Abstract
Discussion on two distinct mechanisms of regulation for membrane levels of CXCR2 as a level of therapeutic control.
Keywords: Neutrophils, G protein-coupled receptors, chemotaxis, metalloproteinase, leukocyte
Approaches that limit the innate immune response by ablating the activation and mobility of an immune cell have emerged as favored therapeutic strategies in the setting of inflammation and vascular damage. In this regard, members of the ADAM family of metalloproteinases may be viable targets, as ADAMs mediate the down-regulation of adhesion receptors (impacting cell localization and movement) and the release of membrane-bound cytokines and chemokines (controlling inflammation and activation responses). In the current issue of JLB, Mishra and colleagues [1] have identified a specialized role for ADAM17 in the regulation of levels of the chemokine receptor CXCR2 on human and mouse neutrophils and in the infiltration of neutrophils to sites of inflammation. CXCR2 has created tremendous interest as a therapeutic target [2], as this receptor plays a central role in inflammation [3] and cancer cell metastasis [4]; however, the use of direct CXCR2 antagonists in the clinic remains unclear as a result of complexities in interpreting data from animal models of inflammatory disease and the widespread involvement of CXCR2 in inflammatory cascades. The work of Mishra and colleagues [1] helps establish a strong rationale for modulation of CXCR2 levels "from afar" by controlling ADAM17 activity in settings of inflammation.
Throughout biology, the shedding of receptor ligand-binding ectodomains is a consistent, recurring mechanism used to regulate the function of adhesion and signaling receptors. Transmembrane metalloproteinases of the ADAM family proteolytically regulate surface and soluble levels of growth factors, cytokines, adhesion receptors, and ectoenzymes. The ADAMs are closely related to the matrix metalloproteinase family of enzymes; however, ADAMs are primarily responsible for regulating "life-essential" molecules, particularly on vascular cells [5]. On human platelets, ADAMs control surface levels of primary platelet adhesion/activation receptors [6], and ADAM activity controls processes of adherence and de-adherence that are essential for movement and migration of a host of cell types, including leukocytes, endothelial and smooth muscle cells, fibroblasts, and metastasising cancer cells [7]. ADAM activity is also essential for the liberation of cytokine and growth-hormone precursors from membrane-bound inactive forms, permitting the interaction of active, soluble cytokines with their receptors.
From the ADAM family of 13 proteolytically active enzymes, ADAM17 is ubiquitously expressed and cleaves numerous biologic substrates, usually colocalized with ADAM17 on membranes. Originally named TNF-α-converting enzyme for its ability to cleave and release TNF-α from membrane surfaces, ADAM17 has emerged as a master regulator of levels of a host of adhesion proteins and ligands, cytokines, chemokines, and growth factors. This family of Zn2+-dependent metalloproteinases shares a number of common features within a multidomain structure comprised of extracellular prodomain, catalytic, disintegrin, and cysteine-rich domains, then a single transmembrane domain, and a cytoplasmic tail. The catalog of substrates cleaved by ADAM17 is sizeable [5, 7], and unsurprisingly, mouse knock-out studies (targeting either protein expression or catalytic activity) demonstrate that ADAM17 is essential for embryogenesis, maturation, reproduction, and survival [7].
