Abstract
The promoter of a gene that is selectively expressed in just a few cell types provides unique opportunities to study: (1) the pleiotropic function of a protein in two different cell types including the cell compartment specific function, and (2) the crosstalk between two cell/tissue types at the systemic level. This is not possible with a ubiquitous or a highly specific gene promoter. The adipocyte protein-2 (aP2) is one such gene. It is primarily expressed in adipocytes, but also selectively in monocytic macrophages and dendritic cells, among various immune cell types. Thus, the adipocyte protein-2 gene promoter provides an opportunity to simultaneously manipulate adipose and immune functions in a transgenic animal. Prohibitin (PHB) is a pleiotropic protein that has roles in both adipocytes and immune cells. Adipocyte specific functions of prohibitin are mediated through its mitochondrial function, whereas its immune functions are mediated in a phosphorylation-dependent manner. We capitalized on this attribute of prohibitin to explore the crosstalk between adipose and immune functions, and to discern mitochondrial and plasma membrane-associated cell signaling functions of prohibitin, by expressing wild type prohibitin (Mito-Ob) and a phospho-mutant form of prohibitin (m-Mito-Ob) from the protein-2 gene promoter, individually. Both transgenic mice develop obesity in a sex-neutral manner, but develop obesity-related metabolic dysregulation in a male sex-specific manner. Subsequently, the male Mito-Ob mice spontaneously developed type 2 diabetes and liver cancer, whereas the male m-Mito-Ob mice developed lymph node tumors or autoimmune diabetes in a context-dependent manner. This review provides a point of view on the role of prohibitin in mediating sex differences in adipose and immune functions at the systemic level. We discuss the unique attributes of prohibitin and provide a new paradigm in adipose-immune crosstalk mediated through a pleiotropic protein.
Impact statement
Prohibitin (PHB) is ubiquitously expressed and plays a role in adipocyte-immune cell cross-talk. Both male and female transgenic mice expressing wild-type PHB in adipose tissue and in macrophages are obese, but only males develop diabetes and liver cancer. When the mice express PHB mutated on tyrosine-114 in adipocytes and macrophages, both males and females are still obese, but none develops liver cancer; instead, males develop lymph node tumors. Adipocyte specific functions of PHB are mediated through its mitochondrial function, whereas its immune functions are mediated in a phosphorylation-dependent manner. Thus, PHB appears to be an important molecule linking obesity, diabetes, and cancer. In addition, this link appears to be affected by sex steroids. Therefore, targeting PHB may lead to a better understanding of the pathogenesis of obesity, diabetes and cancer.
Keywords: Adipocyte, adipose-immune crosstalk, immune cell, mitochondria, sex dimorphic, pleiotropic protein
Introduction
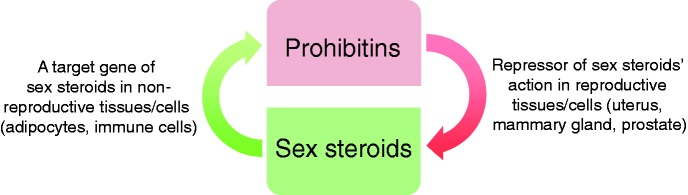
PHB belongs to the SPFH (stomatins, flotillins, and HflK/C) family of proteins, which share an evolutionarily conserved SPFH domain (also known as PHB domain).1 The members of the SPFH family of proteins are evolutionarily conserved and ubiquitously expressed.2 PHB was discovered in a search for anti-proliferative genes—hence the name ‘prohibitin’3—and localizes to the plasma membrane,4 mitochondria5 and the nucleus.6 Subsequently, a homologous protein with almost 50% sequence homology with PHB was identified as a repressor of estrogen activity (REA, also known as PHB2).7 After the discovery of PHB2, PHB gets its alternate name PHB1.1,8 The prohibitins were also identified in a separate study as B cell membrane-associated proteins (BAP)-32 and -37, based on their molecular masses.4 However, B cell specific function of BAP32 (PHB or PHB1) and BAP37 (REA or PHB2) remained largely unexplored. Early evidence of mitochondrial localization of PHB came from studies on baby hamster kidney cells (BHK1) and Saccharomyces cerevisiae.5,9 In mitochondria, PHB1 and PHB2 localize to the inner membrane, where they heterodimerize and function as a mitochondrial chaperones.10,11 In addition, prohibitins localize to the plasma membrane and the nucleus, where they have roles in membrane signaling and transcriptional co-regulation, respectively.4,7,12 In the membrane, PHB has been shown to be involved in PI3K-Akt, MAPK-ERK, and STAT3 signaling pathways in cells/tissues involved in metabolic regulation and immune functions.12–14 The membrane signaling functions of PHB involve phosphorylation and other posttranslational modifications of PHB, such as palmitoylation and glycosylation at different residues.15–18 In addition to membrane signaling, the posttranslational modification of PHB appears to be involved in the trafficking of PHB in different cellular compartments.19,20 However, it is not known whether prohibitins also heterodimerize in other cellular compartments, similar to mitochondrial prohibitins. Furthermore, the steroid hormones repressor activity of prohibitins is mainly demonstrated in reproductive tissues such as uterus,21 mammary glands,22 and cell lines derived from reproductive organs such as prostate cancer cell lines.23 To the best of our knowledge, it remains unclear whether prohibitins have similar functions in non-reproductive tissues. Conversely, it has been reported that prohibitins are also downstream target genes or mediators of sex steroid hormones in reproductive tissues24,25 and their cell line derivatives.26 Collectively, these evidences suggest that the relationship between prohibitins and sex steroid hormones is complex, and both may regulate each other’s function in a cell type, tissue specific, and context-dependent manner (Figure 1). It is also possible that this multifaceted relationship between sex steroid hormones and prohibitins is part of an auto-regulatory loop(s) to maintain tissue homeostasis. The involvement of pleiotropic PHB in the fundamental aspects of mitochondrial biology, in versatile PI3K-Akt and MAPK-ERK signaling, and as a co-regulator of steroid receptor actions creates a unique possibility for a role of PHB in the crosstalk between metabolic and immune functions in physiological and pathophysiological processes such as obesity, diabetes, and cancer. In this review, we will synthesize knowledge related to metabolic and immune functions of PHB in the light of new evidence from tissue specific transgenic mouse models of PHB. We will also provide a viewpoint on some of the early works on PHB, to get a better sense of its evolution from an anti-proliferative/tumor suppressor gene to a pleiotropic protein with roles in adipose and immune functions.
