Abstract
Discussion on leukotoxin A as a potential therapeutic target to halt the recruitment of inflammatory leukocytes in allergic asthma.
Keywords: allergy, integrins, airway remodelling
Asthma is a prevalent inflammatory disorder of the airway, characterized by immune cell infiltration, epithelial barrier defects, mucous hypersecretion, and obstruction of airflow [1]. In allergic asthma, patients are sensitized to inhaled allergens in a Th2-driven process of allergen-specific IgE production. Subsequent exposures to inhaled allergens result in inflammatory mediator release from mast cells and basophils that enhance blood vessel permeability, leukocyte recruitment, and chronic inflammation [2]. Current therapies largely target the symptoms of this disease and are not effective for all allergic asthma patients [3]. New targets that are fundamentally involved in allergic asthma are being pursued to block more effectively early events in the pathogenesis of allergic asthma. In this issue of the Journal of Leukocyte Biology, Gupta et al. [4] report that LFA-1 is a promising target in human allergic asthma patient samples. They test this by use of a mouse model of allergic asthma and an experimental toxin directed against LFA-1, called LtxA (Leukothera).
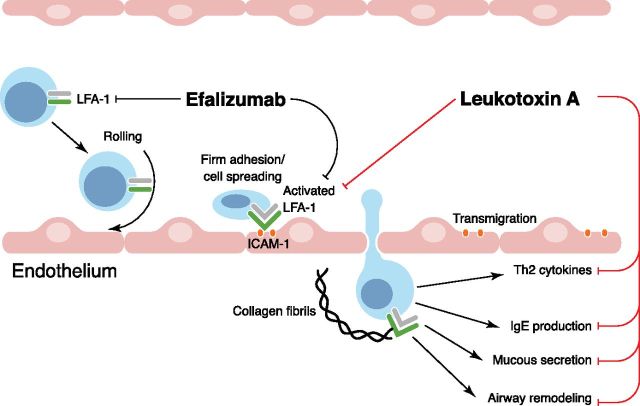
Integrins form heterodimers on the cell surface that regulate cell adhesion and motility through interactions with ligands expressed on activated endothelium or within the extracellular matrix. Leukocyte integrins play key roles in leukocyte adhesion to blood vessel walls, spreading, and transendothelial migration (Fig. 1) [5]. Relevant integrins include VLA-4 (or α4β1), macrophage 1 antigen (αMβ2), and LFA-1 (αLβ2, or CD11a/CD18). In asthmatic airways, ligands for these integrins are expressed at high levels, including VCAM-1 and ICAM-1 [6]. This promotes firm adhesion and tissue invasion by immune cells expressing VLA-4 and LFA-1 and has led research into therapeutic blockade of these integrins in asthma and autoimmune disorders. Efalizumab (Raptiva) is an inhibitory LFA-1 antibody that was approved by the U.S. Food and Drug Administration in 2003 for treatment of an autoimmune disease but was withdrawn in 2009 as a result of risk of developing fatal brain infections [7]. One potential drawback with Efalizumab is that it targets all LFA-1-positive cells, whereas targeting only activated LFA-1 would be preferable for therapeutic applications.
Figure 1. Therapeutic strategies targeting LFA-1-dependent steps in leukocyte recruitment that promote allergic asthma pathophysiology. Leukocyte recruitment to the lungs in allergic asthma patients or mouse models is a multistep process involving multiple classes of cell–cell adhesion molecules. Selectins (not shown here for simplicity) mediate the initial attachment and rolling, whereas integrins, such as VLA-4 and LFA-1, are critical for firm adhesion, cell spreading, and transmigration of inflammatory leukocytes. Efalizumab binds and inhibits LFA-1 in low-avidity state (circulating leukocytes) and high-avidity states (activated LFA-1 on adherent and recruited leukocytes). In contrast, LtxA preferentially inhibits activated LFA-1 and was shown to suppress Th2 cytokines and airway remodeling in a mouse model of allergic asthma.
