Abstract
Purpose
Cervical cancer is the leading cause of cancer death among the 20 million women with HIV worldwide. We sought to determine whether HIV infection affected survival in women with invasive cervical cancer.
Patients and Methods
We enrolled sequential patients with cervical cancer in Botswana from 2010 to 2015. Standard treatment included external beam radiation and brachytherapy with concurrent cisplatin chemotherapy. The effect of HIV on survival was estimated by using an inverse probability weighted marginal Cox model.
Results
A total of 348 women with cervical cancer were enrolled, including 231 (66.4%) with HIV and 96 (27.6%) without HIV. The majority (189 [81.8%]) of women with HIV received antiretroviral therapy before cancer diagnosis. The median CD4 cell count for women with HIV was 397 (interquartile range, 264 to 555). After a median follow-up of 19.7 months, 117 (50.7%) women with HIV and 40 (41.7%) without HIV died. One death was attributed to HIV and the remaining to cancer. Three-year survival for the women with HIV was 35% (95% CI, 27% to 44%) and 48% (95% CI, 35% to 60%) for those without HIV. In an adjusted analysis, HIV infection significantly increased the risk for death among all women (hazard ratio, 1.95; 95% CI, 1.20 to 3.17) and in the subset that received guideline-concordant curative treatment (hazard ratio, 2.63; 95% CI, 1.05 to 6.55). The adverse effect of HIV on survival was greater for women with a more-limited stage cancer (P = .035), those treated with curative intent (P = .003), and those with a lower CD4 cell count (P = .036). Advanced stage and poor treatment completion contributed to high mortality overall.
Conclusion
In the context of good access to and use of antiretroviral treatment in Botswana, HIV infection significantly decreases cervical cancer survival.
INTRODUCTION
Cervical cancer is the most common cause of cancer death among African women,1 and the HIV epidemic intensifies this burden. Cervical cancer incidence is sixfold greater among women with HIV infection than the general population.2 Although antiretroviral therapy (ART) for HIV may reduce the frequency and duration of human papillomavirus (HPV) infections that cause cervical cancer,3 cervical cancer incidence has not decreased since expansion of treatment for HIV.4 With declining infection-related mortality, cervical cancer has become a leading cause of death for the 16 million women with HIV around the globe.5
The impact of HIV on risk of cervical cancer is well described, but the effect of HIV on survival from cervical cancer is poorly understood. In retrospective studies of anal cancer, which is also linked to HPV, outcomes of individuals with and without HIV infection seem to be similar in the context of ART.6-8 However, observations have suggested that outcomes for cervical cancer may be poorer for women with HIV. Two registry-based studies9,10 have suggested possible decreased cervical cancer survival associated with HIV, but trends did not achieve statistical significance, and designs had limited control of possible biases. Treatment of cervical precancers in women with HIV infection is associated with increased relapse or residual disease11,12 compared with women without HIV.13 Treatment of locally advanced cervical cancer requires a combination of external beam radiation therapy (EBRT) and brachytherapy and concurrent chemotherapy to maximize survival.14 In South Africa, women with HIV experience more frequent interruptions of this therapy and lower rates of treatment completion15 and receive concurrent chemotherapy less often.16
With an adult HIV prevalence peaking at 27%, Botswana had the earliest nationwide HIV treatment program in Africa, and its program has achieved HIV treatment coverage and virologic suppression rates higher than even the most successful high-income countries.17 Despite remarkable corresponding declines in HIV-associated mortality in Botswana,18 the incidence of cervical cancer remains among the highest in the world (36.6 per 100,000), and nearly two thirds of cases arise in women with HIV.4 To inform whether novel treatment approaches may be warranted for HIV-associated cervical cancer, we sought to estimate the effect of HIV on survival through a prospective cohort of women with cervical cancer and free access to ART and chemoradiation therapy.
PATIENTS AND METHODS
Study Participants
Women with histologically confirmed invasive cervical cancer treated at Princess Marina Hospital (October 2010 to July 2015), Gaborone Private Hospital (November 2012 to July 2015), and Nyangabgwe Referral Hospital (January 2015 to July 2015) in Botswana were approached for enrollment. Primary therapy for cervical cancer was provided at Gaborone Private Hospital through a public-private partnership with the government of Botswana, which provided the full cost of treatment. Women too ill or unwilling to consent, younger than 18 years, and non-Botswana citizens were excluded. Participants were interviewed and HIV and oncology records abstracted. Cancers were staged on the basis of clinical examination, chest x-ray, and abdominal ultrasound by using the system of the International Federation of Gynecology and Obstetrics. Patients without evidence of HIV infection or a recent test were tested for HIV unless they declined.
After enrollment, participants were contacted every 3 months. Those unable to be contacted by phone were visited in their homes or contacted through the village Kgotla (office of the village chief) or their local clinic. In the event of participant death, families and/or health workers were questioned about the circumstances surrounding the death and causes reported on the death certificate. Cancer treatment details were obtained through review of the electronic radiation treatment planning system (MOSAIQ; Elekta, Stockholm, Sweden) and paper records.
Participants provided written informed consent. The study was reviewed and approved by the ethical review committees of the Harvard T.H. Chan School of Public Health and the Botswana Ministry of Health.
Treatment
Standard treatment of cervical cancer did not vary during the study period and included EBRT of 45 to 50 Gy delivered to the pelvis in 20 to 25 fractions from a single linear accelerator (Elekta Precise). Concurrent cisplatin (35 mg/m2) was given weekly for four to five cycles during EBRT unless the estimated glomerular filtration rate was < 60 mL/min/1.73 m2 or poor performance status or hydronephrosis was present. After completion of EBRT, an additional 14 to 26 Gy was delivered through high-dose-rate brachytherapy (Elekta) in two to four fractions of 5 to 7 Gy per fraction. Before brachytherapy was available in Botswana (January 2012), patients were referred to South Africa for brachytherapy. Patients with poor performance status or stage IV disease received palliative radiation, generally 30 to 50 Gy of EBRT (eg, 10 fractions of 3 Gy to 25 fractions of 2 Gy). Concurrent cisplatin was delayed for women with HIV who had not yet started ART; otherwise, the standard treatment approach did not differ between participants with and without HIV infection or by CD4 cell count.
Toxicities during treatment were noted in the clinical record, and treatment response was assessed by pelvic examination. Follow-up visits are not covered by the public-private partnership, so patients are followed after treatment in their local clinics. Toxicities developing after completion of treatment were not systematically captured.
