Abstract
Hepatocellular carcinoma is one of the most common causes of cancer-related death worldwide. Hepatocellular carcinoma development depends on the inhibition and activation of multiple vital pathways, including the Wnt signaling pathway. The Wnt/β-catenin pathway lies at the center of various signaling pathways that regulate embryonic development, tissue homeostasis and cancers. Activation of the Wnt/β-catenin pathway has been observed frequently in hepatocellular carcinoma. However, activating mutations in β-catenin, Axin and Adenomatous Polyposis Coli only contribute to a portion of the Wnt signaling hyper-activation observed in hepatocellular carcinoma. Therefore, besides mutations in the canonical Wnt components, there must be additional atypical regulation or regulators during Wnt signaling activation that promote liver carcinogenesis. In this mini-review, we have tried to summarize some of these well-established factors and to highlight some recently identified novel factors in the Wnt/β-catenin signaling pathway in hepatocellular carcinoma.
Impact statement
Early recurrence of human hepatocellular carcinoma (HCC) is a frequent cause of poor survival after potentially curative liver resection. Among the deregulated signaling cascades in HCC, evidence indicates that alterations in the Wnt/β-catenin signaling pathway play key roles in hepatocarcinogenesis. In this review, we summarize the potential molecular mechanisms how the microtubule-associated Protein regulator of cytokinesis 1 (PRC1), a direct Wnt signaling target previously identified in our laboratory to be up-regulated in HCC, in promoting cancer proliferation, stemness, metastasis and tumorigenesis through a complex regulatory circuitry of Wnt3a activities.
Keywords: Hepatocellular carcinoma, Wnt signaling, non-canonical regulators, β-catenin, protein regulator of cytokinesis 1
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer mortality worldwide.1 Despite treatment with surgical resection, which provides a limited opportunity for cure, most HCC patients have a dismal prognosis,2 primarily due to the complexity of HCC primary tumors and frequent tumor recurrence.3 The development of HCC is closely associated with chronic hepatitis B or C infection, cirrhosis of any etiology, and/or aflatoxin B1 exposure.4 The etiology of HCC is complicated and includes numerous genetic and epigenetic alterations as well as deregulation of various signaling pathways, including the Wnt/β-catenin, PI3K, HGF, Ras, VEGF, IGF, and PDGF pathways, and is likely to involve other pathways as well.5 Among these growth factor signaling cascades, evidence suggests that the Wnt/β-catenin pathway plays a crucial role in hepatocarcinogenesis as well as in recurrence.3,6
Wnt signaling plays important roles in the regulation of diverse processes, including cell growth, survival, cytoskeleton remodeling, embryonic patterning, cell fate, as well as stem cells maintenance.7 Aberrant activation of Wnt signaling contributes to cancers, including colon cancer,8 gastric cancer,9 esophageal cancer,10 and HCC.6 Approximately 95% of reported HCC cases exhibit deregulation of eight different Wnt/Frizzled (Fzd) signaling cascades.11
Although Wnt/β-catenin signaling is frequently activated in HCC, the causes and mechanisms of its activation are not yet well understood or established. Mutation has been confirmed as one of the most important contributors to activation of aberrant Wnt signaling in HCC. By far, the β-catenin gene has been confirmed as the most frequently mutated gene associated with aberrant activity of the Wnt pathway in HCC. As has also been discovered in many other cancers, these mutations are often dominant gain-of-function (GOF) mutations affecting the N-terminal GSK3 and CK1 phosphorylation sites of β-catenin.12 The recorded frequency of β-catenin gene somatic mutations in HCC ranges from 20 to 40%,13 but the cytoplasmic and nuclear accumulation of β-catenin have been reported more commonly, detected in 40% to 70% of HCC cases.12,14 Mutations of other canonical Wnt pathway components, such as Axin1 and Axin2, have been identified in 10% and 3% of HCC patients, respectively.13 Furthermore, inactivating mutations of the tumor suppressor adenomatous polyposis coli (APC) are unlikely to contribute significantly due to its low mutation rate in HCC.15 These data indicate that, in addition to mutations, there must be other novel regulatory mechanisms contributing to the hyper-activation of Wnt signaling in the liver, leading to hepatocarcinogenesis and even metastasis.