Figure 1.
The relationship between PHB and sex steroids is complex. Schematic diagram showing the relationship between PHB and sex steroids in the regulation of each other’s functions is different in reproductive and non-reproductive cells/tissues. (A color version of this figure is available in the online journal.)
PHB as an anti-proliferative/tumor suppressor gene
Since PHB was discovered as an anti-proliferative gene, the initial works were primarily focused on the mechanisms involved in its anti-proliferative function, and its potential involvement in cancer development. Early on, it was suggested that PHB has cell cycle regulatory activity based on the observation that microinjection of PHB mRNA blocked human fibroblasts from entering the S-phase, whereas antisense oligonucleotides stimulated cell cycle progression.27 Later on, the cell cycle regulatory activity of PHB mRNA was attributed to 3′-untranslated region (3′UTR) of PHB mRNA.28 Subsequently, it was reported that a single nucleotide polymorphism (C-T transition) in the 3′UTR creates a null allele (T allele) lacking the anti-proliferative activity.29 The T allele was shown to be associated with increased risk of breast cancer in North American women.30 However, inconsistency exists in the literature on the association between PHB T allele and increased risk of breast, ovarian, and gastric cancers.31–33
Continuous interest in the anti-proliferative property of PHB led to the discovery of p53 and Rb, two well-known tumor suppressors,34,35 as PHB interacting partners. The interaction of PHB with p53 was found to increase the transcriptional activity of p53, whereas co-transfection of an antisense PHB construct reduced p53-mediated transcriptional activation, in breast cancer cells.34 PHB1 also prevented cell proliferation by binding to Rb and this interaction repressed the activity of E2F transcription factors. This effect was also evident when PHB interacted with other proteins, including histone deacetylase, and the nucleosome remodeling proteins Brg-1 and Brm,35–37 and appeared to facilitate channeling of specific signaling pathways to the cell cycle machinery. It is unlikely that repressive attributes of PHB are involved in its emerging roles in adipose and immune functions, which appear to be mediated through its mitochondrial and plasma membrane-associated functions. Similar to PHB1, some of the initial works on PHB2 were also focused on cell proliferation because of its estrogen receptor co-repressor activity.21,22,38 For example, REA heterozygous mice have been reported to exhibit faster mammary ductal elongation in virgin animals, increased lobuloalveolar development during pregnancy, and delayed mammary gland involution after weaning.38 It has been suggested that a reduction or loss of REA function may cause co-activation of estrogen receptors and increase breast cancer risk in humans.38 Interestingly, such studies also led to the finding that PHB2 is a downstream target gene for estrogen and the relationship between PHB2 and estrogen is bidirectional.24,25 Subsequently, PHB1 was also found to have repressor activity and shown to repress androgen action in prostate cancer cells as well as being a target gene for androgen.23 However, it remains to be determined whether PHB1 and PHB2 function as sex steroid receptor-specific co-repressors or sex steroid receptor neutral co-repressors, and what is the relevance of this attribute of PHB in non-reproductive tissues such as adipose and immune cells.