HIV therapy was provided without cost at government clinics nationwide. During the study period, the CD4 count threshold to initiate ART increased from 250 to 350 cells/μL. Cervical cancer, a World Health Organization HIV stage IV condition, was an absolute indication to start ART irrespective of CD4 count throughout the study period. Standard first-line therapy for HIV was coformulated tenofovir, emtricitabine, and efavirenz. Despite possible additive nephrotoxicity, tenofovir was continued in patients who also received cisplatin.
Analytic Methods
Participants not known to have HIV infection and with negative test results within 1 year of enrollment (or anytime after enrollment) were considered not to have HIV; otherwise, they were considered to have unknown HIV status. Patients were categorized by treatment intent—curative or palliative—as recorded by the treating oncologist. For analytic purposes, women treated with curative intent were considered to have completed the recommended radiation therapy if they received both EBRT and brachytherapy and achieved a combined dose of > 79 Gy in 2 Gy per fraction radiobiologic equivalence (EQD2)19 as recommended by the American Brachytherapy Society.20 Those who received less but within the range for international practice (71.2 to 79 Gy)21 were considered to have received the minimally adequate dose. Those who received < 71.2 Gy were considered to have received an inadequate dose. Participants treated with the recommended radiation therapy and at least one dose of concurrent cisplatin were considered to have received guideline-concordant therapy. Women with unknown HIV status or with < 6 months follow-up were excluded.
The primary analytic objective was to evaluate the effect of HIV infection on survival in women with cervical cancer irrespective of cancer stage, age, and other possible confounding factors. We used inverse probability weighting to develop a Cox marginal structural model22 to simulate a theoretical trial where chronic HIV infection was randomly assigned in women with cervical cancer.23 Anticipating strong associations between HIV status and age, cancer stage, and access to treatment (analogous to confounding by indication), this framework enabled adjustment for a robust set of confounders in the context of a limited number of events, avoided assumptions of relationships among potential confounders, and permitted the modeling of HIV infection and subsequent cervical cancer treatment separately. Baseline factors associated (P < .1) with HIV infection or survival in univariable logistic and Cox models stratified by HIV status were retained in the final model to determine model weights. The final logistic model that produced inverse probability weights included dichotomized age, use of traditional healers, ordinal cancer stage, education beyond primary school, presence of household electricity, marital status, dichotomized income, and employment status. The model included an updated adjustment for radiation dose received. Significance testing was performed by using robust variance estimators. Sensitivity analyses on the basis of standard multivariable and propensity score-adjusted Cox models were performed. The Appendix (online only) provides more details on the analytic methods used in this study.
