Introduction
Acute tubulointerstitial nephritis is demonstrated in 2–3% of all native renal biopsies, increasing to 10–15% if the biopsy is performed in the setting of acute renal failure [1]. It is most commonly related to medication or infection [1,2]. An increasingly recognized entity is tubulointerstitial nephritis with uveitis (TINU) syndrome. We outline the clinical course of a patient with TINU syndrome and review the changes in epidemiology, diagnosis and management.
Case
A 39-year-old man presented with a 6-month history of lethargy and generalized arthralgia. He also complained of urinary frequency and nocturia. His renal function was impaired with a creatinine of 257 μmol/L (estimated glomerular filtration rate, eGFR, 28 mls/min). He had received topical treatment for sudden-onset bilateral anterior uveitis 6 weeks previously but had no other significant past medical history. He took no regular medications and there had been no use of antibiotics or non-steroidal agents in the previous 12 months. The physical examination was unremarkable.
There were red cells but no white cells or casts on urine microscopy. There was normoglycaemic glycosuria. Urine culture was sterile. The protein/creatinine ratio was 66 mg/mmol (normal 0–20 mg/mmol). The white cell count, peripheral eosinophil count and complement levels were normal. Autoimmune and vasculitic screens were negative. The C-reactive protein (CRP) was 12 mg/L. The kidneys were anatomically normal on ultrasound examination. Renal histology was consistent with acute tubulointerstitial nephritis (Figure 1).
Fig. 1.
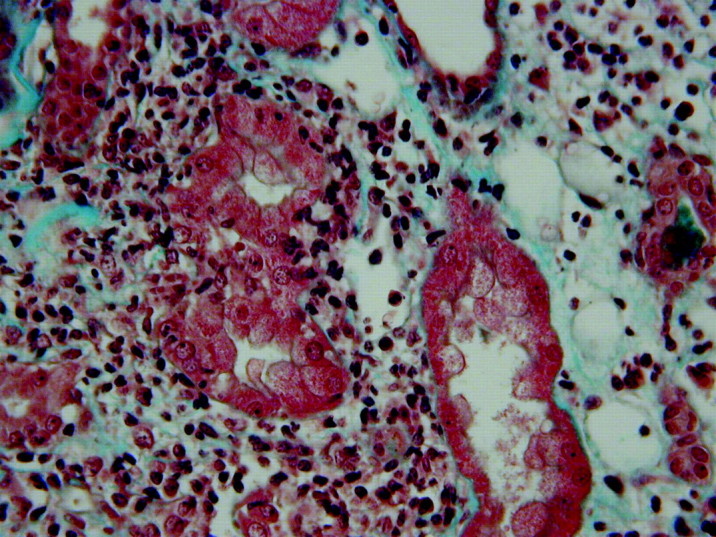
Renal biopsy specimen showing widespread interstitial inflammation with a predominant mononuclear cell infiltrate (haematoxylin and eosin stain).
A reducing course of oral prednisolone (0.8 mg/kg/day) was commenced and tapered to zero over 18 weeks. Two weeks later there was a relapse of bilateral anterior uveitis requiring reinstitution of oral prednisolone at 10 mg daily. His urinalysis was bland and creatinine level stable at this stage (Figure 2). The steroids were slowly withdrawn over the following 6 months. Currently, at 13 months after the diagnosis of TINU syndrome, he remains well without steroid therapy with no further ocular problems and a creatinine of 128 μmol/L (eGFR 56 mls/min).
Fig. 2.
Improvement in renal function from time of diagnosis. Oral corticosteroid dose was tapered from 60 mg daily to zero by 44 weeks.