Importance of canonical Wnt signaling in HCC
Wnt signaling can be classified into canonical (mainly β-catenin-dependent) and non-canonical (β-catenin-independent) pathways. Wnt signaling is primed by cysteine-rich and lipid-modified secreted glycoprotein ligands named Wnts, their corresponding receptors and co-receptors called Frizzleds (Fzds) and low-density lipoprotein receptor-related protein-6 (LRP6).7 Thus far, the non-canonical Wnt signaling pathways (the Planar Cell Polarity/PCP and the Wnt-Ca2+ pathways) are primarily implicated in cytoskeleton remodeling and cell movement, whereas the canonical signaling pathway has functions in embryonic development and the regulation of cell proliferation and metastasis.7 The biological role of non-canonical Wnt-mediated signaling is less well defined in liver cancer. Here, we shall focus on the Wnt/β-catenin pathway because currently there are only a limited number of reports specifically linking non-canonical Wnt signaling with liver cancer.
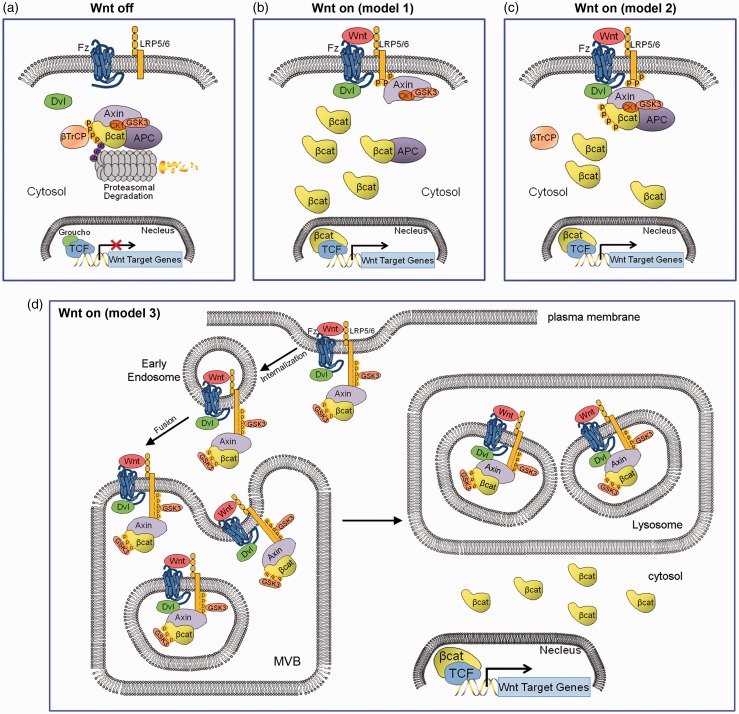
The canonical Wnt signaling pathway controls many biological processes, including cell proliferation, embryonic patterning, and stem cell self-renewal.7 There are currently three models of upstream canonical Wnt signaling (Figure 1). In the absence of ligands such as Wnt3a, newly formed β-catenin is continuously degraded by a multi-factor destruction complex mainly composed of the scaffolding proteins Axin1 and Axin2 (Conductin), APC, casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). The captured β-catenin will be sequentially phosphorylated at Ser45 and Thr41 by CK1γ, followed by Ser37 and Ser33 by GSK3β. The phosphorylated β-catenin in destruction complex will be recognized by the β-transducin repeat-containing protein (β-TrCP)-dependent E3 ligase, and be degraded by the ubiquitin-proteasome system (Figure 1(a)). In the presence of Wnt ligands, β-catenin is stabilized due to either membrane sequestration of the Axin/GSK3 complex through dissociation of the destruction complex (Figure 1(b)) or by membrane sequestration of the complete Axin destruction complex (Figure 1(c)), using the LRP6 signalosome as the clustering center, and thus the expression of Wnt transcriptional targets is primed.7,16 Describing the third model in more detail, Wnt bind to the Fzd receptor and LRP6 or LRP5 co-receptor. This ternary complex clusters with the scaffolding protein dishevelled (Dvls, including Dvl1, 2, 3) to form LRP6-signalosomes.16 After signalosome complex internalized and sorted into early endosomes, LRP6 phosphorylation is primed and sustained at multiple PPPSPxS motifs on its intracellular domain (ICD) by several proline-directed kinases in a GSK3-dependent manner,17 resulting in the subsequent phosphorylation of LRP6 via CK1γ, thereby recruiting the Axin destruction complex to the signalosome.16,18 The signalosomes will be further fused into multivesicular bodies (MVPs) to sequestrate GSK3β, rendering this kinase unable to phosphorylate cytosolic β-catenin, and thereby preventing its degradation by the β-TrCP-ubiquitin ligase system19,20 (Figure 1(d)). Other reports have suggested an association between the protein phosphatases PP1 and PP2A and the Axin complex, which counteracts GSK3β- and/or CK1γ-mediated phosphorylation, thereby dissociating the destruction complex and stabilizing β-catenin.21 Mutations in the components of Axin destruction complex result in cancer, including HCC.