Alterations in PHB protein levels have been reported in different types of tumors from humans and rodents models,39–42 which have been associated with both anti- and pro-tumorigenic roles in a context-dependent manner. However, the mechanisms involved in these two opposite functions of PHB remain to be clarified. It is possible that such conflicting observations are two sides of the same coin and are due to different post-translational modifications of PHB. For example, phosphorylation of PHB at tyrosine-114 or threonine-258 residues has been shown to be associated with opposite effects on PI3K-Akt signaling pathway.13,14 It is anticipated that a better understanding of the mechanisms involved in the regulation of different functions of PHB will be necessary to clarify the controversy related to its function in proliferative and anti-proliferative activities in cells. It would be interesting to know whether proliferative and anti-proliferative effects of PHB are mediated through metabolic switches involving its mitochondrial and membrane signaling functions. This is because metabolic dysregulation is a hallmark of cancer, and PHB is known to be involved in MAPK-ERK and PI3K-Akt pathways, the two predominant signaling arms of insulin/IGF receptors, which are often dysregulated in cancer cells. In breast cancer cells, overexpression of PHB leads to upregulation of MAPK-ERK pathways,12 whereas in preadipocytes/muscle cell lines, PHB has been shown to differentially affect PI3K-Akt signaling in a phosphorylation-dependent manner.43 Furthermore, PHB and m-PHB (Y114F-PHB) have differential effects on MAPK-ERK and PI3K-Akt signaling with and without insulin.43,44 In this context, it is important to note that tumors from PHB transgenic (Mito-Ob) mice showed selective upregulation of MAPK-ERK signaling,45 whereas tumors from m-PHB (m-Mito-Ob) mice had selective increase in Akt signaling.46 Taken together, these evidences would imply that the phosphorylation of PHB at tyrosine-114 has a role in switching the modulatory effect of PHB on MAPK-ERK and PI3K-Akt signaling, and potentially in integrating metabolic aspects of mitochondrial functions. These findings from Mito-Ob and m-Mito-Ob mice are consistent with the known information in the literature about the role of PHB in these two signaling cascades.12–14,47 It is possible that the context dependent effects of PHB on MAPK-ERK and PI3K-Akt signaling play an important role in switching the metabolic and mitogenic effects of insulin/IGF signaling cascade in a tissue specific manner. Collectively, these evidences point towards a role of PHB in the crosstalk between MAPK-ERK and PI3K-Akt signaling, and in switching their metabolic and mitogenic effects.
PHB in metabolic homeostasis
Early indications of a role for PHB in metabolic homeostasis came from C. elegans. Knockdown of PHB in the worm by siRNA was found to differentially affect intestinal fat content in a context-dependent manner.48 For example, siRNA-mediated knockdown of PHB in C. elegans resulted in diminished intestinal fat content, whereas an opposite effect was observed in worms with normal PHB but mutation of dauer formation-2 (daf-2, insulin/IGF signaling equivalent in C. elegans).48 Extrapolation of this finding in rodent models and humans would imply that interfering with PHB function in metabolic tissues might affect metabolic homeostasis differently depending on insulin sensitivity. This is because PHB is important for mitochondrial structure and function, and mitochondria are crucial for normal functioning of tissues involved in metabolic regulation. A similar function of PHB has been reported in cell culture systems. Overexpression of PHB in preadipocyte enhances adipocyte differentiation, whereas silencing PHB results in impairment of mitochondrial function and attenuates adipocyte differentiation.43,49 Collectively, these findings would suggest a role for PHB in the functional regulation of mitochondria during adipogenesis. The obese phenotype of transgenic mice overexpressing PHB in adipocytes (Mito-Ob) confirmed that PHB, indeed, has a role in the regulation of adipose tissue homeostasis, which appears to be primarily mediated though its mitochondrial function.50
In addition to adipocytes, PHB may contribute to metabolic homeostasis through its role in pancreatic β-cells. This is because PHB has important role in mitochondrial function, which is crucial for β-cell function, and PHB level has been reported to be upregulated in murine and human pancreatic β-cell lines as a protective response to oxidative stress.51 A drastic loss of insulin secretion and a rapid onset of type 2 diabetes in β-cell specific Phb2 knockout mice52 further confirmed a crucial role of PHB2 and potentially PHB1 in pancreatic β-cell function. PHB2 knockdown often results in a parallel down regulation of its heterodimerizing partner PHB1 and vice versa.49,52,53 It is possible that the pleiotropic attributes of PHB have a role in metabolic homeostasis mediated through its mitochondria-associated tissue specific functions such as adipogenesis and insulin secretion. The role of PHB in cell metabolism opens a new possibility that the role of PHB in cancer that has been reported in the literature may be related to its metabolic function, which may include mitochondrial and/or membrane signaling functions in a context-dependent manner.
PHB in immune cell function
In addition to the discovery of PHB as an anti-proliferative gene during liver regeneration in the rat,3 it was also identified in association with IgM receptor in murine B cells.4 Although, the precise role of PHB in IgM receptor signaling remains to be determined, a number of studies have reported a diverse array of functions for PHB in different immune cell types. This includes a role as an adaptor molecule in B cell receptor signaling,54 antigen-stimulated signaling in mast cells,15 and an important protein for mitochondrial integrity and maturation of T cells.55 Immune cell specific functions of PHB appear to require its phosphorylation at different residues.15,54,55 Furthermore, PHB has been reported to be involved in thymic involution during pregnancy in mice.56 This function of PHB also appears to require phosphorylation of PHB.56 Expression of PHB from the villin gene promoter attenuates colonic inflammation in experimental model of colitis,57 whereas its deficiency promotes inflammation and increases sensitivity to liver injury.58 Additional evidence suggesting a role of PHB in immune function came from studies demonstrating PHB as a host target protein for a number of pathogens.59–61 A lymphoproliferative phenotype, as observed in m-Mito-Ob mice expressing Y114F-PHB in macrophages and dendritic cells,46is suggestive of a potential role of membrane signaling function of PHB in this process. This is because Y114F-PHB upregulates PI3K-Akt signaling, which plays a role in activation and functional maturation of immune cells.62 However, a potential role of mitochondrial function of PHB may not be ruled out, because metabolic switches play an important role in the activation of immune cells and PI3K-Akt signaling is known to upregulate mitochondrial function.63 Thus, it is possible that the membrane signaling and mitochondrial functions of PHB are mutually exclusive, or interlinked. It is expected that a better understanding of the mechanisms involved in the role of PHB in immune cells may advance our understanding of chronic inflammation in various diseases, such as obesity-linked diabetes and cancer.