Analyses were performed after occurrence of 110 deaths to achieve an estimated 80% power to detect a 70% increased risk of death among women with HIV. Follow-up observations through March 2016 were included in the analysis. Analyses were conducted with SAS 9.3 software (SAS Institute, Cary, NC). All tests were two-tailed, and P < .05 was considered statistically significant.
RESULTS
Study Participants
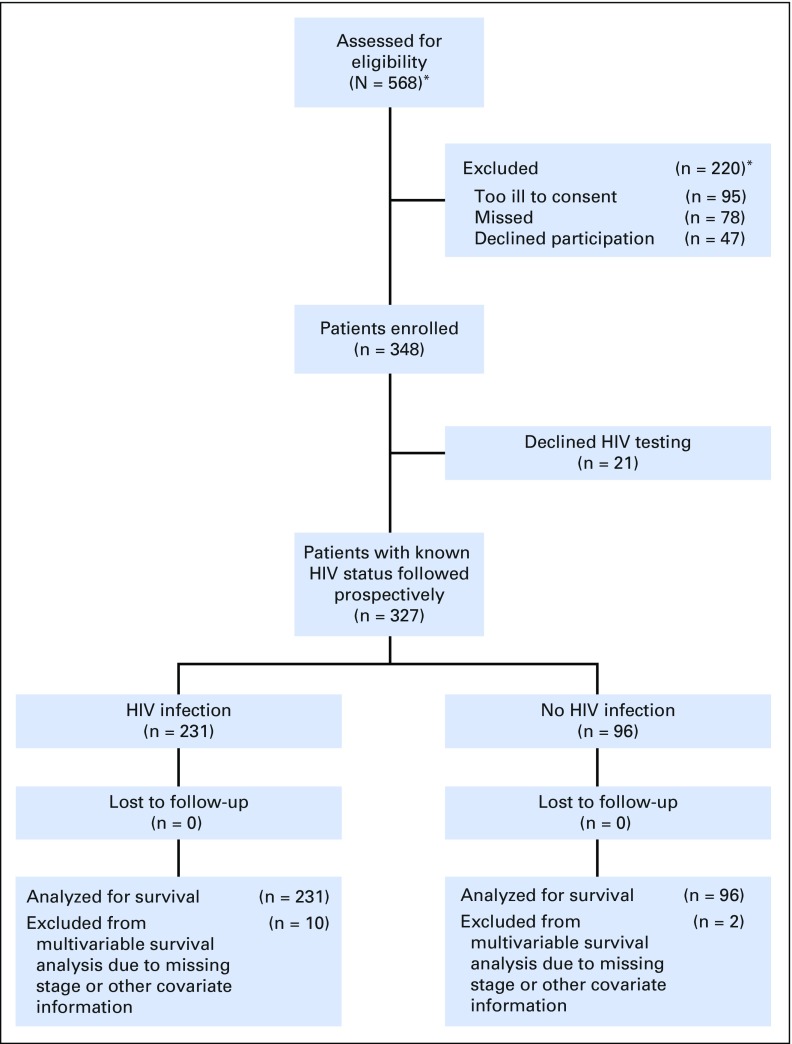
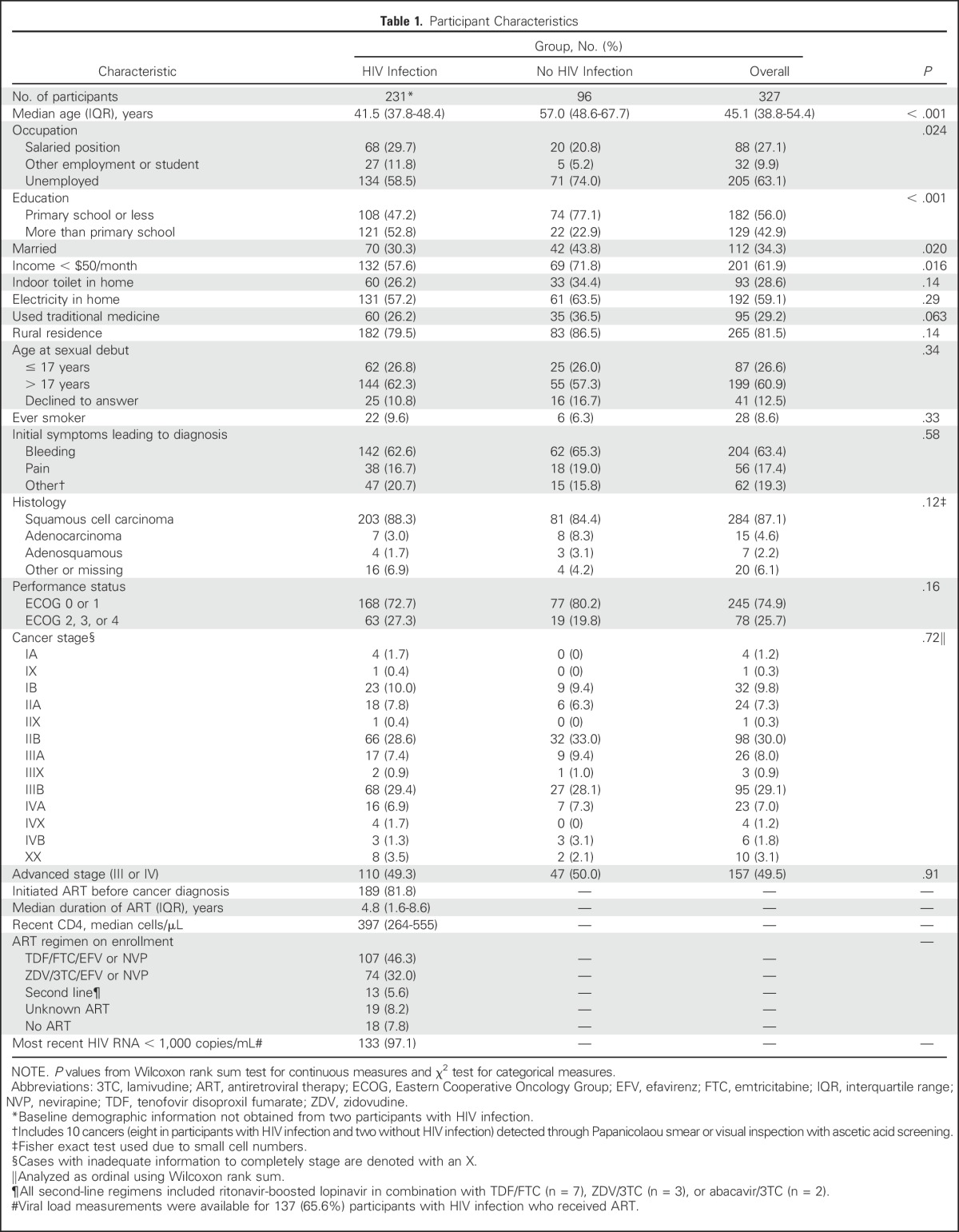
A total of 348 women with a new diagnosis of invasive cervical cancer were enrolled (Fig 1), including 231 (66.4%) with HIV infection, 96 (27.6%) without HIV infection, and 21 (6.0%) with unknown HIV status. Analyses were restricted to 327 with known HIV status. The majority (81.8%) of participants with HIV had started ART before the cervical cancer diagnosis and had received ART for several years (median, 4.8 years; interquartile range [IQR], 1.6 to 8.6 years). Cervical cancer was the initial ART-qualifying condition for 14 (6.1%) participants. Current advanced immunosuppression was uncommon (median CD4 count, 397 cells/μL; IQR, 264 to 554 cells/μL), and only 24 (10.4%) participants had a CD4 count < 200 cells/μL.
Fig 1.
Enrollment and retention. (*)Enrollment registers of the Botswana Prospective Cancer Cohort include all cancers and do not differentiate by cancer site. The number of eligible women with cervical cancer and number excluded were estimated from percentages of the full cohort.
Nearly all (96.9%) participants had symptomatic cancer, with 10 (3.1%) cancers detected through screening and 80.8% with parametrial involvement (stage IIB or greater) at the time of diagnosis. No differences were detected in cancer stage at presentation by HIV status (P = .72). Participants with HIV were substantially younger than those without HIV (median, 42 years [IQR, 37 to 48 years] and 57 years [IQR 48 to 68 years], respectively). Reflective of their younger age and the ongoing economic development in Botswana, participants with HIV infection had significantly greater access to education and higher measures of wealth than those without HIV (Table 1).
Table 1.
Participant Characteristics
Follow-Up of Study Participants
During 5,677 person-months of follow-up (median, 19.7 months; minimum, 6.6; maximum, 64.3 months among survivors), 157 (48.0%) participants died. No participants withdrew from the study or were lost to follow-up for > 6 months, for an overall retention of vital status of 100%.
Primary Outcome
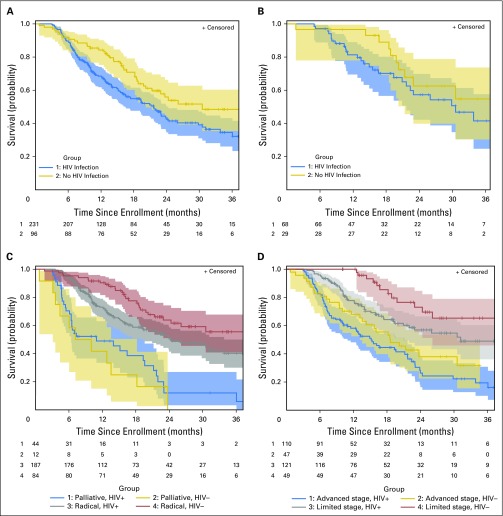
One-hundred seventeen (50.7%) participants with HIV infection and 40 (41.7%) without HIV infection died. The majority of deaths were attributed to cancer (with HIV, 102 [97.1%]; without HIV, 38 [97.4%]; P = 1.0). However, the cause of death was not known for 12 (10.3%) participants with HIV and one (2.5%) without HIV (P = .19). The death of one woman (0.9%) was attributed to HIV infection by treating clinicians. In unadjusted analysis, participants with HIV had shorter overall survival (median, 21.7 months; 95% CI, 16 to 24 months) than those without HIV (median, 30.5 months; 95% CI, 20 months to not estimable; P = .020; Fig 2).