Figure 1.
Models of canonical Wnt signaling. (a) When Wnt receptor complexes are not bound by Wnt ligands on the cell membrane, CK1 and GSK3α/β phosphorylate β-catenin. Phosphorylated β-catenin is recognized by β-TrCP, a component of a dedicated E3 ubiquitin ligase complex. Following ubiquitination, β-catenin is targeted for rapid destruction by the proteasome. In the nucleus, the binding of Groucho to TCF (T cell factor) inhibits the transcription of Wnt target genes. Once bound by Wnt ligand, the Frizzled (Fz)/LRP5/6 coreceptor complex activates the canonical signalling pathway. Fz interacts with Dsh, a cytoplasmic protein that functions upstream of β-catenin. Wnt signaling controls phosphorylation of Dishevelled (Dsh). Wnts are thought to induce the phosphorylation of LRP by GSK3β and casein kinase I-γ (CK1γ), thus regulating the docking of Axin. (b) The recruitment of Axin away from the destruction complex (destruction complex dissociation), (c) or the recruitment of the intact Axin1 destruction complex will lead to the stabilization of β-catenin. In the nucleus, β-catenin displaces Groucho from Tcf/Lef to promote the transcription of Wnt target genes. (d) Canonical Wnt signaling through the sequestration of GSK3 inside multivesicular endosomes. Binding of GSK3 to the Wnt receptor complex, including phospho-LRP6, phospho-β-catenin, and other GSK3 substrates such as Dvl, Axin and APC, sequesters GSK3 inside small intraluminal multivesicular body (MVB) vesicles, causing its cytosolic substrates such as β-Catenin (in yellow) and many other Wnt/STOP target proteins to become stabilized. The initial GSK3 molecules are recruited to the receptor complex bound to Axin, ensuring that the GSK3 fraction bound to the destruction complex is first being depleted. (A color version of this figure is available in the online journal.)
Non-classical regulators of Wnt signaling in HCC
Recently, there have been some unexpected discoveries of novel Wnt regulators that have not been considered classical Wnt signaling players, but are now confirmed as important regulators for Wnt signaling activation by experimental and biological evidence, especially in the case of HCC. Moreover, there is increasing evidence that the stabilization of proteins (STOP) primed by non-β-catenin-mediated Wnt signaling is also an unsolved but potentially important pathway in liver cancer. Here, we have summarized these recently identified novel regulators and have termed them tentatively as non-classical regulators for Wnt signaling for their distinct subcellular localization to the microtubule (MT) cytoskeleton, plasma membrane, and centrosomal region and because they present atypical functions including regulation of protein stabilization.
Cytoskeleton-associated regulators and Wnt signaling
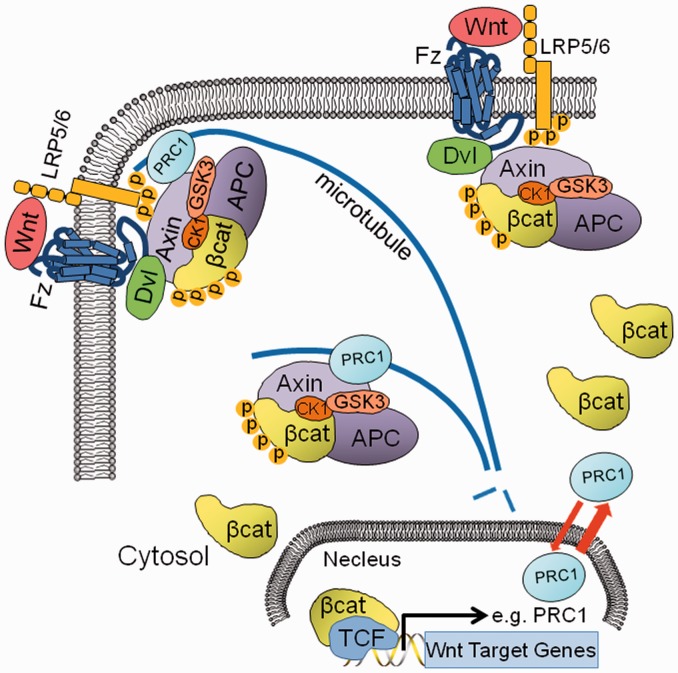
Thus far, many different signaling pathways including Wnt,22 Shh,23 FGF,24 Notch,25 mTor,26 PDGF,27 and Hippo28 signaling have been confirmed in association with the cytoskeleton. However, little is known about the mechanistic organization of Wnt signaling and the cytoskeletal factors that are modulated in the development of HCC. Recently, our group has reported that the microtubule-associated protein (MAP) PRC1 is linked to Wnt/β-catenin signaling in HCC.6 PRC1 is highly enriched in HCC tumor tissues, and its expression is associated with early HCC recurrence and poor patient prognosis.6 PRC1 promotes proliferation of the HCC cells harboring wild-type (WT) β-catenin but shows no effects in HCC cells with the mutated β-catenin.6 Interestingly, PRC1 was further confirmed as a novel Wnt/β-catenin signaling transcriptional target, and its localization at the cytoskeleton was dynamically regulated by Wnt3a signaling, indicating that PRC1 may have a role in Wnt3a signaling (Figure 2). Indeed, PRC1 regulates Wnt signaling via interaction with the destruction complex on MTs, promoting cytoskeletal sequestration of the destruction complex and thus stabilizing cytoplasmic β-catenin and activating Wnt signaling (Figure 2).6,29 Our data further indicate that PRC1 MT localization promotes Wnt/β-catenin signaling and promote cell proliferation. PRC1 MT localization domain deleted mutant (PRC1-CC) shows dot-like localization of PRC1.6 Moreover, PRC1-CC cells grew slower than cells harboring WT-PRC1 (unpublished data), further indicating that PRC1 MT localization mediates the sequestration of the destruction complex to stabilize the cytoplasmic β-catenin to promote the cell growth and Wnt signaling function.
