. CD4+CD25+Foxp3+ Tregs develop in the thymus and provide an endogenous, long-lived population of self-antigen-specific T cells in the periphery that are poised to prevent autoimmune reactions [34]. Consistent with this, neonatally thymectomized mice that are deficient in CD4+CD25+Foxp3+ Tregs develop multiorgan autoimmune diseases [38]. Our results suggest that an impairment in thymic CD19+CD5+ B cell numbers or function results in an up-regulation of CD8−CD4+ and CD8+CD4− T cell differentiation by reducing Foxp3+CD4+ Tregs in the thymus (Fig. 3). On the other hand, transferred thymic B220+CD19+CD5+CD1dhi Bregs promoted the expansion of Foxp3+CD4+ Tregs, which in turn, suppressed CD4+ and CD8+ T cell differentiation in CD19−/− mice. Of note, this was seen in the thymus and secondary lymphoid tissues, such as spleen and LN but not in the BM (Fig. 4) or in lupus-prone NZB/NZW F1 recipient mice (Fig. 5). These results suggest that Bregs are an important early factor that controls immune homeostasis. In addition, transferred splenic CD5+CD1dhi Bregs promoted the expansion of Foxp3+CD4+ Tregs in the peripheral lymphoid tissues in CD19−/− mice, whereas this was not seen in the thymus or the BM (data not shown). Furthermore, CD4+ and CD8+ T cell differentiation was not suppressed in the thymus from splenic CD5+CD1dhi Breg-transferred CD19−/− mice (data not shown). These results suggest that thymic Bregs are specific in their control of thymic immune homeostasis.
As Bregs are rare and lack a specific marker, it is of significant interest to discover factors involved in the generation and induction of this cell type. IL-10 itself is not required for Breg development, as B cells develop normally in IL-10−/− mice [48]. Our data show that CD4+CD25+ Tregs induced or maintained B220+IL-10+ Bregs (Fig. 6D and E). It is possible that this enhanced survival is a result of interactions with Tregs. Further studies demonstrated that the CD5–CD72 interaction is involved in the expansion/maintenance of B220+IL-10+ Bregs induced by Foxp3+CD4+ Tregs (Fig. 6F and G). In turn, CD5+CD1dhi Bregs could increase the frequency of CD4+Foxp3+ Tregs (Fig. 2A and B). These results suggest that the interaction of Bregs–Tregs is critical for the expansion/maintenance of each population.
The ex vivo expansion and reinfusion of autologous Bregs and/or Tregs may provide a novel and effective in vivo treatment for severe autoimmune diseases that are resistant to current therapies [49]. Our data demonstrated that transferred thymic B220+CD19+CD5+CD1dhi Bregs suppressed antibody production (Fig. 7) via induction of Foxp3+CD4+ Tregs and CD5+CD1dhi Bregs (Figs. 5 and 6) in lupus-prone NZB/NZW F1 mice. These results suggest that thymic B220+CD19+IL-10+CD5+CD1dhi Bregs effectively suppress peripheral autoimmune responses, such as autoantibody production, by induction of Tregs and Bregs.
SLE is a prototype autoimmune disease characterized by abundant production of autoantibodies against a series of nuclear antigens and subsequent formation of immune complexes that lead to tissue damage [50, 51]. Recent compelling evidences have suggested that T cells are actually crucial in the pathogenesis of SLE in that they enhance the production of autoantibodies by offering substantial help to B cells through stimulating the latter to differentiate, proliferate, and mature, in addition to their support on class-switching of autoantibodies, which B cells are expressing [52]. In SLE, the number and function of Tregs are perturbed and therefore, unable to counteract autoreactive T lymphocytes. Elimination of CD4+CD25+ T cells accelerates the onset of glomerulonephritis during the preactive phase in autoimmune-prone female NZB × NZW F1 hybrid mice [53], whereas transfer of Treg effectively abrogate the progress of lupus [54, 55]. Thus, Treg therapy might be a rational approach for the treatment of lupus. Our previous study has suggested that there may be a feedback loop between Tregs and Bregs that depends on IL-35 for amplification of the immunosuppressive response [7]. We here demonstrated that thymic B220+CD19+IL-10+CD5+CD1dhi Bregs effectively suppress peripheral autoimmune responses, such as autoantibody production to alleviate lupus by induction of Tregs and Bregs (Figs. 6 and 7). Thus, immunotherapy with Bregs induced ex vivo has the potential to recruit 2 physiologic pathways (Tregs and Bregs) of immune regulation with a single agent—a rare opportunity in the treatment of autoimmunity [56].