Discussion
Epidemiology
TINU syndrome was first documented in 1975 [3]. Approximately 200 cases have since been reported worldwide, primarily in the ophthalmic and paediatric medical literature. We reviewed all the renal pathology reports from 1986 to 2006 in a single region of a population of 1.7 million (Northern Ireland); all of these were analysed at a single central pathology laboratory. Details regarding the ophthalmic diagnosis and management were obtained from written correspondence from the patients’ hospital ophthalmologist. In total, six cases of TINU were identified, with an approximate prevalence of 3.5 cases per million population (pmp), and incidence of 0.2 cases pmp per year. The first case was identified in 1988; all subsequent cases were reported after 2000 with the last two in 2006. This may indicate an increase in the prevalence of TINU syndrome. Table 1 summarizes the cases identified in our region.
Table 1.
Summary of cases of TINU identified in our region
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Year of diagnosis | 1988 | 2000 | 2003 | 2004 | 2006 | 2006 | |
| Characteristics | |||||||
| Age (years) | 46 | 53 | 56 | 46 | 39 | 40 | |
| Gender | M | F | F | F | M | F | |
| Investigations at the time of renal biopsy | |||||||
| Urinalysis | Protein | N/A | 2+ | N/A | 2+ | 3+ | 2+ |
| Blood | − | 4+ | 2+ | 2+ | |||
| Glucose | 1+ | − | 3+ | − | |||
| Leukocytes | − | 2+ | − | − | |||
| Creatinine (μmol/L) | 200 | 491 | 215 | 371 | 257 | 459 | |
| Protein (g/24 h) | 1.7 | 0.97 | 2.42 | 0.84 | 0.66 | 0.09 | |
| Haemoglobin (g/dL) | N/A | 8.3 | 8.3 | 10.5 | 14.6 | 9.7 | |
| White cell count (×109/L) | N/A | 4.0 | 7.7 | 10.0 | 11.8 | 9.0 | |
| CRP (mg/L) | N/A | <6 | 91 | 4 | 12 | 95 | |
| Treatment | |||||||
| Oral steroid therapy | Yes | Yes | Yes | Yes | Yes | Yes | |
| Duration (weeks) | N/A | 18 | 17a | 23 | 44 | 15 | |
| Follow-up | |||||||
| Time (months) | N/A | 85 | 52 | 37 | 13 | 12 | |
| Creatinine (μmol/L) | N/A | 85 | 104 | 73 | 128 | 92 | |
N/A: information not available.
aPatient also received a reducing dose of mycophenolate mofetil which was withdrawn over 28 months.
There is a female predominance (3:1 ratio), but the proportion of male patients appears to be increasing (18% prior to 1990, 34% since 1990) [2,4]. TINU syndrome has been reported in all age groups, though the median age of onset was 15 years in one report [4,5]. In our series 4/6 of the cases were female and the average age at diagnosis was 47 years (range 39–56 years). This is older than previously reported in the medical literature [4].
Pathophysiology
The pathogenic mechanisms are poorly understood although abnormalities in cell-mediated immunity are likely to play an important role [6]. Prior infections with Epstein-Barr virus, herpes zoster virus and Chlamydia trachomatis have been linked in some cases [4,7,8]. Other reports have speculated that the use of drugs such as non-steroidal agents or antibiotics is important in the development of TINU syndrome [4]. Autoimmune conditions such as rheumatoid arthritis [4], sacroileitis [7] and hyperthyroidism [9] have also been implicated, but it is unclear whether these associations are coincidental or causative. None of these factors were identified in our case. Several investigators have reported associations with certain human leucocyte antigen (HLA) phenotypes and the possibility that TINU is an autoimmune process. One such study by Levinson and co-workers [10] noted that the haplotype HLA-DQA1*01/DQB1*05/DRB1*01 was identified in 13/18 patients (72.2%) with TINU and postulated that it may be important as a risk factor for the development of this condition.