Figure 2.
Role of PRC1 in canonical Wnt signaling in HCC cells. As a transcriptional target of Wnt signaling in HCC cells, PRC1 expression is activated by the β-catenin/TCF complex. Upon Wnt signaling activation, PRC1 is transferred to MTs and the membrane cortex, which helps to sequester the destruction complex of Axin, APC, GSK3, CK1 and β-catenin at the MTs and the membrane, leading to Wnt signalosome activation and stabilization of β-catenin in HCC cells. (A color version of this figure is available in the online journal.)
The MT cytoskeleton has a critical regulatory function that affects not only mitosis but also cytoskeletal shape, cell motility, and intracellular protein and organelle transport.30 MTs and MAPs are attractive drug targets, for example, Paclitaxel (Taxol), a chemotherapy drug, has been offered as a systemic treatment for unresectable HCC patients.31 Wnt pathway components have been proposed to regulate the dynamics of MTs. Many of them were found in the mitotic spindle, centrosome and centromere, which are all associated with MTs, and all are necessary for cell division and survival.32 Centromere APC, with its partners end-binding 1 (EB1) and the formin mDia, can stabilize microtubules oriented towards the front end of migrating cells.33 APC can also target Microtubule-Actin Crosslinking Factor 1 (MACF1) to the cell cortex to capture microtubule ends.34 Additionally, GSK3β and CK1, two spindle-localized factors, can phosphorylate several MAPs leading to MT destabilization35; however, upon GSK3β inhibition mediated by Dvl, Axin can bind and stabilize microtubules.36 Reciprocally, MTs can affect Wnt signaling through spatiotemporal regulation of the destruction complex.29 Upon Wnt stimulation, cytoplasmic APC partially translocates to MTs, together with PRC1, Axin and GSK3β (Figure 2). These interactions with the cytoskeleton further sequester the destruction complex to MTs and the membrane-associated LRP6 signalosome (Figure 2), thus contributing to the stabilization of newly translated β-catenin in the cytoplasm.29 Therefore, the MAPs can function in Wnt signaling activation by regulating MT stability to affect the MT localization of the Axin/APC/GSK3 complex. KIF17, another MAP that stabilizes MTs by acting at MT plus ends with APC, has also been indicated in Wnt signaling regulation.37
In addition to MTs, the cell membrane is also an important cytoskeleton-based structure. The complex interplay between events and factors of membrane-associated Wnt signaling is difficult to ascertain, as cells develop individual receptors, co-activators and specialized structures, such as primary cilia to mediate Wnt-specific, tissue-specific and cell-specific functions in development and tumorigenesis. One notable example in HCC is membrane-associated Glypican-3 (GPC3), a member of the glypican family and a vital regulator of canonical Wnt signaling.38 GPC3 is anchored to the cell membrane via glycosyl-phosphatidylinositol and is highly enriched in HCC tumor tissues.38 The expression pattern of GPC3 alters with the degree of cell differentiation, thus making it a potential diagnostic marker and drug target.38 In HCC tissues, immunohistochemistry revealed a tendency for overall localization of GPC3 in the cytoplasm of HCC cells. However, GPC3 was more preferentially localized in the cell membrane in poorly differentiated HCC when compared with well-differentiated HCC. Moreover, GPC3 was more preferentially stained in the cell membrane in the metastatic lesions of HCC when compared with primary HCC lesions.39 GPC3 regulates the signaling several growth factors, including Wnts.38 It has been confirmed that GPC3 can enhance the binding of Wnt and Fzd receptors, to enhance canonical Wnt signaling in HCC.40 Thus, the membrane localization of GPC3 in metastatic lesions could indicate an important role of GPC3 in Wnt signaling-mediated metastasis of HCC, and targeting GPC3 might offer a new strategy for the treatment of HCC. Currently, there are two therapeutic approaches targeting membrane-associated GPC3 in HCC that are under phase II clinical trials. One is the application of a humanized GPC3 specific antibody to inhibit the HCC cells growth by the induction of antibody-dependent cellular cytotoxicity, while the second application employs a vaccine consisting of GPC3-derived peptides to trigger cytotoxic T cells against GPC3.38