Metabolic and immune dysregulation—Common to obesity-linked diabetes and cancer
There are numerous evidences in the literature suggesting obesity is a risk factor for the development of type 2 diabetes and certain types of cancer.64–66 The development of obesity-associated type 2 diabetes has been attributed to insulin resistance and β-cell insufficiency as a consequence of adipose tissue dysregulation, including chronic low-grade adipose inflammation.67 However, the relationship between obesity and cancer remains unclear. This is because the relationship between obesity and cancer is more complex and intertwined, than the progression from obesity to type 2 diabetes (Figure 2). For example, obesity is associated with substantial metabolic and endocrine abnormalities, including alterations in sex hormone metabolism, insulin and insulin-like growth factor (IGF) signaling, and adipokines or inflammatory pathways.68,69 Each one of them is known to contribute to the development of cancer and all of them may coexist in the obese state. So, one of the major challenges is to discern the major drivers from the bystanders. Furthermore, obesity has been linked to more than ten different types of cancer.70 It is not known how the same set of abnormalities, which generally comes with obesity, leads to different types of cancer. In addition to obesity, type 2 diabetes itself is a risk factor for certain types of cancer, and in patients with type 2 diabetes, cancer may develop with or without obesity, making it more difficult to discern the main drivers from the confounders. A closer look at the evidence available in the literature suggests that evidence for a role of sex hormone metabolism and of chronic inflammation in mediating the obesity–cancer relation is strong, whereas evidence for a role of insulin and IGF signaling is moderate.70 Keeping all these points into consideration, it is highly likely that the relationship between obesity and cancer is not linear like obesity-linked type 2 diabetes, which progresses from obesity through chronic low-grade inflammation-insulin resistance–β cell insufficiency–type 2 diabetes, but much more complex and interwoven. In this context, evidence from Mito-Ob mice and m-Mito-Ob mice provides a new perspective in the development of obesity-linked cancer. Because adipocyte and immune cell (macrophages and dendritic cells) specific functions of PHB were manipulated simultaneously by expressing PHB or m-PHB from the aP2 gene promoter in these mice, this would imply that preexisting immune cell dysfunction increases the likelihood of obesity-linked cancer development. There are a number of obese mouse models available, which develop obesity-related metabolic dysregulation, including type 2 diabetes, but they do not develop obesity-linked cancer like Mito-Ob and m-Mito-Ob mice. Conversely, Mito-Ob and m-Mito-Ob mice share obesity and metabolic phenotype, but differ in immune phenotype and, as a result, develop two different, obesity-linked, types of tumor in a mutually exclusive manner.46,50 Moreover, the development of adult onset type 1 diabetes or tumor in the male m-Mito-Ob mice in a mutually exclusive and context-dependent manner46,71 further supports the notion that pre-existing immune status plays a crucial role in obesity-related diseases, including diabetes and different types of cancer. Furthermore, it is possible that the development of insulin resistance as a result of immune dysregulation may be one of the mechanisms for the development of type 2 diabetes and its associated cancer in lean subjects.
Figure 2.
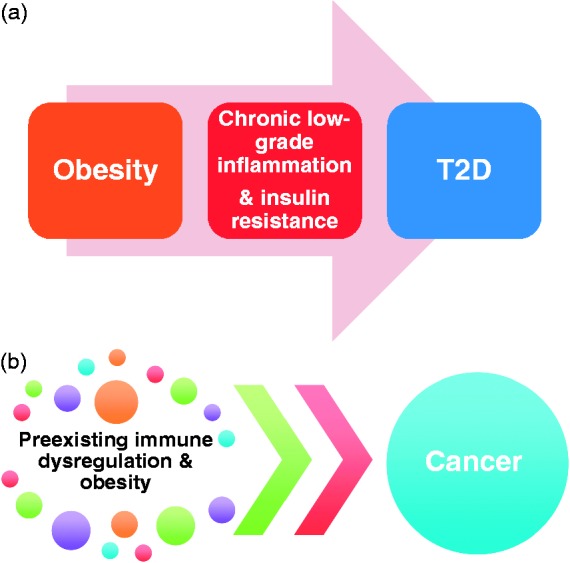
Schematic diagram showing known and potential relationship between obesity-linked type 2 diabetes and cancer. (a) The relationship between obesity and type 2 diabetes is linear, which progress from obesity-related adipose tissue abnormalities to insulin resistance, β-cell insufficiency, and eventually type 2 diabetes. (b) Preexisting immune dysregulation may play a role in obesity-linked cancer development. (A color version of this figure is available in the online journal.)