Fig 2.
Kaplan-Meier estimated survival by HIV status (A) overall, (B) among participants who received guideline-concordant curative treatment, (C) by treatment intent, and (D) by stage. Number at risk is shown under the curves, and shaded areas indicate 95% confidence bands. Survival significantly differed by HIV status in these univariable analyses among all participants (P = .022), among those treated with radical intent (P = .019), and among those with limited-stage cancer (stage IA to IIB; P = .036). HIV+, with HIV infection; HIV–, without HIV infection.
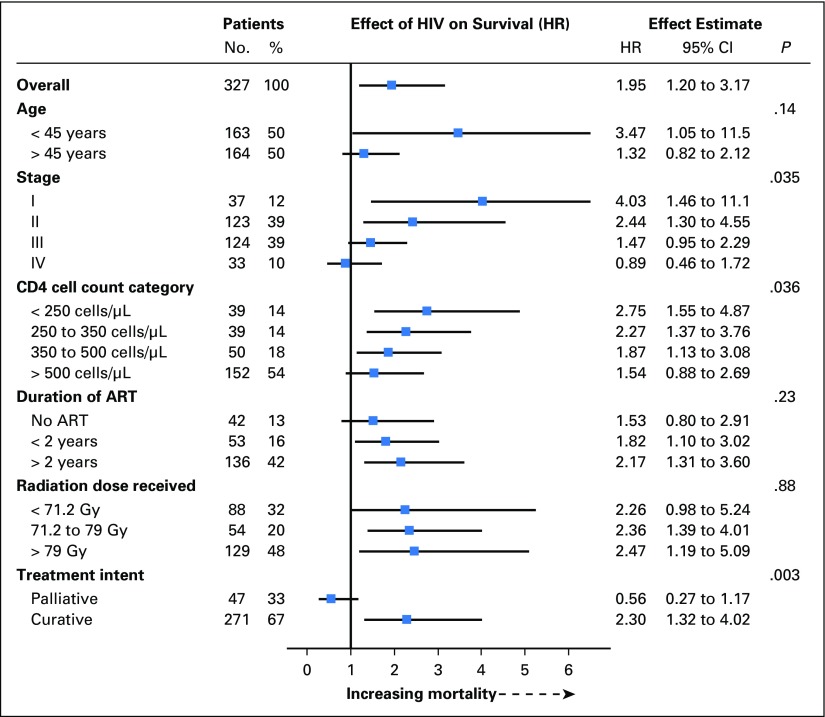
In the primary adjusted model, HIV infection nearly doubled the risk of death (hazard ratio [HR] 1.95; 95% CI, 1.20 to 3.17; P = .007). Analysis restricted to participants who received curative intent therapy resulted in similar findings (HR, 2.35; 95% CI, 1.36 to 4.06; P = .002). Similarly, analysis restricted to participants with HIV who received guideline-concordant curative intent treatment showed higher mortality (HR, 2.63; 95% CI, 1.05 to 6.55; P = .037). The effect of HIV differed significantly (P = .035 for interaction) by cancer stage, with a much greater adverse effect of HIV infection for participants with more localized disease (Fig 3). The effect of HIV was significantly attenuated by increasing CD4 cell count category (P = .036 for interaction), but the adverse effect of HIV was apparent even in strata of near-normal CD4 cell counts. Although the survival curves suggest a possibility that the adverse effect of HIV may decline with follow-up time, no significant interaction was observed with time detected (P = .33). A similar but smaller estimated effect of HIV was obtained in sensitivity analyses on the basis of multivariable and propensity score–adjusted Cox models that used the same covariates as the primary inverse probability weighted model (HR, 1.57 [95% CI, 1.03 to 2.41; P = .037] and 1.54 [95% CI, 1.01 to 2.35; P = .043], respectively).
Fig 3.
Effect of HIV on overall survival within subgroups. The P value is from the statistic for testing the interaction between HIV status and the subgroup variable. Only participants treated with curative intent are included in the comparison of effect of HIV by radiation dose received. ART, antiretroviral therapy; HR, hazard ratio.
Treatment Delivered and Response
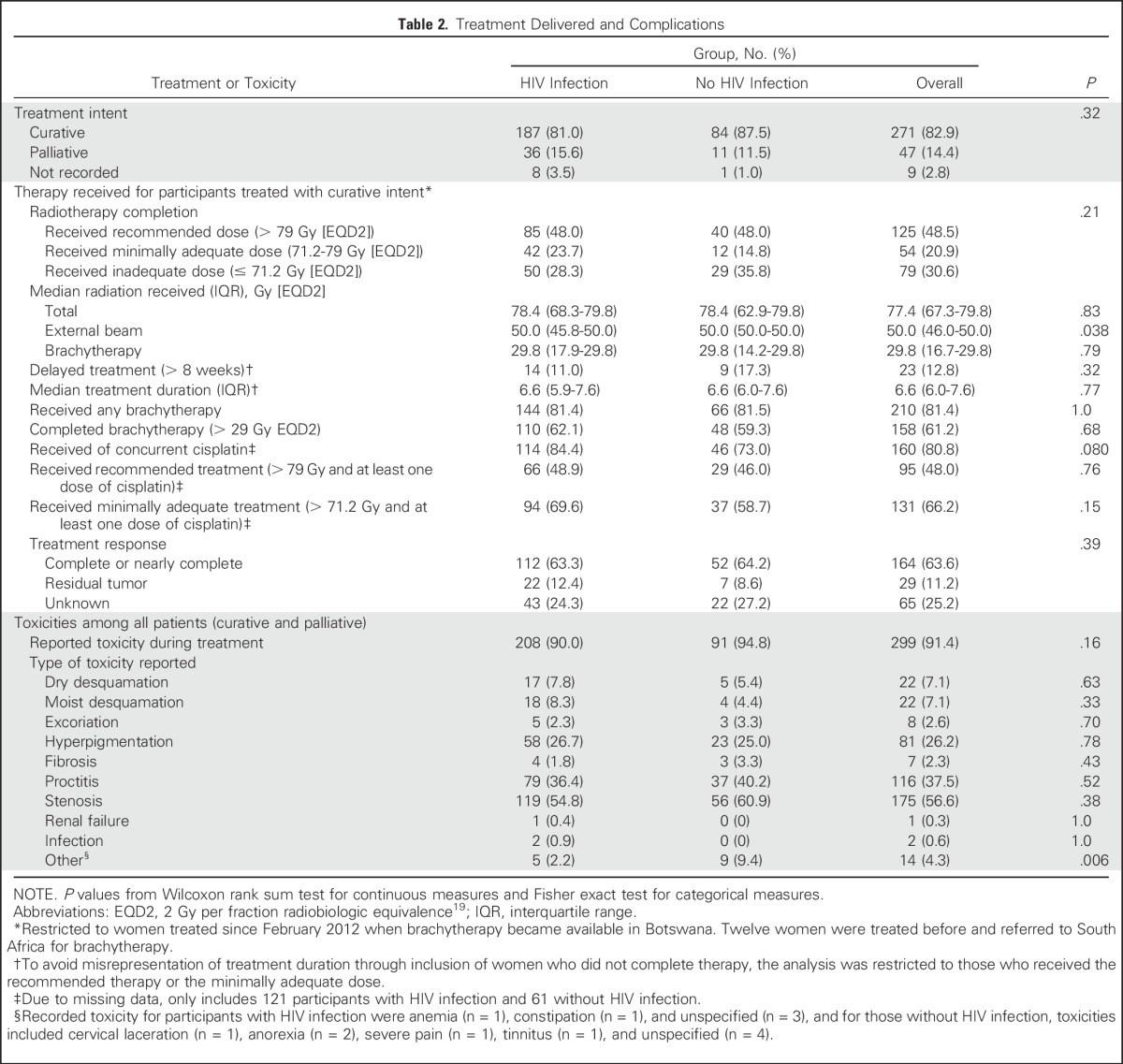
The majority of participants (271 [82.9%]) were candidates for potentially curative therapy, as determined by the treating oncologist, and 47 (14.4%) were treated with palliative intent. No significant differences (P = .32) were found in the proportion eligible for definitive therapy by HIV status. Among those treated with curative intent, 85 (48.0%) participants with HIV infection and 40 (48.0%) without HIV infection completed the recommended radiation therapy (> 79 Gy [EQD2]). A sizable minority received an inadequate radiation dose (< 71.2 Gy [EQD2]; 50 [28.3%] v 29 [35.8%] for participants with v without HIV infection, respectively). Failure to achieve the target dose was the result of inadequately received fractions of brachytherapy (62.2%), EBRT (26.3%), or both (27.8%). No significant differences in radiation treatment completion, dose received, or treatment duration by HIV status were found.
Information on chemotherapy received was available for 198 (76.7%) participants treated with curative intent. A total of 114 (84.4%) participants with HIV infection and 46 (73.0%) without HIV infection received at least one cycle of concurrent cisplatin (P = .080). Treatment response was documented for 193 (74.8%) participants treated with curative intent. Among these participants, complete or nearly complete tumor response was seen in 115 (83.9%) with HIV and 54 (88.5%) without HIV (P = .52).
Complications