Some additional membrane-associated proteins may also be required for Wnt signaling activation in HCC. These could include members of the ubiquitin specific peptidase (USP) family, of which some have shown potential membrane localization (Genecards) and have recently been confirmed to be able to deubiquitinate the Wnt receptors Fzds to further promote the stability of Fzds and activate canonical Wnt signaling.41 We have further confirmed that USP1, USP21 and USP49 genes are significantly up-regulated in HCC tumor tissues (Data not shown), indicating that these USP family members might be involved in Wnt signaling activation by stabilizing the Fzd receptors on HCC cells. However, a more comprehensive study is needed to understand the detailed functions of these USPs in HCC Wnt signaling and their potential therapeutic values. Overexpression of the membrane-associated Wnt receptor Fzds may provide another solution for Wnt signaling hyper-activation in HCC. It was reported that Fzd7 is overexpressed in HCC and activates Wnt signaling to stabilize β-catenin.42 Furthermore, the membrane-associated kinase cyclin-dependent kinase 14 (CDK14) and its activators Cyclin Y/Cyclin Y-like have been suggested to play important roles in the activation of the Wnt pathway in HCC.43 Their function in Wnt signaling has been supported by experiments conducted in cell lines and animal models44 and by bioinformatics analysis in human cancers.43
The centrosome is the MT organization center, and its function partially depends on the MTs. Centrosome-based proteins, such as kinases, were also found to have a function in the regulation of Wnt signaling. Recently, NEK2 was discovered to link centrosome separation and maturation with regulation of Wnt signaling via Dvl and β-catenin.45 Moreover, NEK2 has already been reported as an oncogenic factor in HCC, promoting cancer progression and drug resistance by enhancing Wnt activation,46 indicating that centrosome-based proteins may be involved in local regulation of Wnt signaling, although there are currently limited examples. Besides NEK2 regulation on Dvl protein, tumor suppressor WW domain-containing oxidoreductase (WWOX) has also been confirmed to regulate Dvl function via the sequestration of Dvl2 in the cytoplasm.47 Moreover, WWOX has been proven to be an crucial negative regulator of canonical Wnt signaling via the destabilization of β-catenin in HCC, and the down-regulation and suppressive function of WWOX have further been confirmed in HCC.48–50
Another important discovery concerning the membrane-related processes associated with Wnt signaling is endocytosis-mediated Wnt signaling activation.51 Endocytosis is a dynamic process during which the membrane cargo complex is organized and invaginates into the cytoplasm, leading to the formation of a vesicle which then can fuse with the early endosomes and can be sorted into the endo-lysosomal membrane system.52 During endocytosis, cytoskeletal modulation is one of the most crucial process.52 Currently, there are mainly two reported important endocytosis regulators. These are Caveolin 1 (CAV1) and Clathrin, both of which show membrane and/or cytoskeleton subcellular distribution.53,54 Recently, some reports have suggested that CAV1 and Clathrin directly interact and regulate endocytosis and trafficking of the Wnt signalosome. Although it has been verified that CAV1 is involved in Wnt signaling activation in HCC cells,55 the function of Clathrin in Wnt-mediated hyper-activation of HCC has not yet been well established, indicating that this regulation might be cell line dependent. As endocytosis is a dynamic, cooperative process involving multiple cytoskeleton molecules, it can respond to stimulation by growth factors and other environmental signals. Abnormal expression or modification of the endocytic regulators leads to aberrant signaling transduction, which has been repeatedly observed in cancers, including HCC.