Sex differences in metabolic and immune functions
In addition to sexual maturity, puberty is a crucial stage in life in relation to adipose and immune functions.72 For example, puberty leads to a significant change in the development and distribution of adipose tissue, and sex steroid hormones have an important role in this process.73 Similarly, puberty is marked by the appearance of sex differences in immune functions, with again an important role of sex steroid hormones.74 There are numerous examples for this in the literature, from vaccination to malaria and tuberculosis infection, pre- and post-puberty.74 In general, males are more susceptible to infectious diseases and cancer, whereas females are more susceptible to autoimmune diseases, indicating sex differences in immune functions. This would imply that marked differences in adipose and immune function that appear during puberty have long lasting effects in physiology and pathophysiology. Thus, puberty appears to be a defining moment for sex differences in adipose and immune functions. However, our precise knowledge of sex steroids and their downstream mediators in these fundamental aspects of body physiology remains limited. It remains unclear whether sex differences in adipose and immune functions are intrinsic to sex steroids, or due to intrinsic differences in target tissue response, or a combination of both. Irrespective of the underlying mechanisms involved, a crucial role of sex steroid hormones in adipose and immune functions leads to a thought provoking question—why do hormones, whose primary functions are to promote reproductive functions, have so much influence on metabolic and immune functions? Most importantly, what is the importance of this relationship between adipose and immune functions during critical stages of development on metabolic status later in life, especially overweight and obese conditions? New findings from PHB transgenic mice suggest a crucial role of PHB in mediating the effects of sex steroids on adipose and immune functions during the defining moment of puberty, which warrants further investigations. It is possible that dysregulation of the intricate relationship between sex steroid hormones and adipose-immune function may be a major driver in the development of diabetes and cancer later in life. The appearance of metabolic dysregulation and lymph node tumor development in the gonadectomized female m-Mito-Ob transgenic mice, despite the reversal of obesity,46 suggests a crucial role of PHB in mediating the effects of sex steroids on adipose and immune functions at the systemic level. These novel transgenic mice have created unique opportunities to further define the relationship between sex steroids and adipose-immune functions, especially in the context of obesity, and their relative contribution to the development of obesity-linked diabetes and cancer. It would be interesting to know whether gender differences in adipose and immune functions in humans have a role in gender differences in cancer incidence.
Relative contribution of environmental and genetic factors in obesity-linked cancer?
There has been a constant debate on the relative contribution of extrinsic/environmental and intrinsic/genetic factors in cancer development. This debate was further reinvigorated after the publication of two recent articles supporting one or the other view.75,76 An obvious question in this context would be, which one is operative in obesity-linked different types of cancer. The development of lymph node tumors only in m-Mito-Ob may be in favor of intrinsic factor or so called “bad luck” theory, because Mito-Ob mice share the metabolic features of m-Mito-Ob mice but they do not develop lymph node tumors. This would imply that lymph node tumor development in m-Mito-Ob mice requires m-PHB.46 However, m-PHB by itself is not sufficient to initiate tumor development and requires metabolic dysregulation, because female m-Mito-Ob mice that develop obesity and carry m-PHB do not develop tumors.46 Thus, obesity-associated abnormalities may facilitate the manifestation of preexisting dormant clones, which may not manifest by themselves. On the other hand, the development of liver tumors in Mito-Ob mice45 may be in favor of environmental factor theory, because they develop tumor in obesity-related hyperinsulinemia-dependent manner, as female Mito-Ob mice share obese phenotype with male Mito-Ob mice but do not develop obesity-related metabolic dysregulation and consequently liver tumor. However, a possibility remains that obesity-associated alterations in the liver such as oxidative and mitochondrial damage may induce genetic changes and facilitate obesity-linked tumor development. An in-depth analysis of tumor development in Mito-Ob and m-Mito-Ob mice may shed new lights on the underlying mechanisms and the relative contribution of extrinsic and intrinsic factors in obesity-linked different types of cancer development.
Concluding remarks
A recent report by the WHO-IACR Working Group on obesity-linked cancer concluded that evidence for a role of sex hormone metabolism and chronic inflammation in mediating obesity-linked cancer is strong.70 In this context, it is important to note that sex differences are known to exist in adipose and immune functions, and in cancer incidence. The identities of various proteins that mediate sex dimorphic effects of sex steroid hormones in adipose and immune function, and biological mechanisms of sex differences in cancer incidence are largely unknown. Identification of a common protein in mediating sex differences in adipocyte and immune cell function is a first step in a new direction. The discovery of PHB as an important protein in this context, as revealed by novel transgenic mice, has opened a new way(s) of looking at the relationship between adipose and immune functions and their roles in disease processes. Till now, the focus has been on direct interactions between adipocytes and immune cells by cell–cell contact and via their secreted products such as adipokine and cytokines, respectively.72 The cell and tissue type specific diverse functions of PHB suggest a potential role of pleiotropic proteins in the crosstalk between adipose and immune functions, which may work at early events in their crosstalk. Targeting PHB may be a useful therapeutic approach for the treatment of metabolic and immune diseases including obesity, diabetes, and cancer. Further study on role of PHB in various adipose depots and different immune cell types is crucial, because it integrates several concepts of metabolic and cell signaling events in the relation to functional switch in adipocytes and immune cells in physiological and pathophysiological processes.
