Introduction
Focal segmental glomerulosclerosis (FSGS) can recur after renal transplantation and is associated with a reduced graft survival. In the case of recurrent FSGS, treatment with plasma exchange (PE) results in a remission of proteinuria in up to 85% of patients, especially if started shortly after the onset of recurrence [1,2]. However, many patients require repeated courses of PE because of frequent relapses [1,3]. Recently, a 7-year-old boy with recurrent FSGS after renal transplantation responded to rituximab, a monoclonal anti-CD20 antibody, that was administered for a transplantation-related lymphoma [4]. Following this report, several other cases with recurrent FSGS after renal transplantation were published, showing varying degrees of success after treatment with rituximab [5]. We describe the results of treatment with rituximab in a 24-year-old female patient, with recurrent FSGS after renal transplantation.
Case
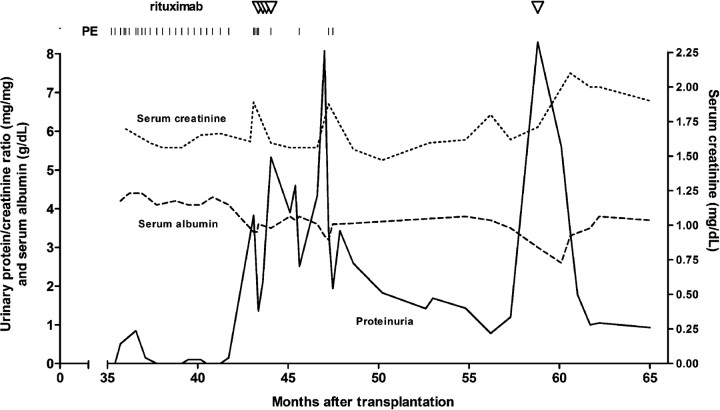
At the age of 10 years, the patient presented with a nephrotic syndrome due to biopsy-proven FSGS. Treatment with prednisone, cyclophosphamide and cyclosporine was unsuccessful and end-stage renal disease developed within 3 years after presentation. At the age of 13 years the patient received a renal graft, which failed after 1 year due to recurrent FSGS. Seven years later, she received a second renal graft. Baseline immunosuppressive therapy consisted of prednisone (10 mg), tacrolimus (target through level 15–20 mg/l) and mycophenolate mofetil (MMF 750 mg bid). There was almost immediate graft function. One week after transplantation, the patient developed nephrotic range proteinuria. A presumptive diagnosis of recurrent FSGS was made and treatment with PE was started, which resulted in a complete remission. Three months after cessation of PE, proteinuria recurred. At that moment, the immunosuppressive regimen consisted of prednisone (15 mg) and tacrolimus (target through level 5–10 mg/l). A second course of PE (eight sessions) again resulted in a complete remission. A third relapse occurred 2 years later. A biopsy of the renal graft demonstrated diffuse foot process effacement, without significant lesions on light microscopy and IF, favouring a diagnosis of recurrent FSGS. Although treatment with PE induced a remission of proteinuria, repeated trials of cessation of PE failed (Figure 1). A remission of proteinuria could only be maintained with continuous PE, even though the patient was treated with a more intensive immunosuppressive regimen consisting of prednisone (10 mg), tacrolimus (target through level 5–10 mg/l) and MMF (500 mg bid), which was replaced by azathioprine (2 mg/kg/day) because of gastrointestinal side effects. Because of PE dependence, the decision was made to start treatment with rituximab. She tolerated the entire course of four weekly infusions (375 mg/m2) without significant side effects, although we observed a temporary neutropenia. After treatment B-cell markers CD19+ and CD20+ were undetectable. In the first 4 months after treatment with rituximab, three PE sessions were necessary because of increasing proteinuria. Thereafter, proteinuria gradually decreased without further interventions. Seven months after treatment with rituximab a partial remission was attained. Nine months later, she experienced a relapse of proteinuria (Figure 1). At that time, CD19+ and CD20+ B-cells were still undetectable. The patient was re-treated with a single infusion of rituximab 1000 mg. Proteinuria gradually decreased, and a partial remission was reached 2 months after treatment.
Fig. 1.
Clinical course of proteinuria after third relapse of FSGS and response to plasma exchange (PE) and rituximab.
Our case report indicates that rituximab may be effective in patients with recurrent FSGS after renal transplantation. Admittedly, we cannot exclude that PE contributed to the response after initial therapy; however, the efficacy of rituximab was virtually proven by the response to rituximab monotherapy after relapse.