LtxA is a toxin secreted by the gram-negative bacterium Aggregatibacter actinomycetemcomitans that binds activated LFA-1 on leukocytes leading to cell death [8]. The therapeutic potential of LtxA was first demonstrated in a leukemia model, showing that LtxA preferentially targets leukemic cells expressing activated LFA-1 compared with normal leukocytes [8]. Moreover, LtxA treatment was effective in treating leukemias that had relapsed on standard chemotherapy [9]. In addition, LtxA was tested in a xenograft model of human psoriasis and shown to allow resolution of disease by preferential targeting of proinflammatory leukocytes [10]. In this issue of the Journal of Leukocyte Biology, Gupta et al. [4] have now extended studies of LFA-1 expression in allergic asthma patient samples and effects of LFA-1 blockade with LtxA in a mouse model of allergic asthma [4]. They identified a circulating cell population with elevated levels of LFA-1 (CD11ahi) in asthma patients that were not found in healthy controls. This population was composed of CD14+CD4−CD11ahi monocytes and CD3+CD4−CD11ahi non-Th cells. The authors went on to show that these CD11ahi cells undergo apoptosis upon LtxA treatment ex vivo, whereas CD11alo cells within the same samples were unaffected. These findings were tested further in mice chronically exposed to HDM extract. The authors validate high expression of LFA-1 in leukocytes from broncheoalveolar lavage fluid of allergic mice compared with control mice. This provides further evidence for the potential targeting of LFA-1hi cells in allergic asthma. The authors go on to compare treatment of HDM mice directly with LtxA or dexamethasone, the current gold standard corticosteroid used as a general immunosuppressant. Importantly, the authors demonstrate that LtxA outperforms dexamethasone by several metrics. LtxA-treated mice were protected from airway infiltration of all major leukocyte subtypes, including neutrophils, eosinophils, macrophages, and B and T cells. LtxA-treated mice were protected from airway remodeling induced by HDM exposure and had reduced expression of several cytokines implicated in asthma pathogenesis within the lung. LtxA treatment was more effective than dexamethasone in blocking neutrophil infiltration and expression of Th2 cytokines (IL-4, IL-5), which are key drivers of IgE production, mucous secretion, and allergic inflammation. As LtxA targets only activated leukocytes, it may provide a viable alternative to corticosteroids, whose chronic use can lead to multiple side-effects. However, an important extension of this work will involve testing of LtxA for efficacy against other allergens or pathogens that induce asthma. It would also be worth comparing LtxA with Efalizumab in both allergic asthma models and in mice infected with bone fide pathogens. For LtxA to progress beyond an experimental tool, it will be important to demonstrate that LtxA does not increase risk of infections, as observed in patients treated with Efalizumab [7]. Future studies will also be required to test the efficacy of LtxA across a larger cohort of allergic asthma patients as a result of the genetic heterogeneity of this disease.
Overall, the study by Gupta et al. [4] will undoubtedly spur further testing of LtxA as a new therapeutic in allergic asthma. If LtxA continues to outperform current therapies and lacks side-effects associated with Efalizumab, then it may prove to be a more targeted approach to treat LFA-1-driven diseases.
Glossary
- HDM
house dust mite
- LtxA
Leukotoxin A
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 439
REFERENCES
- 1.Trevor J. L., Deshane J. S. (2014) Refractory asthma: mechanisms, targets, and therapy. Allergy 69, 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fireman P. (2003) Understanding asthma pathophysiology. Allergy Asthma Proc. 24, 79–83. [PubMed] [Google Scholar]
- 3.Bonfield T. L., Ross K. R. (2012) Asthma heterogeneity and therapeutic options from the clinic to the bench. Curr. Opin. Allergy Clin. Immunol. 12, 60–67. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A., Espinosa V., Galusha L. E., Rahimian V., Miro K. L., Rivera-Medina A., Kasinathan C., Capitle E., Aguila H. A., Kachlany S. C. (2015) Expression and targeting of lymphocyte function-associated antigen 1 (LFA-1) on white blood cells for treatment of allergic asthma. J. Leukoc. Biol. 97, XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreiro O., de la Fuente H., Mittelbrunn M., Sánchez-Madrid F. (2007) Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol. Rev. 218, 147–164. [DOI] [PubMed] [Google Scholar]
- 6.Gosset P., Tillie-Leblond I., Janin A., Marquette C. H., Copin M. C., Wallaert B., Tonnel A. B. (1995) Expression of E-selectin, ICAM-1 and VCAM-1 on bronchial biopsies from allergic and non-allergic asthmatic patients. Int. Arch. Allergy Immunol. 106, 69–77. [DOI] [PubMed] [Google Scholar]
- 7.Berger J. R., Houff S. A., Major E. O. (2009) Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs 1, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kachlany S. C. (2010) Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J. Dent. Res. 89, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A., Le A., Belinka B. A., Kachlany S. C. (2011) In vitro synergism between LFA-1 targeting leukotoxin (Leukothera™) and standard chemotherapeutic agents in leukemia cells. Leuk. Res. 35, 1498–1505. [DOI] [PubMed] [Google Scholar]
- 10.Stenderup K., Rosada C., Dam T. N., Salerno E., Belinka B. A., Kachlany S. C. (2011) Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J. Invest. Dermatol. 131, 2033–2039. [DOI] [PubMed] [Google Scholar]