Acknowledgements
SM is supported by the Natural Sciences and Engineering Research Council of Canada, Research Manitoba and University Collaborative Research Program.
Authors’ contributions
SM and BLGN wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mishra S, Murphy LC, Murphy LJ. The Prohibitins: Emerging roles in diverse functions. J Cell Mol Med 2006; 10: 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: More than lipid raft markers. Trends Cell Biol 2007; 17: 394–402. [DOI] [PubMed] [Google Scholar]
- 3.McClung JK, Danner DB, Stewart DA, Smith JR, Schneider EL, Lumpkin CK, Dell’Orco RT, Nuell MJ. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem Biophys Res Commun 1989; 164: 1316–22. [DOI] [PubMed] [Google Scholar]
- 4.Terashima M, Kim KM, Adachi T, Nielsen PJ, Reth M, Köhler G, Lamers MC. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO J 1994; 13: 3782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikonen E, Fiedler K, Parton RG, Simons K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett 1995; 358: 273–7. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene 1999; 18: 3501–10. [DOI] [PubMed] [Google Scholar]
- 7.Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA 1999; 96: 6947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT, Brügger B, Westermann B, Langer T. The genetic interactome of prohibitins: Coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol 2009; 184: 583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Choy JS, VanderWeele DJ, Brace JL, Harris MH, Bauer DE, Prange B, Kron SJ, Thompson CB, Rudin CM. Bcl-x(L) complements Saccharomyces cerevisiae genes that facilitate the switch from glycolytic to oxidative metabolism. J Biol Chem 2002; 277: 44870–6. [DOI] [PubMed] [Google Scholar]
- 10.Back JW, Sanz MA, De Jong L, De Koning LJ, Nijtmans LG, De Koster CG, Grivell LA, Van Der Spek H, Muijsers AO. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Sci 2002; 11: 2471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J 2000; 19: 2444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol 2005; 7: 837–43. [DOI] [PubMed] [Google Scholar]
- 13.Ande SR, Gu Y, Nyomba BL, Mishra S. Insulin induced phosphorylation of prohibitin at tyrosine 114 recruits Shp1. Biochim Biophys Acta 2009; 1793: 1372–8. [DOI] [PubMed] [Google Scholar]
- 14.Ande SR, Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Biophys Res Commun 2009; 390: 1023–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim DK, Kim HS, Kim AR, Jang GH, Kim HW, Park YH, Kim B, Park YM, Beaven MA, Kim YM, Choi WS. The scaffold protein prohibitin is required for antigen-stimulated signaling in mast cells. Sci Signal 2013; 6: ra80–ra80. [DOI] [PubMed] [Google Scholar]
- 16.Ande SR, Moulik S, Mishra S. Interaction between O-GlcNAc modification and tyrosine phosphorylation of prohibitin: Implication for a novel binary switch. PLoS One 2009; 4: e4586–e4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ande SR, Mishra S. Palmitoylation of prohibitin at cysteine 69 facilitates its membrane translocation and interaction with Eps 15 homology domain protein 2 (EHD2). Biochem Cell Biol 2010; 88: 553–8. [DOI] [PubMed] [Google Scholar]
- 18.Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol 2008; 10: 1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem 2003; 278: 47853–61. [DOI] [PubMed] [Google Scholar]
- 20.Dong P, Jiang L, Liu J, Wu Z, Guo S, Zhang Z, Zhou F, Liu Z. Induction of paclitaxel resistance by ERα mediated prohibitin mitochondrial-nuclear shuttling. PLoS One 2013; 8: e83519–e83519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Kim TH, Kommagani R, Feng Q, Lanz RB, Jeong JW, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O'Malley BW. Estrogen-regulated prohibitin is required for mouse uterine development and adult function. Endocrinology 2011; 152: 1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhance estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA 2006; 103: 16716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O'Malley BW. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol 2008; 22: 344–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Zhao Y, Yoon S, Xu J, Liao L, Lydon J, DeMayo F, O'Malley BW, Katzenellenbogen BS. Repressor of estrogen receptor activity (REA) is essential for mammary gland morphogenesis and functional activities: Studies in conditional knockout mice. Endocrinology 2011; 152: 4336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble SC, Chotai D, Odontiadis M, Dart DA, Brooke GN, Powell SM, Reebye V, Varela-Carver A, Kawano Y, Waxman J, Bevan CL. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene 2007; 26: 1757–68. [DOI] [PubMed] [Google Scholar]
- 26.Gamble SC, Odontiadis M, Waxman J, Westbrook JA, Dunn MJ, Wait R, Lam EW, Bevan CL. Androgens target prohibitin to regulate proliferation of prostate cancer cells. Oncogene 2004; 23: 2996–3004. [DOI] [PubMed] [Google Scholar]