Rituximab was used with apparent success in children with recurrent FSGS [4,6]. These children were treated with rituximab for EBV-related posttransplant lymphoproliferative disease (PTLD). Coincident with the remission of the PTLD, proteinuria also disappeared. In contrast, two children without PTLD failed to respond to treatment with rituximab for recurrent FSGS after renal transplantation [7]. Data in adults are also conflicting (Table 1) [5]. A remission of proteinuria after treatment with rituximab was also reported in two adult patients with recurrent FSGS after transplantation [8,9]. However, since both patients were simultaneously treated with PE, it cannot be excluded that PE alone induced the remission. In contrast, Gossmann reported a patient who was resistant to treatment with PE but who responded to rituximab [10]. Similarly, another report by Meyer also showed a response to rituximab independent of PE treatment [11]. However, Kamar, Yabu and El-Firjani reported no effect of treatment with rituximab in six adult patients with recurrent FSGS after transplantation, independent of the response to PE treatment [9,12,13].
Table 1.
Results of treatment with rituximab in adult patients with recurrent FSGS after renal transplantation
| Study | Donor source | Immuno-suppression | Age at transplantation | Time to recurrencea | Concurrent disease? | Plasma exchange? | Rituximab dose | Comments |
|---|---|---|---|---|---|---|---|---|
| Hristea [8] | LRD | Pred; Tac; MMF; Basiliximab | 22 years | 2 days | None | Yes, partial response | 375 mg/m2 once weekly; two doses | Complete remission; PE and rituximab were used concomitantly |
| Gossmann [10] | Post-mortal | Pred; Tac; MMF; ATG | 48 years | 40 days | None | Yes, no response | 375 mg/m2 once weekly; two doses | Complete remission after rituximab |
| Meyer [11] | LRD | Pred; Tac; MMF; Basiliximab | 31 years | 2 months | None | Yes, preemptive, partial response | 375 mg/m2 once weekly; three doses | Partial remission after rituximab |
| El-Firjani [13] | LRD | Pred; Tac; MMF; ATG | 48 years | 1 month | None | Yes, no response | 375 mg/m2; six doses in 8 weeks | No remission after rituximab |
| Kamar [9] | Post-mortal | Pred; CsA; MMF; Basiliximab | 25 years | 1 day | None | Yes, preemptive, partial response | 375 mg/m2 once weekly; two doses | Complete remission; however, PE and rituximab were used concomitantly; A relapse was also treated with rituximab and PE, proteinuria had already decreased significantly before rituximab treatment |
| Post-mortal | Pred; CsA; MMF; Basiliximab | 46 years | 1 day | None | Yes, no response | 375 mg/m2 once weekly; four doses | No remission after rituximab | |
| Yabu [12] | Post-mortal | Pred; Tac; MMF | 41 years | 3 days | None | Yes, partial response | 1000 mg fortnightly; two doses | No remission after rituximab |
| LRD | Pred; Tac; MMF; ATG | 43 years | 2 months | None | Yes, >50% decrease in proteinuria, no remission | 375 mg/m2 once weekly; four doses | No remission after rituximab | |
| LRD | Total lymphoid irradiation; ATG; CsA; MMF; pred | 41 years | <14 days | None | Yes, no response | 375 mg/m2 once weekly; six doses | No remission after rituximab | |
| LRD | JAK3 inhibitor; MMF; Pred | 47 years | 1 month | None | Yes, partial response | 375 mg/m2 once weekly; six doses | No remission after rituximab |
aExact time to recurrence not available.
Definitions: complete remission = proteinuria <0.2 g/24 h; partial remission/response = proteinuria between 0.2 g/24 h and 2.0 g/24 h.
LRD = living related donor; ATG = anti-thymocyte globulin; Aza = azathioprine; CsA = Cyclosporine; MMF = mycophenolate mofetil; Pred = prednisone; Tac = tacrolimus; PE = plasma exchange.
Recurrent FSGS has been attributed to a thus far unidentified circulating permeability factor, and T-cells have been implicated as a source of the permeability factor [2,14,15]. The efficacy of rituximab might suggest that recurrent FSGS is dependent on a B-cell derived antibody or cytokine. However, B-cells are important for T-cell activation; thus rituximab may exert its effect by indirectly affecting T-cell function [16,17]. In our patient, the first remission was attained more than 5 months after treatment with rituximab. The time to remission is clearly longer compared to previous case reports in recurrent FSGS and minimal change disease, but similar to studies performed in patients with refractory SLE or ANCA-associated vasculitis (AAV) [4,6,8]. In these patients, especially those with SLE, attainment of a remission can take up to 8 months after treatment with rituximab [18,19]. The slower response has been attributed to different kinetics of tissue and peripheral blood B-cells [20]. Studies involving a murine model for human CD20 expression demonstrated that depletion is slower for tissue B-cells than for peripheral blood B-cells [21]. In humans with rheumathoid arthritis, treatment with rituximab also resulted in a variable degree of depletion of B-cells in bone marrow [22]. Slow depletion of tissue B-cells may thus explain the slower response in our patient.