Clinical features
The clinical presentation can be varied. Systemic symptoms such as weight loss, fatigue, arthralgia and fever may predominate. Renal manifestations include sterile pyuria, haematuria, sub-nephrotic proteinuria and renal insufficiency. Polyuria and nocturia have been reported in up to 8% of cases [4], and multiple tubular defects may also be present, such as normoglycaemic glucosuria as in this case. Approximately two-third of patients have a creatinine of at least 184 μmol/L at the time of presentation [4]. In our series the mean serum creatinine was 332 μmol/L (range 200–491 μmol/L) at the time of biopsy. Mild anaemia and elevated inflammatory markers can also be present [2,4].
The concurrent onset of anterior uveitis and interstitial nephritis occurs in a minority (15%) of cases. Ocular symptoms can precede (20%) or follow (65%) the renal diagnosis. In the latter scenario the average delay in developing uveitis is 3 months, but up to 14 months has been documented [2,4]. This may lead to underdiagnosis of TINU syndrome, and be responsible for cases labelled as ‘idiopathic’ acute interstitial nephritis. The incidence of TINU syndrome in our region may be greater than recorded as all cases had one episode of uveitis prior to the detection of renal disease with an average lag time of 4 weeks (range 2–8 weeks) between the ophthalmic and biopsy-proven renal diagnoses. Given the time discordance between clinical features in TINU syndrome it is imperative that practitioners in both specialties have a high degree of awareness of this condition.
No single diagnostic test is available for TINU syndrome. It remains a diagnosis of exclusion based on the presence of uveitis and findings consistent with acute tubulointerstitial nephritis in the absence of other disease entities known to cause both of these disorders. Ocular and renal disease can co-exist in Wegener's granulomatosis, systemic lupus erythematous, Sjorgren's syndrome, sarcoidosis and Behçet's disease; clinical features and serological testing should readily distinguish these conditions from TINU syndrome.
Histological evidence of acute tubulointerstitial nephritis should be sought for a definitive diagnosis of TINU syndrome. Typical biopsy findings include interstitial oedema with active interstitial inflammation mainly composed of plasma cells, lymphocytes and eosinophils. However, an invasive procedure such as renal biopsy may not be suitable for all patients and this should be judged on an individual basis. In such cases, a clinical diagnosis of tubulointerstitial nephritis together with typical uveitis features would be sufficient to make a ‘probable’ diagnosis of TINU syndrome. Mandeville and colleagues [4] wrote helpfully on this topic and suggested that the clinical diagnosis of tubulointerstitial nephritis could be made if three criteria were fulfilled: (1) abnormal renal function; (2) abnormal urinalysis with the presence of white cells, red cells or mild proteinuria and (3) history of an acute systemic illness lasting at least 2 weeks.
Krebs von den Lunge-6 (KL-6) is a human glycoprotein; serum concentrations rise in response to various respiratory pathologies and it has been used to monitor the activity of sarcoidosis [11]. More recently, Kase and colleagues [12] noted that serum KL-6 levels in patients with TINU syndrome were significantly elevated when compared with age-matched patients with uveitis from other causes (363 ± 51 U/mL versus 213 ± 10 U/mL, P < 0.001). On renal biopsy the distal tubules of the patients with TINU syndrome stained strongly with anti-KL-6 antibody suggesting that the elevated KL-6 levels reflect the underlying renal lesion [12]. However, there has not yet been a comparison of serum KL-6 levels in those with TINU syndrome versus patients with acute tubulointerstitial nephritis without uveitis. If there was a significant difference between these groups, then serum KL-6 levels may prove to be a valuable tool in the diagnosis and follow-up of patients with TINU syndrome.
Another potential diagnostic marker is β2 microglobulin. Goda and co-workers [13] noted that in 11/12 patients (91.7%) with TINU syndrome, urinary β2 microglobulin was increased with 8 of these patients having values 10 times the upper limit of normal. Although both these markers may prove useful in the future, there is, as yet, not enough data available regarding their sensitivity or specificity to support their use in clinical practice.