- 27.Nuell MJ, Stewart DA, Walker L, Friedman V, Wood CM, Owens GA, Smith JR, Schneider EL, Dell' Orco R, Lumpkin CK, Danner DB, McClung JK. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol Cell Biol 1991; 11: 1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell'Orco RT. The 3' untranslated region of prohibitin and cellular immortalization. Exp Cell Res 1996; 224: 128–35. [DOI] [PubMed] [Google Scholar]
- 29.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell'Orco RT. Prohibitin in breast cancer cell lines: Loss of antiproliferative activity is linked to 3' untranslated region mutations. Cell Growth Differ 1996; 7: 871–8. [PubMed] [Google Scholar]
- 30.Jupe ER, Badgett AA, Neas BR, Craft MA, Mitchell DS, Resta R, Mulvihill JJ, Aston CE, Thompson LF. Single nucleotide polymorphism in prohibitin 3' untranslated region and breast-cancer susceptibility. Lancet 2001; 357: 1588–9. [DOI] [PubMed] [Google Scholar]
- 31.Spurdle AB, Hopper JL, Chen X, McCredie MR, Giles GG, Newman B, Chenevix-Trench G. Prohibitin 3' untranslated region polymorphism and breast cancer risk in Australian women. Lancet 2002; 360: 925–6. [DOI] [PubMed] [Google Scholar]
- 32.Spurdle AB, Purdie DM, Chen X, Chenevix-Trench G. The prohibitin 3' untranslated region polymorphism is not associated with risk of ovarian cancer. Gynecol Oncol 2003; 90: 145–9. [DOI] [PubMed] [Google Scholar]
- 33.Leal MF, Cirilo PD, Mazzotti TK, Calcagno DQ, Wisnieski F, Demachki S, Martinez MC, Assumpção PP, Chammas R, Burbano RR, Smith MC. Prohibitin expression deregulation in gastric cancer is associated with the 3' untranslated region 1630 C>T polymorphism and copy number variation. PLoS One 2014; 9: e98583–e98583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem 2003; 278: 47853–61. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Zhang B, Faller DV. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J 2002; 21: 3019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Nath N, Fusaro G, Chellappan S. Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol Cell Biol 1999; 19: 7447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene 2002; 21: 8388–96. [DOI] [PubMed] [Google Scholar]
- 38.Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA 2006; 103: 16716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, Troup S, Adeyinka A, Watson PH, Murphy LC. Expression of a repressor of estrogen receptor activity in human breast tumors: Relationship to some known prognostic markers. Cancer Res 2000; 60: 2796–9. [PubMed] [Google Scholar]
- 40.Ummanni R, Junker H, Zimmermann U, Venz S, Teller S, Giebel J, Scharf C, Woenckhaus C, Dombrowski F, Walther R. Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer Lett 2008; 266: 171–85. [DOI] [PubMed] [Google Scholar]
- 41.Liao Q, Guo X, Li X, Xiong W, Li X, Yang J, Chen P, Zhang W, Yu H, Tang H, Deng M, Liang F, Wu M, Luo Z, Wang R, Zeng X, Zeng Z, Li G. Prohibitin is an important biomarker for nasopharyngeal carcinoma progression and prognosis. Eur J Cancer Prev 2013; 22: 68–76. [DOI] [PubMed] [Google Scholar]
- 42.Jia L, Ren JM, Wang YY, Zheng Y, Zhang H, Zhang Q, Kong BH, Zheng WX. Inhibitory role of prohibitin in human ovarian epithelial cancer. Int J Clin Exp Pathol 2014; 7: 2247–55. [PMC free article] [PubMed] [Google Scholar]
- 43.Ande SR, Xu Z, Gu Y, Mishra S. Prohibitin has an important role in adipocyte differentiation. Int J Obes 2012; 36: 1236–44. [DOI] [PubMed] [Google Scholar]
- 44.Mishra S, Ande SR, Nyomba BL. The role of prohibitin in cell signaling. FEBS J 2010; 277: 3937–46. [DOI] [PubMed] [Google Scholar]
- 45.Ande SR, Nguyen KH, Nyomba BL, Mishra S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci Rep 2016; 6: 23608–23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ande SR, Nguyen KH, Padilla-Meier GP, Nyomba BL, Mishra S. Expression of a mutant prohibitin from the aP2 gene promoter leads to obesity-linked tumor development in insulin resistance-dependent manner. Oncogene 2016; 35: 4459–70. [DOI] [PubMed] [Google Scholar]
- 47.Han EK, Mcgonigal T, Butler C, Giranda VL, Luo Y. Characterization of Akt overexpression in MiaPaCa-2 cells: Prohibitin is an Akt substrate both in vitro and in cells. Anticancer Res 2008; 28: 957–63. [PubMed] [Google Scholar]
- 48.Artal-Sanz M, Tavernarakis N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature 2009; 461: 793–7. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Lin Y, Kang T, Huang B, Xu W, Garcia-Barrio M, Olatinwo M, Matthews R, Chen YE, Thompson WE. Mitochondrial dysfunction and adipogenic reduction by prohibitin silencing in 3T3-L1 cells. PLoS One 2012; 7: e34315–e34315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ande SR, Nguyen KH, Padilla-Meier GP, Wahida W, Nyomba BL, Mishra S. Prohibitin overexpression in adipocytes induces mitochondrial biogenesis, leads to obesity development, and affects glucose homeostasis in a sex-specific manner. Diabetes 2014; 63: 3734–41. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Nguyen KH, Mishra S, Nyomba BL. Prohibitin is expressed in pancreatic beta-cells and protects against oxidative and proapoptotic effects of ethanol. FEBS J 2010; 277: 488–500. [DOI] [PubMed] [Google Scholar]