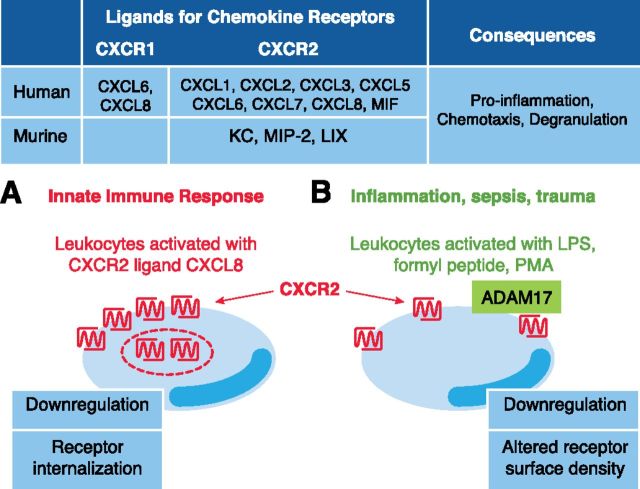
CXCR2 is a 7-transmembrane GPCR on leukocytes and endothelial cells that binds CXCL8 (IL-8) with high affinity. Numerous studies have shown that CXCR2 is internalized rapidly upon ligand binding and under certain experimental conditions, can be recycled to the surface of neutrophils [8]. Levels of CXCR2 and the related CXCR1 can also be regulated by other neutrophil activators, such as LPS and TNF-α; however, this ligand-independent process involves metalloproteinase activity [9] and is irreversible, helping to explain the decreased sensitivity for CXCL8 of neutrophils that have been pre-exposed to neutrophil activators, such as LPS and TNF-α. In the current study, Mishra and colleagues [1] use specific ADAM17 inhibitors, as well as mice with leukocytes deficient in ADAM17 protein to demonstrate a role for ADAM17 in the down-regulation of CXCR2 levels on neutrophils exposed to non-CXCR2 ligands, such as LPS, formyl peptide, or phorbol ester. This down-regulation appeared to be specific for CXCR2, as similar effects on CXCR1 were not evident, and there was no detectable reduction in CXCR4 levels. Notably, the authors showed that maintenance of CXCR2 levels on neutrophils had an important functional consequence in vivo, as ADAM17-null neutrophils were able to infiltrate an inflamed peritoneal cavity at much greater levels than control neutrophils, and this effect was largely reversed by pretreatment with a CXCR2 inhibitor.
The mechanism by which ADAM17 controls surface levels of CXCR2 on neutrophils is unclear. GPCRs can be proteolyzed, and CXCR1 is cleaved by serine proteases, for example [10]. Nevertheless, direct ADAM17-mediated cleavage, liberating CXCR2 from the neutrophil surface, would be unusual, as these types of receptors are not known as substrates for ADAMs. Release of CXCR1 from LPS- or TNF-α-treated neutrophils by an unidentified metalloproteinase has been reported, but the mechanism was not defined and was complicated by the fact that soluble proteolytic fragments of 20 and 40 kDa are immunoreactive toward antibodies against the intracellular C-terminal region of the receptor [9].
It is conceivable that removal of CXCR2 from the neutrophil surface, mediated by active ADAM17, is indirect, involving 1 or more intermediary factors; however, definition of the precise mechanism requires further work. What is clear from the current study, however, is that CXCR2 is regulated via 2 distinct mechanisms, depending on the agonist used (Fig. 1). The ligand-based, CXCL8-dependent CXCR2 internalization is reversible, whereas clearance triggered by conditions mimicking bacterial sepsis and mediated by ADAM17 is irreversible. The understanding of distinctions between the 2 mechanisms may allow a discrete molecular, therapeutic control of this central inflammatory receptor. Modulators of myeloid cell ADAM17 activity are already under development for inflammatory disease [11], highlighting the feasibility of this approach. Conceivably, reagents that trigger an ablation of ADAM17 activity would be appropriate under conditions of acute systemic inflammation (sepsis, coagulopathy); however, in situations of chronic inflammation with prolonged invasion of neutrophils, the current study by Mishra and colleagues [1] would support development of reagents that are able to up-regulate ADAM17 activity. New therapeutic reagents would ideally target ADAM activity toward specific substrates (for example, selectively bind to substrate cleavage sites and modulate shedding) or target ADAMs on specific cell types [11]. Ultimately, the targeting of ADAMs by use of novel, therapeutic delivery systems that enable selective control of enzymatic activity within high, shear environments or specific vascular beds [12] seems feasible.