Participants with and without HIV infection commonly experienced cervical stenosis, proctitis, and hyperpigmentation during cervical cancer treatment (Table 2). No significant differences in incidence of any complication or frequency of specific complications by HIV status were found. Because participants received subsequent follow-up care at local facilities, we were unable to reliably ascertain complications that occurred after treatment completion.
Table 2.
Treatment Delivered and Complications
DISCUSSION
In this prospective study of women with cervical cancer in Botswana, HIV infection nearly doubled the risk of death. Excess risk for women with HIV infection was greater for those with earlier stage cancer, although survival was poorer for all with HIV infection except those with stage IV cancer. With the exception of one death attributed to HIV, all deaths with available information were attributed to cancer. Treatment completion was a challenge for all participants, with nearly one third of those treated with curative intent receiving less radiation than international norms.21
To our knowledge, this study is the first to prospectively compare cervical cancer outcomes by HIV status. Prior clinical cohort studies in anal carcinoma,6-8 diffuse large B-cell lymphoma,24 and hepatocellular carcinoma25 have demonstrated similar survival between patients with and without HIV infection and cancer in the modern ART era. However, potentially related to increased sample size, population-based studies that used US cancer registries or health care systems have documented 1.2- to threefold decreased survival for individuals with HIV for several cancers,26-28 including an estimated 1.4-fold increased risk of death as a result of cervical cancer in contemporaneous patients.10 Poorer survival observed in population studies may be related to confounding due to decreased access to treatment and other factors.29,30 The current study based on prospective observation and improved adjustment for confounding factors and treatment received adds to the existing evidence that suggests an elevated risk due to concurrent HIV infection and extends this evidence to the African setting where the bulk of HIV-associated cancers occur.
HIV is believed to increase incident cervical cancer primarily through an increased risk of developing the initial precancerous lesion31 through impaired clearance of high-risk HPV infections32-36 and requisite infection of cervical basal cells.37 However, these mechanisms would not be expected to affect survival after the cancer develops. Regression of intraepithelial lesions seems to be a process mediated by tissue CD4+ and CD8+ lymphocytes, which lead to the killing of infected cells.38,39 We speculate that immune responses from tissue lymphocytes also contribute to the clearance of residual cancerous cells after treatment. Mucosal CD4+ cells are decimated early in HIV infection and do not recover substantially with ART, despite an increased circulating CD4 cell count.40,41 The residual deficiency in cellular immunity could contribute to poorer responses to cervical cancer therapy in women with HIV infection. The impact may be larger for those with more localized and more curable cancers, which would account for the striking modification of the effect of HIV by cancer stage. Further investigation into the role of cellular immunity to maintain cervical cancer remission could lead to improvements in cervical cancer survival for women with and without HIV.
Alternatively, ART could affect response to radiation as some infrequently used protease inhibitors potently sensitize cells to radiation.42-44 In vitro results have suggested that the commonly used combination of tenofovir, emtricitabine, and efavirenz sensitizes tumors to EBRT (< 4 Gy per fraction) but protect tumors from brachytherapy (≥ 4 Gy per fraction).45 However, early responses to treatment and toxicity were similar between groups, which suggests that these effects are not a major contributor to the observed survival difference.