- 52.Supale S, Thorel F, Merkwirth C, Gjinovci A, Herrera PL, Scorrano L, Meda P, Langer T, Maechler P. Loss of prohibitin induces mitochondrial damages altering b-cell function and survival and is responsible for gradual diabetes development. Diabetes 2013; 62: 3488–99.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merkwirth C, Martinelli P, Korwitz A, Morbin M, Brönneke HS, Jordan SD, Rugarli EI, Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphos-phorylation and neurodegeneration. PLoS Genet 2012; 8: e1003021.–e1003021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucas CR, Cordero-Nieves HM, Erbe RS, McAlees JW, Bhatia S, Hodes RJ, Campbell KS, Sanders VM. Prohibitins and the cytoplasmic domain of CD86 cooperate to mediate CD86 signaling in B lymphocytes. J Immunol 2013; 190: 723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross JA, Nagy ZS, Kirken RA. The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem 2008; 283: 4699–713. [DOI] [PubMed] [Google Scholar]
- 56.Dixit VD, Sridaran R, Edmonsond MA, Taub D, Thompson WE. Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology 2003; 144: 1496–505. [DOI] [PubMed] [Google Scholar]
- 57.Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 2009; 137: 199–208, 208.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sánchez-Quiles V, Segura V, Bigaud E, He B, O'Malley BW, Santamaría E, Prieto J, Corrales FJ. Prohibitin-1 deficiency promotes inflammation and increases sensitivity to liver injury. J Proteomics 2012; 75: 5783–92. [DOI] [PubMed] [Google Scholar]
- 59.Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA 2004; 101: 17492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emerson V, Holtkotte D, Pfeiffer T, Wang IH, Schnölzer M, Kempf T, Bosch V. Identification of the cellular prohibitin 1/prohibitin 2 heterodimer as an interaction partner of the C-terminal cytoplasmic domain of the HIV-1 glycoprotein. J Virol 2010; 84: 1355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wintachai P, Wikan N, Kuadkitkan A, Jaimipuk T, Ubol S, Pulmanausahakul R, Auewarakul P, Kasinrerk W, Weng WY, Panyasrivanit M, Paemanee A, Kittisenachai S, Roytrakul S, Smith DR. Identification of prohibitin as a Chikungunya virus receptor protein. J Med Virol 2012; 84: 1757–70. [DOI] [PubMed] [Google Scholar]
- 62.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 2014; 153: 145–52. [DOI] [PubMed] [Google Scholar]
- 63.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38: 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010; 15: 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity and mortality from cancer in a prospective studied cohort of U.S. adults. N Engl J Med 2003; 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 66.Tilg H, Moschen AR. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract Res Clin Gastroenterol 2014; 28: 599–610. [DOI] [PubMed] [Google Scholar]
- 67.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallagher EJ, Alikhani N, Tobin-Hess A, Blank J, Buffin NJ, Zelenko Z, Tennagels N, Werner U, LeRoith D. Insulin receptor phosphorylation by endogenous insulin or the insulin analog AspB10 promotes mammary tumor growth independent of IGF-I receptor. Diabetes 2013; 62: 3553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, Zhang R, Salatino M, Tchou J, Rabinovich GA, Conejo-Garcia JR. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015; 27: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer – Viewpoint of the IARC working group. N Engl J Med 2016; 375: 794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen KH, Ande SR, Mishra S. Obesity-related abnormalities couple environmental triggers with genetic susceptibility in adult-onset T1D. Biochem Biophys Res Commun 2016; 470: 94–100. [DOI] [PubMed] [Google Scholar]
- 72.Ande SR, Nguyen KH, Nyomba BL, Mishra S. Prohibitin in adipose and immune functions. Trends Endocrinol Metab 2016; 27: 531–41. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Sex hormone imbalances and adipose tissue dysfunction impacting on metabolic syndrome: A paradigm for the discovery of novel adipokines. Horm Mol Biol Clin Investig 2014; 17: 89–97. [DOI] [PubMed] [Google Scholar]
- 74.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014; 35: 97–104. [DOI] [PubMed] [Google Scholar]
- 75.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016; 529: 43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomasetti C, Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015; 347: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]