Alternatively, the difference in response to rituximab may be associated with different types of plasma cells. It is known that some plasma cells have short life spans, whereas other plasma cells are able to live for extended periods of time, continuously secreting antibodies [23,24]. Rituximab only results in depletion of immature and mature B-cells, but not plasma cells. Thus, depending on the type of plasma cells that are involved (short- or long-lived), time to remission may differ.
Our patient relapsed before the return of circulating B-cells. This has also been described in a few patients with SLE and AAV [18]. This finding also underscores the possible role of tissue B-cells. Stimulation of T-cells by tissue B-cells that return before peripheral blood B-cells is measurable could explain the development of a relapse in the presence of undetectable CD19 positive B-cells [18].
In conclusion, rituximab appears to be beneficial in some patients with recurrent FSGS after renal transplantation. Further studies are necessary to determine which patients may benefit from rituximab and to elucidate the pathogenetic role of B-cells. Our case report shows that patients who are responsive to rituximab can be effectively treated with another course in the case of a relapse, even in the absence of CD19/20+ cells.
Acknowledgments
J.K.J.D. is supported by a grant from the Dutch Kidney Foundation (PV 02). Rituximab was supplied by Roche Netherlands B.V.
Conflict of interest statement. None declared.
References
- 1.Deegens JK, Andresdottir MB, Croockewit S, et al. Plasma exchange improves graft survival in patients with recurrent focal glomerulosclerosis after renal transplant. Transpl Int. 2004;17:151–157. doi: 10.1007/s00147-003-0679-y. [DOI] [PubMed] [Google Scholar]
- 2.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 3.Matalon A, Markowitz GS, Joseph RE, et al. Plasmapheresis treatment of recurrent FSGS in adult renal transplant recipients. Clin Nephrol. 2001;56:271–278. [PubMed] [Google Scholar]
- 4.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354:1961–1963. doi: 10.1056/NEJMc055495. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed MS, Wong CF. Rituximab and nephrotic syndrome: a new therapeutic hope? Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm683. [DOI] [PubMed] [Google Scholar]
- 6.Nozu K, Iijima K, Fujisawa M, et al. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660–1663. doi: 10.1007/s00467-005-2013-7. [DOI] [PubMed] [Google Scholar]
- 7.Marks SD, McGraw M. Does rituximab treat recurrent focal segmental glomerulosclerosis post-renal transplantation? Pediatr Nephrol. 2007;22:158–160. doi: 10.1007/s00467-006-0260-x. [DOI] [PubMed] [Google Scholar]
- 8.Hristea D, Hadaya K, Marangon N, et al. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20:102–105. doi: 10.1111/j.1432-2277.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamar N, Faguer S, Esposito L, et al. Treatment of focal segmental glomerular sclerosis with rituximab: 2 case reports. Clin Nephrol. 2007;67:250–254. doi: 10.5414/cnp67250. [DOI] [PubMed] [Google Scholar]
- 10.Gossmann J, Scheuermann EH, Porubsky S, et al. Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transpl Int. 2007;20:558–562. doi: 10.1111/j.1432-2277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 11.Meyer TN, Thaiss F, Stahl RA. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl Int. 2007;20:1066–1071. doi: 10.1111/j.1432-2277.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 12.Yabu JM, Ho B, Scandling JD, et al. Rituximab failed to improve nephrotic syndrome in renal transplant patients with recurrent focal segmental glomerulosclerosis. Am J Transplant. 2007 doi: 10.1111/j.1600-6143.2007.02021.x. [DOI] [PubMed] [Google Scholar]
- 13.El-Firjani A, Hoar S, Karpinski J, et al. Post-transplant focal segmental glomerulosclerosis refractory to plasmapheresis and rituximab therapy. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm616. [DOI] [PubMed] [Google Scholar]
- 14.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 15.Koyama A, Fujisaki M, Kobayashi M, et al. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 16.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanz I, Anolik J. Reconstitution of the adult B cell repertoire after treatment with rituximab. Arthritis Res Ther. 2005;7:175–176. doi: 10.1186/ar1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KG, Jones RB, Burns SM, et al. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 19.Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 20.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66:1933–1948. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Gong Q, Ou Q, Ye S, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 22.Leandro MJ, Cooper N, Cambridge G, et al. Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatology (Oxford) 2007;46:29–36. doi: 10.1093/rheumatology/kel148. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer BF, Moser K, Hauser AE, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manz RA, Radbruch A. Plasma cells for a lifetime? Eur J Immunol. 2002;32:923–927. doi: 10.1002/1521-4141(200204)32:4<923::AID-IMMU923>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]