Naïve CD4+ T cells from the thymus migrate to peripheral secondary lymphoid tissues (e.g., spleen and LNs), where they become into effector Th cells. A previous study suggested that lupus-prone mice with CD4+CD25+ Treg depletion from thymectomy have an enhanced expansion of autoreactive T cells and accelerated autoantibody production in peripheral tissue [57]. Our study here demonstrated that thymic Bregs could control thymic Tregs and peripheral effector T cells (Figs. 3 and 4). These studies suggest that thymic Bregs may play a critical role in protecting SLE.
The potential therapeutic effect of IL-10-producing Bregs in lupus is highlighted by the prolonged survival of CD19−/− NZB/NZW recipients following the adoptive transfer of splenic CD5+CD1dhi Bregs from WT NZB/NZW mice [58]. Studies in the NZB/NZW spontaneous lupus model suggest that IL-10-producing Bregs have protective and potentially therapeutic effects [4]. However, in the MRL.Fas (lpr) mouse lupus model, B cell-derived IL-10 does not regulate spontaneous autoimmunity [59]. These studies suggest fundamental differences in the pathogenesis and immune dysregulation in the NZB/NZW lupus model compared with the MRL.Fas (lpr) model [4].
In conclusion, we identified a population of B220+CD19+IL-10+CD5+CD1dhi Bregs in the murine thymus. Thymic B220+CD19+CD5+CD1dhi Bregs regulated CD4+CD8− or CD4−CD8+ T-cell homeostasis by up-regulating CD4+Foxp3+ Tregs. Finally, thymic B220+CD19+CD5+CD1dhi Bregs suppressed autoimmune responses in lupus-prone mice by up-regulating CD4+Foxp3+ Tregs, as well as IL-10-producing Bregs. Our study suggests that thymic B220+CD19+IL-10+CD5+CD1dhi Bregs play a critical role in maintaining immune homeostasis.
AUTHORSHIP
R.W., B.S., and Y.L. conceived of and designed the studies. C.X., N.M., H.X., X.W., M.Z., G.H., G.C., and C.H. carried out or contributed essential reagents and materials for the experiments. All authors contributed to data analysis and manuscript preparation.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Basic Research Program 973 grants (2013CB530506), Beijing Natural Science Foundation (7132139 and 7132151), and National Nature and Science Fund (81471529, 81272320, and 81172800).
Glossary
- BM
bone marrow
- Breg
regulatory B cell
- CD19−/−
mice that lack expression of cluster of differentiation 19
- CD62L
cluster of differentiation 62 ligand
- LN
lymph node
- MZP
marginal zone precursor
- NZB/NZW
New Zealand Black/New Zealand White
- SLE
systemic lupus erythematosus
- T2
transitional 2
- Treg
regulatory T cell
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Lipsky P. E. (2001) Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat. Immunol. 2, 764–766. [DOI] [PubMed] [Google Scholar]
- 2.Mauri C., Bosma A. (2012) Immune regulatory function of B cells. Annu. Rev. Immunol. 30, 221–241. [DOI] [PubMed] [Google Scholar]
- 3.Vadasz Z., Haj T., Kessel A., Toubi E. (2013) B-Regulatory cells in autoimmunity and immune mediated inflammation. FEBS Lett. 587, 2074–2078. [DOI] [PubMed] [Google Scholar]
- 4.Kalampokis I., Yoshizaki A., Tedder T. F. (2013) IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 15 (Suppl 1), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pistoia V. (1997) Production of cytokines by human B cells in health and disease. Immunol. Today 18, 343–350. [DOI] [PubMed] [Google Scholar]
- 6.Yanaba K., Bouaziz J. D., Haas K. M., Poe J. C., Fujimoto M., Tedder T. F. (2008) A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28, 639–650. [DOI] [PubMed] [Google Scholar]
- 7.Wang R. X., Yu C. R., Dambuza I. M., Mahdi R. M., Dolinska M. B., Sergeev Y. V., Wingfield P. T., Kim S. H., Egwuagu C. E. (2014) Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas K. M., Watanabe R., Matsushita T., Nakashima H., Ishiura N., Okochi H., Fujimoto M., Tedder T. F. (2010) Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J. Immunol. 184, 4789–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanaba K., Bouaziz J. D., Matsushita T., Tsubata T., Tedder T. F. (2009) The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 182, 7459–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Q., Yeung M., Camirand G., Zeng Q., Akiba H., Yagita H., Chalasani G., Sayegh M. H., Najafian N., Rothstein D. M. (2011) Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 121, 3645–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillatreau S., Gray D., Anderton S. M. (2008) Not always the bad guys: B cells as regulators of autoimmune pathology. Nat. Rev. Immunol. 8, 391–397. [DOI] [PubMed] [Google Scholar]