Figure 1. Ligands for CXCR1 and CXCR2 regulate human or mouse neutrophil functon. (A) Engagement of CXCR2 by ligand CXCL8 triggers a transient receptor internalization into storage vesicles, enabling CXCR2 surface levels to be replenished later. (B) However, after exposure of neutrophils to nonligand-based activators that recapitulate aspects of sepsis and trauma, ADAM17 directs irreversible reduction in CXCR2 density on the surface of mouse and human neutrophils. MIF, Macrophage inhibitory factor; KC, keratinocyte-derived chemokine; LIX, LPS-induced CXC chemokine.
Glossary
- ADAM
a disintegrin and metalloproteinase
- GPCR
G protein-coupled receptor
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 447
REFERENCES
- 1.Mishra H. K., Long C., Bahaie N. S., Walcheck B. (2014) Regulation of CXCR2 expression and function by a disintegrin and metalloprotease-17 (ADAM17). J. Leukoc. Biol. 97, XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwyer M. P., Yu Y. (2014) CXCR2 modulators: a patent review (2009–2013). Expert Opin. Ther. Pat. 24, 519–534. [DOI] [PubMed] [Google Scholar]
- 3.Boppana N. B., Devarajan A., Gopal K., Barathan M., Bakar S. A., Shankar E. M., Ebrahim A. S., Farooq S. M. (2014) Blockade of CXCR2 signalling: a potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp. Biol. Med. (Maywood) 239, 509–518. [DOI] [PubMed] [Google Scholar]
- 4.Sharma B., Singh S., Varney M. L., Singh R. K. (2010) Targeting CXCR1/CXCR2 receptor antagonism in malignant melanoma. Expert Opin. Ther. Targets 14, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann M., Herrlich A., Herrlich P. (2013) Who decides when to cleave an ectodomain? Trends Biochem. Sci. 38, 111–120. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner E. E., Karunakaran D., Shen Y., Arthur J. F., Andrews R. K., Berndt M. C. (2007) Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J. Thromb. Haemost. 5, 1530–1537. [DOI] [PubMed] [Google Scholar]
- 7.Lisi S., D’Amore M., Sisto M. (2014) ADAM17 at the interface between inflammation and autoimmunity. Immunol. Lett. 162(1PA), 159–169. [DOI] [PubMed] [Google Scholar]
- 8.Samanta A. K., Oppenheim J. J., Matsushima K. (1990) Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J. Biol. Chem. 265, 183–189. [PubMed] [Google Scholar]
- 9.Khandaker M. H., Mitchell G., Xu L., Andrews J. D., Singh R., Leung H., Madrenas J., Ferguson S. S., Feldman R. D., Kelvin D. J. (1999) Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-α-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93, 2173–2185. [PubMed] [Google Scholar]
- 10.Hartl D., Latzin P., Hordijk P., Marcos V., Rudolph C., Woischnik M., Krauss-Etschmann S., Koller B., Reinhardt D., Roscher A. A., Roos D., Griese M. (2007) Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 13, 1423–1430. [DOI] [PubMed] [Google Scholar]
- 11.Issuree P. D., Maretzky T., McIlwain D. R., Monette S., Qing X., Lang P. A., Swendeman S. L., Park-Min K. H., Binder N., Kalliolias G. D., Yarilina A., Horiuchi K., Ivashkiv L. B., Mak T. W., Salmon J. E., Blobel C. P. (2013) iRHOM2 is a critical pathogenic mediator of inflammatory arthritis. J. Clin. Invest. 123, 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korin N., Kanapathipillai M., Matthews B. D., Crescente M., Brill A., Mammoto T., Ghosh K., Jurek S., Bencherif S. A., Bhatta D., Coskun A. U., Feldman C. L., Wagner D. D., Ingber D. E. (2012) Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 337, 738–742. [DOI] [PubMed] [Google Scholar]