Virus infection has been demonstrated to play an important role in the regulation of Wnt signaling in HCC through membrane and/or cytoskeleton associated-factors. Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) core proteins have been proven to enhance the canonical Wnt signaling via different mechanisms. The HVB core protein HBx can promote membrane Src kinase activity to inhibit the GSK3 kinase and promote β-catenin stability.56 HBx can directly interact with APC on cytoskeleton where PRC1 interacts with the APC complex to stabilize β-catenin,57 indicating HBx may link to PRC1 function in Wnt signaling regulation. On the other hand, direct activation of the Wnt/β-catenin pathway has been demonstrated for the core (C) protein of HCV. In the SMMC-7721 cells, HCV core protein can up-regulate the expression of Wnt ligands and their receptors, including Wnt-2, -3, -3a, -10a, -10b, Fzd-1, -2, -3, -6, -7, -9 and LRP5/6 co-receptors. Furthermore, the HCV core protein can also epigenetically silence the secreted frizzled-related protein (SFRP) in the SMMC-7721 cells leading to the activation of the Wnt signaling.58 As the expression and localization of PRC1 are also modulated by Wnt3a-signaling,6 a potential mechanism of HCV regulating Wnt signaling can be through the promotion of PRC1 expression and MT localization.
Mitotic Wnt and Wnt/STOP signaling pathway
Wnt signaling is tightly associated with cell cycle progression, and Wnt signaling can transcriptionally regulate key growth regulators such as cyclin D1 or c-Myc, which is reinforced by distinct cell cycle- regulators such as PRC1, NEK2 and CDK14/CyclinY. Increasing evidence has reaffirmed the tight link between cell cycle and the regulation of Wnt signaling. As a notable example, Wnt coreceptor-LRP6 phosphorylation is under strict regulation by the cell cycle and peaks in G2/M.59 LRP6 is phosphorylated by CDK14, as well as by associated G2/M Cyclin Y or Cyclin Y-like activators, on its ICD domain, which primes LRP6 for Wnt-dependent phosphorylation.59 Hence, the ability of LRP6 to respond to Wnt is under the control of the cell cycle and peaks at G2/M phase, which can explain why cytoplasmic β-catenin, AXIN2, and PRC1 expression all oscillate with the cell cycle and peak at G2/M phase.
It is intriguing that Wnt signaling should peak in mitosis when it is transcriptionally silent, and the observed mitotic activation of LRP6, whose major effects are thought to require β-catenin-dependent gene transcription, is also puzzling.32 Interestingly, there is mounting evidence showing that some of the physiological consequences of canonical Wnt signaling are β-catenin-independent.60 GSK3 is one of the most important downstream kinases in canonical Wnt signaling, and it is inhibited through multiple mechanisms.10,19,20 Bioinformatics assay has confirmed that up to 20% of the GSK3 phospho-degrons in the proteome are dependent on ubiquitin-mediated proteasomal degradation.19,61 Thus, the suppression of GSK3β activity by Wnt signaling activation might stabilize these proteins.19 In some cells lines, such as the HeLa cell line, Wnt-dependent stabilization of proteins (Wnt/STOP), rather than transcriptional activation, appears to be the dominant mode of Wnt signaling.60 Activation of Wnt/STOP signaling increases cellular protein content, especially during G2/M phase, in which these Wnt/STOP targets may help to sustain the proliferative capacity of daughter cells. Abnormal regulation of Wnt/STOP signaling may contribute to cancers, including HCC. Indeed, some evidence has shown that many of the mitotic regulators, such as Aurora kinases,62 Abnormal spindle-like microcephaly associated (ASPM) protein,63 Epithelial Cell Transforming 2 (ECT2),64 centromere protein F (CENPF)65 and many others, exhibit abnormal enrichment in HCC, indicating that these regulators may be potential targets of Wnt/STOP signaling. Based on published bioinformatics resources, we found that ASPM, ECT2 and CENPF all contain three putative GSK3 sites,19 indicating that they may be potential targets of Wnt/STOP signaling (data not shown). However, the abnormal enrichment of these Wnt/STOP targets may also be due to aberrant changes in the master controlling genes such as p53, FoxM1 or β-catenin in liver cancer. Further experimental validation is needed to define the molecular roles of these HCC-associated Wnt/STOP targets. In our earlier study, PRC1, a Wnt target, was also found to be highly expressed in cells during mitosis compared to cells in interphase. As we have reported, PRC1’s regulatory role in Wnt signaling is upstream of β-catenin, indicating that PRC1 may also be involved in mitotic Wnt/STOP signaling by sequestering the Wnt destruction complex to MTs and even spindles. Indeed, it was demonstrated that knock-down of PRC1 completely eliminated the c-Myc protein and yet only inhibited 40% of its mRNA expression.6 A similar effect was also observed for the protein level of LEF1 and its mRNA expression,6 indicating that PRC1 has a potential role in stabilizing the proteins of Wnt/STOP signaling targets in HCC. The PRC1 regulated Wnt/STOP proteome of HCC cells warrants further investigation.