- 12.Carter N. A., Vasconcellos R., Rosser E. C., Tulone C., Muñoz-Suano A., Kamanaka M., Ehrenstein M. R., Flavell R. A., Mauri C. (2011) Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J. Immunol. 186, 5569–5579. [DOI] [PubMed] [Google Scholar]
- 13.Tadmor T., Zhang Y., Cho H. M., Podack E. R., Rosenblatt J. D. (2011) The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol. Immunother. 60, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauri C., Ehrenstein M. R. (2008) The ‘short’ history of regulatory B cells. Trends Immunol. 29, 34–40. [DOI] [PubMed] [Google Scholar]
- 15.Schioppa T., Moore R., Thompson R. G., Rosser E. C., Kulbe H., Nedospasov S., Mauri C., Coussens L. M., Balkwill F. R. (2011) B Regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 108, 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olkhanud P. B., Damdinsuren B., Bodogai M., Gress R. E., Sen R., Wejksza K., Malchinkhuu E., Wersto R. P., Biragyn A. (2011) Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4⁺ T cells to T-regulatory cells. Cancer Res. 71, 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amu S., Saunders S. P., Kronenberg M., Mangan N. E., Atzberger A., Fallon P. G. (2010) Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 125, 1114–1124, e8. [DOI] [PubMed] [Google Scholar]
- 18.Mangan N. E., Fallon R. E., Smith P., van Rooijen N., McKenzie A. N., Fallon P. G. (2004) Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173, 6346–6356. [DOI] [PubMed] [Google Scholar]
- 19.Das A., Ellis G., Pallant C., Lopes A. R., Khanna P., Peppa D., Chen A., Blair P., Dusheiko G., Gill U., Kennedy P. T., Brunetto M., Lampertico P., Mauri C., Maini M. K. (2012) IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 189, 3925–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siewe B., Wallace J., Rygielski S., Stapleton J. T., Martin J., Deeks S. G., Landay A. (2014) Regulatory B cells inhibit cytotoxic T lymphocyte (CTL) activity and elimination of infected CD4 T cells after in vitro reactivation of HIV latent reservoirs. PLoS ONE 9, e92934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akashi K., Richie L. I., Miyamoto T., Carr W. H., Weissman I. L. (2000) B Lymphopoiesis in the thymus. J. Immunol. 164, 5221–5226. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson P. G., Norton A. J., Addis B. J. (1987) The human thymus contains a novel population of B lymphocytes. Lancet 330, 1488–1491. [DOI] [PubMed] [Google Scholar]
- 23.Miyama-Inaba M., Kuma S., Inaba K., Ogata H., Iwai H., Yasumizu R., Muramatsu S., Steinman R. M., Ikehara S. (1988) Unusual phenotype of B cells in the thymus of normal mice. J. Exp. Med. 168, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S., Inaba M., Sugihara A., Taketani S., Doi H., Fukuba Y., Yamamoto Y., Adachi Y., Inaba K., Fukuhara S., Ikehara S. (1997) Presence of B cell progenitors in the thymus. J. Immunol. 158, 4193–4199. [PubMed] [Google Scholar]
- 25.Ceredig R. (2002) The ontogeny of B cells in the thymus of normal, CD3 epsilon knockout (KO), RAG-2 KO and IL-7 transgenic mice. Int. Immunol. 14, 87–99. [DOI] [PubMed] [Google Scholar]
- 26.Frommer F., Waisman A. (2010) B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS ONE 5, e15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleindienst P., Chretien I., Winkler T., Brocker T. (2000) Functional comparison of thymic B cells and dendritic cells in vivo. Blood 95, 2610–2616. [PubMed] [Google Scholar]
- 28.Ray A., Basu S., Williams C. B., Salzman N. H., Dittel B. N. (2012) A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 188, 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng M., Xing C., Xiao H., Ma N., Wang X., Han G., Chen G., Hou C., Shen B., Li Y., Wang R. (2014) Interaction of CD5 and CD72 is involved in regulatory T and B cell homeostasis. Immunol. Invest. 43, 705–716. [DOI] [PubMed] [Google Scholar]
- 30.Rickert R. C., Roes J., Rajewsky K. (1997) B Lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25, 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rickert R. C., Rajewsky K., Roes J. (1995) Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376, 352–355. [DOI] [PubMed] [Google Scholar]
- 32.Engel P., Zhou L. J., Ord D. C., Sato S., Koller B., Tedder T. F. (1995) Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39–50. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita T., Yanaba K., Bouaziz J. D., Fujimoto M., Tedder T. F. (2008) Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118, 3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggi E., Cosmi L., Liotta F., Romagnani P., Romagnani S., Annunziato F. (2005) Thymic regulatory T cells. Autoimmun. Rev. 4, 579–586. [DOI] [PubMed] [Google Scholar]