This study is strengthened by prospective data collection from the largest cohort of HIV-associated cervical cancers to our knowledge and complete vital status ascertainment. However, the findings should be considered in the context of study limitations. Fewer than half of the participants received guideline-concordant treatment, and although many came close to this target and there were no differences by HIV status, receipt of a suboptimal dose may explain poorer-than-expected survival overall. Further work is needed to better understand barriers to treatment completion. Due to limited documentation, we are unable to determine with confidence that access to and tolerability of cisplatin was not affected by HIV status or to determine the proportion of participants who received guideline-concordant therapy. Further study could determine whether patients with HIV infection receive fewer doses of cisplatin and whether this could partially explain the adverse impact of HIV on survival.46 Staging evaluation did not include cross-sectional imaging, so analyses did not include tumor volume or nodal involvement. Finally, information about relapses and cause of death was limited, which restricted analysis to overall survival. With an observed annual mortality rate of 0.9% for women with HIV infection in a Botswana cohort with similar CD4 cell counts and access to ART but without cancer,47,48 only a small portion of the observed 31.6% mortality at 1 year in the current study is expected to be due to noncancer causes.
In conclusion, even in the context of good access to and use of ART, HIV infection more than doubled the risk of death among women who received curative guideline-concordant therapy. Competing mortality from HIV-associated infections seemed to contribute minimally to the excess risk of death; rather, earlier oncologic progression among women with HIV seemed to account for the excess mortality. Challenges in retention and completion of guideline-concordant therapy were observed irrespective of HIV status and likely contributed to overall low survival. Improved treatment approaches for women with HIV-associated cervical cancer and novel retention strategies for all women are urgently needed to address the rising burden of cervical cancer in sub-Saharan Africa and other low-income regions.
Appendix
Statistical Analyses
Objective.
The primary objective was to assess the effect of HIV infection on the overall survival among women with cervical cancer irrespective of possible confounders. Secondary objectives were to assess the effect of HIV among women treated with curative intent and those treated with curative intent who received guideline-concordant treatment.
Analytic models.
An inverse probability weighted Cox marginal structural model (IPW MSM) was chosen as the primary analytic approach to simulate a theoretical trial where baseline HIV infection was randomly assigned to eliminate bias from confounders. Although less commonly used in cohort studies, we anticipated considerable confounding from age (population with HIV infection younger than that without HIV infection in Botswana), socioeconomic factors (younger citizens have benefited from brisk economic development), cancer stage (possible earlier detection in women with HIV infection due to regular care or more rapid tumor progression in women with HIV), and potential bias in treatment decisions (women with HIV infection observed to be offered less-aggressive care in other settings). The relationship between these key confounders and unmeasured factors were not known. Rather than use a standard conditional approach, we used the IPW MSM approach to break the relationship between HIV and the measured factors without needing to make assumptions of the causal structure.
Model weights were generated from the inverse probability of HIV infection as estimated by a logistic model. All baseline factors associated (P < .1) with overall survival in a univariable Cox model stratified by HIV status were included in the logistic model. To further limit confounding, all baseline factors associated with HIV infection (P < .1) were included, even if not significantly associated with survival (Table A1). To improve precision, model weights were stabilized by overall probability of HIV infection in the cohort. Where L represents all included covariates (dichotomized age, use of traditional healers, ordinal cancer stage, education beyond primary school, presence of household electricity, marital status, dichotomized income, and three-category employment status), the weights used were as follows:
The resulting model weights are summarized in Table A2.
Because women with HIV infection may not be offered the same treatment as those without HIV infection, the final MSM included adjustment for radiation dose received:
where A is HIV infection and R1 and R2 are two of three radiation dose levels.
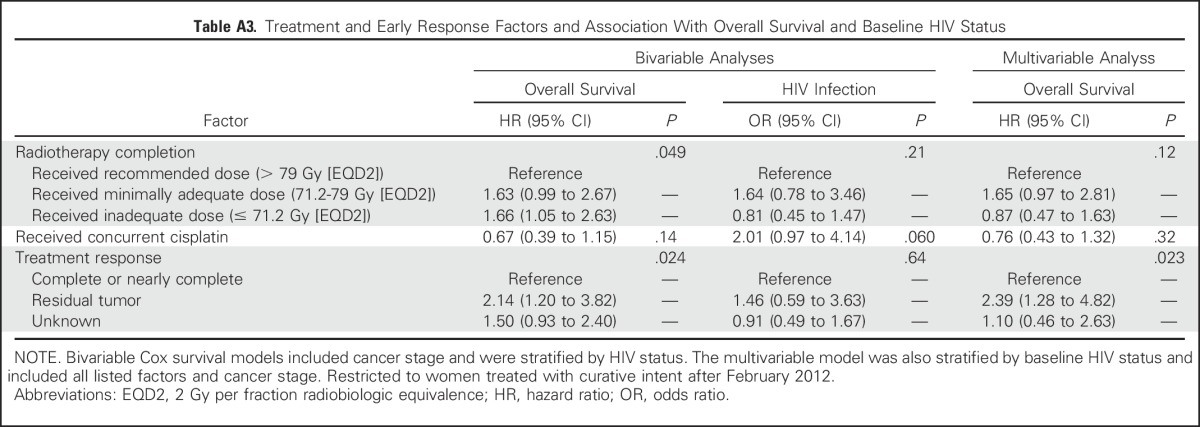
Given the marginal association between survival and receipt of cisplatin (Table A3), inclusion of this factor was explored. However, the effect estimate from this model was similar to the model restricted to those with known cisplatin status who received curative therapy (only patients treated with curative intent received standard cisplatin routinely; hazard ratio, 2.57 [95% CI, 1.38 to 4.78] and 2.61 [95% CI, 1.41 to 4.83], respectively). Final models were executed with proc genmod in SAS software (SAS Institute, Cary, NC) by using Anderson-Gill formatted data and robust variance estimators.
Effect modification, including over follow-up time, was assessed by introducing a product term for the possible effect modifier (M) to the model:
A propensity score–adjusted Cox model was used for sensitivity analysis. The probability of HIV infection based on the same covariates included in the IPW MSM was used as the propensity score. The model included adjustment for the radiation dose received separate from the propensity score (P):
A standard conditional Cox pLroportional hazards model was also used for sensitivity analysis. The same covariates included in the IPW MSM (L1 to L10) were used in the model, including radiation dose received:
Table A1.