Conclusion and perspectives
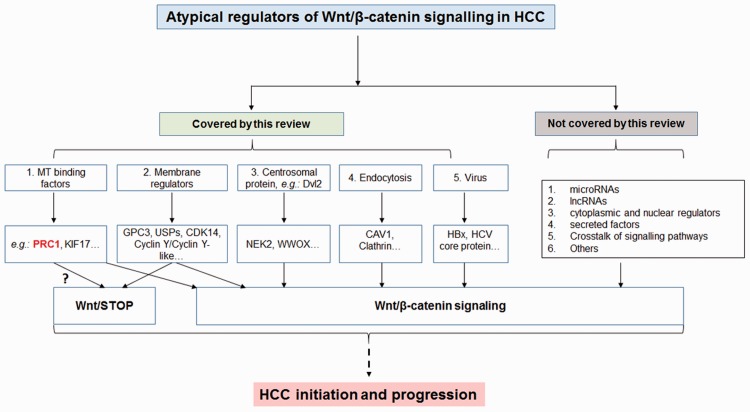
Altogether, the present review focuses on recent published information on the non-classical regulators of Wnt signaling in HCC. In addition to these regulators, there are other published Wnt regulators, such as microRNAs, lncRNAs, cytoplasmic and nuclear regulators, secreted proteins and RNAs, as well as the existence of cross-talk with other signaling pathways that have not been covered in this review (Figure 3). This review highlights the evidence for a cytoskeletal protein – PRC1 – to be a novel regulator of Wnt signaling in HCC with a potential Wnt/STOP signaling function (Figure 3). Nevertheless, there are still many outstanding unresolved issues concerning Wnt signaling in HCC. For example, some of the outstanding challenges that remain unresolved are (1) what is (are) the HCC-specific regulator(s) or target(s) in canonical Wnt signaling? (2) Which Wnt ligand(s) and Fzd receptor(s) mediate(s) local Wnt signaling activation, contributing to heterogeneity in HCC? (3) What is the level of active nuclear WT β-catenin and mutated β-catenin on the level of the transcriptome in HCC? (4) What is the physiological function of Wnt/STOP in HCC development, and what is (are) the Wnt/STOP regulator(s) and target(s)? The Wnt signaling pathway is complex, and we are still in the early stage of mapping and identifying novel regulators that play a role in the different stages of liver cancer development.
Figure 3.
A flow diagram to summarize the topic areas of the present review. (A color version of this figure is available in the online journal.)
Acknowledgements
We thank Dr. Yiting Qiao for her input regarding Figures 1 and 2. This work was supported by grants from the National Medical Research Council of Singapore (to KMH) and from the SingHealth Foundation (to JC).
Author contributions
All authors contributed to the design and writing of the manuscript.