- 35.Bach J. F. (1995) Organ-specific autoimmunity. Immunol. Today 16, 353–355. [DOI] [PubMed] [Google Scholar]
- 36.Pearson C. I., McDevitt H. O. (1999) Redirecting Th1 and Th2 responses in autoimmune disease. Curr. Top. Microbiol. Immunol. 238, 79–122. [DOI] [PubMed] [Google Scholar]
- 37.Maloy K. J., Powrie F. (2001) Regulatory T cells in the control of immune pathology. Nat. Immunol. 2, 816–822. [DOI] [PubMed] [Google Scholar]
- 38.Shevach E. M. (2000) Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18, 423–449. [DOI] [PubMed] [Google Scholar]
- 39.Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. (1978) Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med. 148, 1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwata Y., Matsushita T., Horikawa M., Dilillo D. J., Yanaba K., Venturi G. M., Szabolcs P. M., Bernstein S. H., Magro C. M., Williams A. D., Hall R. P., St Clair E. W., Tedder T. F. (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenert P., Brummel R., Field E. H., Ashman R. F. (2005) TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J. Clin. Immunol. 25, 29–40. [DOI] [PubMed] [Google Scholar]
- 42.Gray M., Miles K., Salter D., Gray D., Savill J. (2007) Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl. Acad. Sci. USA 104, 14080–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brummel R., Lenert P. (2005) Activation of marginal zone B cells from lupus mice with type A(D) CpG-oligodeoxynucleotides. J. Immunol. 174, 2429–2434. [DOI] [PubMed] [Google Scholar]
- 44.Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R. S., Bhan A. K. (2002) Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16, 219–230. [DOI] [PubMed] [Google Scholar]
- 45.Evans J. G., Chavez-Rueda K. A., Eddaoudi A., Meyer-Bahlburg A., Rawlings D. J., Ehrenstein M. R., Mauri C. (2007) Novel suppressive function of transitional 2 B cells in experimental arthritis. J. Immunol. 178, 7868–7878. [DOI] [PubMed] [Google Scholar]
- 46.Perera J., Meng L., Meng F., Huang H. (2013) Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc. Natl. Acad. Sci. USA 110, 17011–17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164. [PubMed] [Google Scholar]
- 48.Maseda D., Smith S. H., DiLillo D. J., Bryant J. M., Candando K. M., Weaver C. T., Tedder T. F. (2012) Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J. Immunol. 188, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshizaki A., Miyagaki T., DiLillo D. J., Matsushita T., Horikawa M., Kountikov E. I., Spolski R., Poe J. C., Leonard W. J., Tedder T. F. (2012) Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson A., Diamond B. (2001) Autoimmune diseases. N. Engl. J. Med. 345, 340–350. [DOI] [PubMed] [Google Scholar]
- 51.Rahman A., Isenberg D. A. (2008) Systemic lupus erythematosus. N. Engl. J. Med. 358, 929–939. [DOI] [PubMed] [Google Scholar]
- 52.Shlomchik M. J., Craft J. E., Mamula M. J. (2001) From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 1, 147–153. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi T., Hasegawa K., Adachi C. (2005) Elimination of CD4(+)CD25(+) T cell accelerates the development of glomerulonephritis during the preactive phase in autoimmune-prone female NZB x NZW F mice. Int. J. Exp. Pathol. 86, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scalapino K. J., Tang Q., Bluestone J. A., Bonyhadi M. L., Daikh D. I. (2006) Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 177, 1451–1459. [DOI] [PubMed] [Google Scholar]
- 55.Weigert O., von Spee C., Undeutsch R., Kloke L., Humrich J. Y., Riemekasten G. (2013) CD4+Foxp3+ regulatory T cells prolong drug-induced disease remission in (NZBxNZW) F1 lupus mice. Arthritis Res. Ther. 15, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mauri C., Nistala K. (2014) Interleukin-35 takes the ‘B’ line. Nat. Med. 20, 580–581. [DOI] [PubMed] [Google Scholar]
- 57.Kyttaris V. C., Juang Y. T., Tsokos G. C. (2005) Immune cells and cytokines in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 17, 518–522. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe R., Ishiura N., Nakashima H., Kuwano Y., Okochi H., Tamaki K., Sato S., Tedder T. F., Fujimoto M. (2010) Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J. Immunol. 184, 4801–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teichmann L. L., Kashgarian M., Weaver C. T., Roers A., Müller W., Shlomchik M. J. (2012) B Cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J. Immunol. 188, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.