Management
The tubulointerstitial nephritis can resolve spontaneously [4,7,14], and dialysis therapy is not usually required. However, a reducing dose of oral prednisolone starting at 1 mg/kg/day is usually prescribed for those with progressive renal impairment [2,4,9] although the evidence to support this approach is not robust. There have been no prospective, randomized trials comparing steroid therapy with placebo, or addressing the optimum dose and duration of treatment.
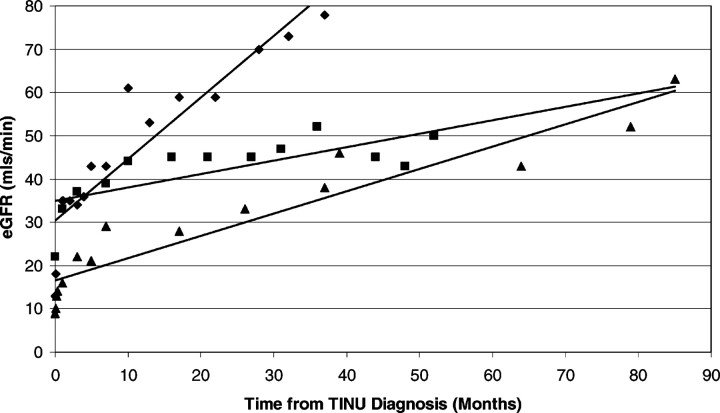
Most patients recover normal renal function although some have persistent mild renal insufficiency following the resolution of the systemic symptoms [2,4]. The course of the renal disease appears to be independent from that of the ocular condition but long-term follow-up data are unavailable in most reports. It remains uncertain from the available literature if the renal and ocular symptoms correlate in severity. All cases in our series were given a reducing dose of corticosteroid therapy. The average fall in creatinine in the first week of treatment was 141 μmol/L (range 87–223 μmol/L) in the five cases for which data were available. In the 3/6 cases with follow-up data over 2 years, the subsequent improvement in renal function was more gradual (Figure 3) and continued even after the withdrawal of steroid therapy. The average duration of therapy was 19 weeks and the mean creatinine and eGFR at the last follow-up (range 37–85 months after diagnosis) were 87 μmol/L (range 73–104 μmol/L) and 64 mls/min (range 50–78 mls/min), respectively.
Fig. 3.
Graph with superimposed trend lines representing the ongoing improvement in renal function (eGFR was calculated by the four-variable MDRD equation) in three cases of TINU (represented by three differing symbols) over a prolonged period of time. This improvement continued after withdrawal of oral corticosteroids (mean corticosteroid duration = 19 weeks; range 17–23 weeks).
The anterior uveitis of TINU is treated with topical steroids and cycloplegic agents to avoid the development of posterior synechiae. Uveitis in the setting of TINU syndrome appears to be more persistent and troublesome than the nephritis [4]. Mackensen and colleagues [15] reviewed 33 cases of TINU, 32 of whom had presented with bilateral sudden-onset uveitis. The ocular inflammation was present for a median of 7 months but persisted for over 2 years in four patients (12%). Twelve patients (36%) were treated with topical steroids alone and 21 patients (64%) received oral corticosteroid therapy (median duration of therapy 2.5 months). For severe or refractory uveitis, oral steroids or steroid-sparing agents (cyclosporine, mycophenolate mofetil, azathioprine or methotrexate) may be required [4,14].
This case series and literature review highlights some important features of TINU syndrome.
Teaching points
The incidence of TINU syndrome may be increasing and the epidemiology is possibly changing with a higher proportion of men and older persons affected.
There are potential diagnostic markers, such as serum KL-6 and urinary β2 microglobulin, which may prove beneficial in the future.
Temporal discordance can exist between the systemic, renal and ocular symptoms. TINU syndrome should therefore be considered in cases of ‘idiopathic’ acute interstitial nephritis and in those with the sudden-onset of bilateral anterior uveitis.
The renal prognosis is variable with incomplete recovery and persistent chronic kidney disease, despite steroid therapy, in some cases.