Predictors of Overall Survival and HIV Infection
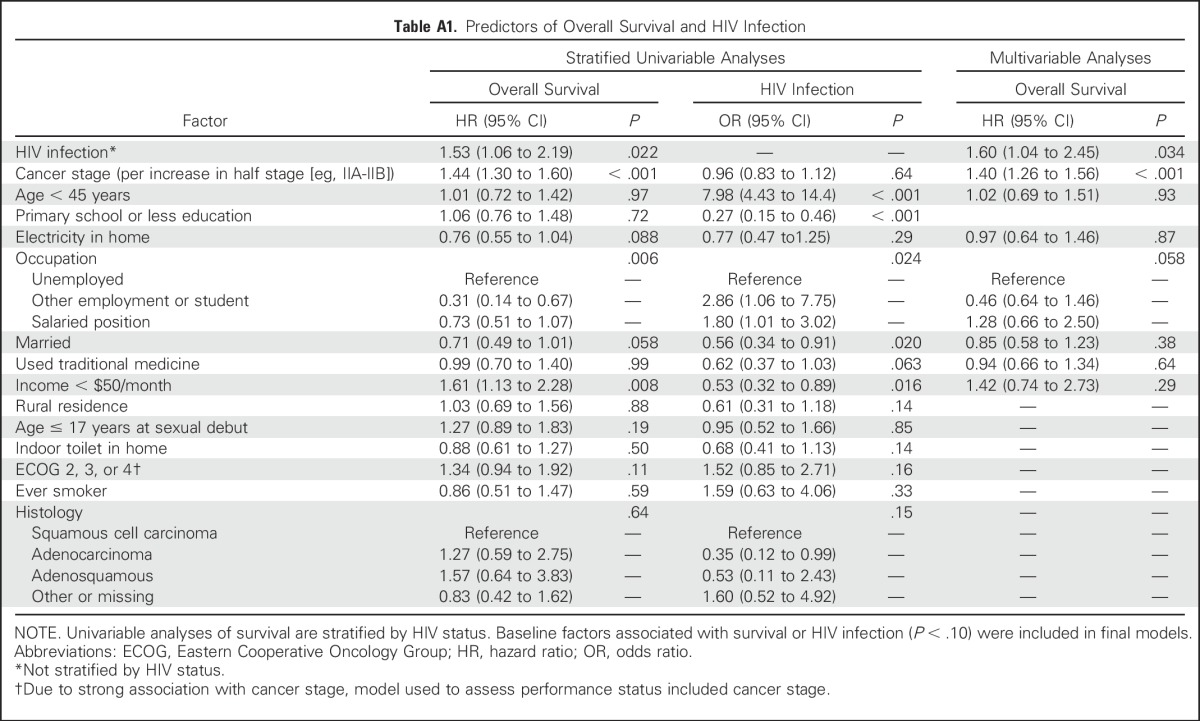
Table A2.
Inverse Probability Model Weights by HIV Status and Selected Covariates
Table A3.
Treatment and Early Response Factors and Association With Overall Survival and Baseline HIV Status
Footnotes
Supported by National Institutes of Health Grants No. P30AI060354, P30AI045008, and K23AI091434; National Cancer Institute Federal Share of program income earned by Massachusetts General Hospital on C06CA059267; and the Paul G. Allen Family Foundation (11689).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 3719
AUTHOR CONTRIBUTIONS
Conception and design: Scott Dryden-Peterson, Gita Suneja, Jason A. Efstathiou, Shahin Lockman
Financial support: Scott Dryden-Peterson, Gita Suneja, Jason A. Efstathiou
Collection and assembly of data: Scott Dryden-Peterson, Memory Bvochora-Nsingo, Rebecca Clayman, Abigail C. Mapes, Mukendi K.A. Kayembe
Data analysis and interpretation: Scott Dryden-Peterson, Gita Suneja, Jason A. Efstathiou, Surbhi Grover, Sebathu Chiyapo, Doreen Ramogola-Masire, Malebogo Kebabonye-Pusoentsi, Neo Tapela, Aida Asmelash, Heluf Medhin, Akila N. Viswanathan, Anthony H. Russell, Lilie L. Lin, Mukendi K.A. Kayembe, Mompati Mmalane, Thomas C. Randall, Bruce Chabner, Shahin Lockman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
HIV Infection and Survival Among Women With Cervical Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Scott Dryden-Peterson
Patents, Royalties, Other Intellectual Property: Royalties for article published by UpToDate
Memory Bvochora-Nsingo
Employment: Gaborone Private Hospital
Stock or Other Ownership: Gaborone Private Hospital
Speakers’ Bureau: AstraZeneca
Travel, Accommodations, Expenses: Roche
Gita Suneja
No relationship to disclose
Jason A. Efstathiou
Consulting or Advisory Role: Medivation, Astellas Pharma, Bayer Healthcare Pharmaceuticals, Genentech
Surbhi Grover
No relationship to disclose
Sebathu Chiyapo
No relationship to disclose
Doreen Ramogola-Masire
No relationship to disclose
Malebogo Kebabonye-Pusoentsi
No relationship to disclose
Rebecca Clayman
No relationship to disclose
Abigail C. Mapes
No relationship to disclose
Neo Tapela
No relationship to disclose
Aida Asmelash
No relationship to disclose
Heluf Medhin
No relationship to disclose
Akila N. Viswanathan
Research Funding: Augmenix
Anthony H. Russell
No relationship to disclose
Lilie L. Lin
No relationship to disclose
Mukendi K.A. Kayembe
No relationship to disclose
Mompati Mmalane
No relationship to disclose
Thomas C. Randall
No relationship to disclose
Bruce Chabner
No relationship to disclose
Shahin Lockman
No relationship to disclose
REFERENCES
- 1. doi: 10.1016/j.vaccine.2012.07.092. De Vuyst H, Alemany L, Lacey C, et al: The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine 31:F32-F46, 2013 (suppl 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 3.Blitz S, Baxter J, Raboud J, et al. Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis. 2013;208:454–462. doi: 10.1093/infdis/jit181. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1371/journal.pone.0135602. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al: Cancer Incidence following expansion of HIV treatment in Botswana. PLoS One 10:e0135602, 2015 [Erratum: PLoS One 10:e0138742, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joint United National Programme on HIV/AIDS (UNAIDS). The Gap Report. Geneva, Switzerland, UNAIDS, 2014. [Google Scholar]
- 6.Seo Y, Kinsella MT, Reynolds HL, et al. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–149. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Fraunholz IB, Haberl A, Klauke S, et al. Long-term effects of chemoradiotherapy for anal cancer in patients with HIV infection: Oncological outcomes, immunological status, and the clinical course of the HIV disease. Dis Colon Rectum. 2014;57:423–431. doi: 10.1097/DCR.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 8.Fraunholz I, Rabeneck D, Gerstein J, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for anal carcinoma: Are there differences between HIV-positive and HIV-negative patients in the era of highly active antiretroviral therapy. Radiother Oncol. 2011;98:99–104. doi: 10.1016/j.radonc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Coghill AE, Newcomb PA, Madeleine MM, et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS. 2013;27:2933–2942. doi: 10.1097/01.aids.0000433236.55937.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coghill AE, Shiels MS, Suneja G, et al. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vuyst H, Mugo NR, Franceschi S, et al. Residual disease and HPV persistence after cryotherapy for cervical intraepithelial neoplasia grade 2/3 in HIV-positive women in Kenya. PLoS One. 2014;9:e111037. doi: 10.1371/journal.pone.0111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimers LL, Sotardi S, Daniel D, et al. Outcomes after an excisional procedure for cervical intraepithelial neoplasia in HIV-infected women. Gynecol Oncol. 2010;119:92–97. doi: 10.1016/j.ygyno.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreimer AR, Guido RS, Solomon D, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006;15:908–914. doi: 10.1158/1055-9965.EPI-05-0845. [DOI] [PubMed] [Google Scholar]