The uveitis activity sometimes requires treatment for a longer period of time than that of the nephritis.
Conflict of interest statement. None declared.
References
- 1.Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticoid steroid therapy. Nephrol Dial Transplant. 2004;19:2778–2783. doi: 10.1093/ndt/gfh485. [DOI] [PubMed] [Google Scholar]
- 2.Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8–11. doi: 10.1093/ndt/gfg464. [DOI] [PubMed] [Google Scholar]
- 3.Dobrin RS, Vernier RL, Fish AL. Acute eosinophilic interstitial nephritis and renal failure with bone marrow-lymph node granulomas and anterior uveitis. A new syndrome. Am J Med. 1975;59:325–333. doi: 10.1016/0002-9343(75)90390-3. [DOI] [PubMed] [Google Scholar]
- 4.Mandeville JTH, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 5.Sessa A, Meroni M, Battini G, et al. Acute renal failure due to idiopathic tubulo-interstitial nephritis and uveitis; ‘TINU syndrome’. Case report and review of the literature. J Nephrol. 2000;13:377–380. [PubMed] [Google Scholar]
- 6.Birnbacher R, Balzar E, Aufricht C, et al. Tubulointerstitial nephritis and uveitis: an immunological disorder? Paed Nephrol. 1995;9:193–195. doi: 10.1007/BF00860744. [DOI] [PubMed] [Google Scholar]
- 7.Cigni A, Soro G, Faedda R, et al. A case of adult-onset tubulointerstitial nephritis and uveitis (‘TINU syndrome’) associated with sacroileitis and Epstein-Barr virus infection with good spontaneous outcome. Am J Kidney Dis. 2003;42:E4–E10. doi: 10.1016/s0272-6386(03)00795-9. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mihatsch MJ, Matter L, et al. Acute tubulo-interstial nephritis with uveitis (TINU syndrome) in a patient with serological evidence for Chlamydia infection. Klin Wochenschr. 1990;68:971–975. doi: 10.1007/BF01646656. [DOI] [PubMed] [Google Scholar]
- 9.Ebihara I, Hirayama K, Usui J, et al. Tubulointerstitial nephritis and uveitis syndrome associated with hyperthyroidism. Clin Exp Nephrol. 2006;10:216–221. doi: 10.1007/s10157-006-0423-x. [DOI] [PubMed] [Google Scholar]
- 10.Levinson RD, Park MS, Rikkers SM, et al. Strong associations between specific HLA-DQ and HLA-DR alleles and the tubulointerstitial nephritis and uveitis syndrome. Invest Ophthalmil Vis Sci. 2003;44:653–657. doi: 10.1167/iovs.02-0376. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi J, Kitamura S. Serum KL-6 for the evaluation of active pneumonitis in pulmonary sarcoidosis. Chest. 1996;109:1276–1282. doi: 10.1378/chest.109.5.1276. [DOI] [PubMed] [Google Scholar]
- 12.Kase S, Kitaichi N, Namba K, et al. Elevation of serum Krebs von den Lunge-6 levels in patients with tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis. 2006;48:935–941. doi: 10.1053/j.ajkd.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Goda C, Kotake S, Ichiishi A, et al. Clinical features in tubulointerstitial nephritis and uveitis (TINU) syndrome. Am J Ophthalmol. 2005;140:637–641. doi: 10.1016/j.ajo.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Takemura T, Okada M, Hino S, et al. Course and outcome of tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis. 1999;34:1016–1021. doi: 10.1016/S0272-6386(99)70006-5. [DOI] [PubMed] [Google Scholar]
- 15.Mackensen F, Smith JR, Rosenbaum JT. Enhanced recognition, treatment, and prognosis of tubulointerstital nephritis and uveitis syndrome. Ophthalmology. 2007;114:995–999. doi: 10.1016/j.ophtha.2007.01.002. [DOI] [PubMed] [Google Scholar]