- 14.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 15.Simonds HM, Wright JD, du Toit N, et al. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer. 2012;118:2971–2979. doi: 10.1002/cncr.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonds HM, Neugut AI, Jacobson JS. HIV status and acute hematologic toxicity among patients with cervix cancer undergoing radical chemoradiation. Int J Gynecol Cancer. 2015;25:884–890. doi: 10.1097/IGC.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: A population-based survey. Lancet HIV. 2016;3:e221–e230. doi: 10.1016/S2352-3018(16)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farahani M, Vable A, Lebelonyane R, et al. Outcomes of the Botswana national HIV/AIDS treatment programme from 2002 to 2010: A longitudinal analysis. Lancet Glob Health. 2014;2:e44–e50. doi: 10.1016/S2214-109X(13)70149-9. [DOI] [PubMed] [Google Scholar]
- 19.Nag S, Gupta N. A simple method of obtaining equivalent doses for use in HDR brachytherapy. Int J Radiat Oncol Biol Phys. 2000;46:507–513. doi: 10.1016/s0360-3016(99)00330-2. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52. doi: 10.1016/j.brachy.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viswanathan AN, Creutzberg CL, Craighead P, et al. International brachytherapy practice patterns: A survey of the Gynecologic Cancer Intergroup (GCIG) Int J Radiat Oncol Biol Phys. 2012;82:250–255. doi: 10.1016/j.ijrobp.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baptista MJ, Garcia O, Morgades M, et al. HIV-infection impact on clinical-biological features and outcome of diffuse large B-cell lymphoma treated with R-CHOP in the combination antiretroviral therapy era. AIDS. 2015;29:811–818. doi: 10.1097/QAD.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 25.Lim C, Goutte N, Gervais A, et al. Standardized care management ensures similar survival rates in HIV-positive and HIV-negative patients with hepatocellular carcinoma. J Acquir Immune Defic Syndr. 2012;61:581–587. doi: 10.1097/QAI.0b013e31826ebdc7. [DOI] [PubMed] [Google Scholar]
- 26.Sigel K, Crothers K, Dubrow R, et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer. 2013;109:1974–1980. doi: 10.1038/bjc.2013.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus JL, Chao C, Leyden WA, et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev. 2015;24:1167–1173. doi: 10.1158/1055-9965.EPI-14-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao C, Xu L, Abrams D, et al. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. AIDS. 2010;24:1765–1770. doi: 10.1097/QAD.0b013e32833a0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookfield KF, Cheung MC, Lucci J, et al. Disparities in survival among women with invasive cervical cancer: A problem of access to care. Cancer. 2009;115:166–178. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- 30.Suneja G, Shiels MS, Melville SK, et al. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS. 2013;27:459–468. doi: 10.1097/QAD.0b013e32835ad56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: Epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep. 2015;12:6–15. doi: 10.1007/s11904-014-0254-4. [DOI] [PubMed] [Google Scholar]
- 32.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 33.Denny L, Boa R, Williamson AL, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol. 2008;111:1380–1387. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- 34.Ahdieh L, Muñoz A, Vlahov D, et al. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151:1148–1157. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 35.Delmas MC, Larsen C, van Benthem B, et al. Cervical squamous intraepithelial lesions in HIV-infected women: Prevalence, incidence and regression. AIDS. 2000;14:1775–1784. doi: 10.1097/00002030-200008180-00013. [DOI] [PubMed] [Google Scholar]
- 36.Leitao MM, Jr, White P, Cracchiolo B. Cervical cancer in patients infected with the human immunodeficiency virus. Cancer. 2008;112:2683–2689. doi: 10.1002/cncr.23504. [DOI] [PubMed] [Google Scholar]
- 37.Tugizov SM, Herrera R, Chin-Hong P, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446:378–388. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 39.van der Burg SH, Palefsky JM. Human immunodeficiency virus and human papilloma virus—why HPV-induced lesions do not spontaneously resolve and why therapeutic vaccination can be successful. J Transl Med. 2009;7:108. doi: 10.1186/1479-5876-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. doi: 10.1111/j.1365-2567.2009.03077.x. Nkwanyana NN, Gumbi PP, Roberts L, et al: Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology 128:e746-e757, 2009 (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta AK, Cerniglia GJ, Mick R, et al. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 43.Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–2706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 44.Rengan R, Mick R, Pryma D, et al. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: A report of toxicities and clinical response. J Thorac Oncol. 2012;7:709–715. doi: 10.1097/JTO.0b013e3182435aa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrike K, Markus H, Thomas H, et al. NNRTI-based antiretroviral therapy may increase risk of radiation induced side effects in HIV-1-infected patients. Radiother Oncol. 2015;116:323–330. doi: 10.1016/j.radonc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. Cochrane Database Syst Rev. 2010;(1):CD008285. doi: 10.1002/14651858.CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro RL, Kitch D, Ogwu A, et al. HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS. 2013;27:1911–1920. doi: 10.1097/qad.0b013e32836158b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]