Declaration Of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2: 16018–16018. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008; 48(Suppl 1): S20–37. [DOI] [PubMed] [Google Scholar]
- 3.Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 2001; 120: 1763–73. [DOI] [PubMed] [Google Scholar]
- 4.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012; 56: 1384–91. [DOI] [PubMed] [Google Scholar]
- 5.Imbeaud S, Ladeiro Y, Zucman-Rossi J. Identification of novel oncogenes and tumor suppressors in hepatocellular carcinoma. Semin Liver Dis 2010; 30: 75–86. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL, Hong W, Hui KM. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/beta-catenin signalling pathway. Gut 2016; 65: 1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen L, De Sousa E Melo, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–76. [DOI] [PubMed] [Google Scholar]
- 9.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis 2014; 5: e1039–e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Luo Y, Tian H, Yu KZ, He JX, Shen WY. The tumor suppressor LKB1 antagonizes WNT signaling pathway through modulating GSK3beta activity in cell growth of esophageal carcinoma. Tumour Biol 2014; 35: 995–1002. [DOI] [PubMed] [Google Scholar]
- 11.Bengochea A, de Souza MM, Lefrancois L, Le RE, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, Merle P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer 2008; 99: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer 2001; 92: 136–45. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, Ross JA, Liu W. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002; 21: 4863–71. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 1999; 155: 1795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizaki Y, Ikeda S, Fujimori M, Shimizu Y, Kurihara T, Itamoto T, Kikuchi A, Okajima M, Asahara T. Immunohistochemical analysis and mutational analyses of beta-catenin, Axin family and APC genes in hepatocellular carcinomas. Int J Oncol 2004; 24: 1077–83. [PubMed] [Google Scholar]
- 16.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007; 316: 1619–22. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005; 438: 873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 2005; 438: 867–72. [DOI] [PubMed] [Google Scholar]
- 19.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010; 143: 1136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinyoles M, Del Valle-Perez B, Curto J, Vinas-Castells R, Alba-Castellon L, Garcia de HA, Dunach M. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol Cell 2014; 53: 444–57. [DOI] [PubMed] [Google Scholar]
- 21.Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC is essential for targeting phosphorylated beta-catenin to the SCFbeta-TrCP ubiquitin ligase. Mol Cell 2008; 32: 652–61. [DOI] [PubMed] [Google Scholar]
- 22.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 2011; 25: 201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nurnberg G, Nurnberg P, Zentgraf H, Koerber F, Addicks K, Elsobky E, Benzing T, Schermer B, Bolz HJ. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J Clin Invest 2011; 121: 2662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 2005; 435: 172–7. [DOI] [PubMed] [Google Scholar]
- 25.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 2011; 145: 1129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, Muller K, Herbst M, Hornung M, Doerken M, Kottgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 2010; 12: 1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 2005; 15: 1861–6. [DOI] [PubMed] [Google Scholar]
- 28.Habbig S, Bartram MP, Muller RU, Schwarz R, Andriopoulos N, Chen S, Sagmuller JG, Hoehne M, Burst V, Liebau MC, Reinhardt HC, Benzing T, Schermer B. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol 2011; 193: 633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoit B, Pous C. MAPping the Wnt pathway to hepatocellular carcinoma recurrence. Gut 2016; 65: 1397–400. [DOI] [PubMed] [Google Scholar]
- 30.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253–65. [DOI] [PubMed] [Google Scholar]
- 31.Chao Y, Chan WK, Birkhofer MJ, Hu OY, Wang SS, Huang YS, Liu M, Whang-Peng J, Chi KH, Lui WY, Lee SD. Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma patients. Br J Cancer 1998; 78: 34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. EMBO J 2012; 31: 2705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 2004; 6: 820–30. [DOI] [PubMed] [Google Scholar]
- 34.Zaoui K, Benseddik K, Daou P, Salaun D, Badache A. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc Natl Acad Sci USA 2010; 107: 18517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol 2007; 17: 333–42. [DOI] [PubMed] [Google Scholar]
- 36.Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 2004; 164: 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol 2010; 190: 443–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J 2013; 280: 2471–6. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M, Sugimoto K, Tanaka J, Tameda M, Inagaki Y, Kusagawa S, Nojiri K, Beppu T, Yoneda K, Yamamoto N, Ito M, Yoneda M, Uchida K, Takase K, Shiraki K. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res 2010; 30: 5055–61. [PubMed] [Google Scholar]
- 40.Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS, Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 2014; 60: 576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madan B, Walker MP, Young R, Quick L, Orgel KA, Ryan M, Gupta P, Henrich IC, Ferrer M, Marine S, Roberts BS, Arthur WT, Berndt JD, Oliveira AM, Moon RT, Virshup DM, Chou MM, Major MB. USP6 oncogene promotes Wnt signaling by deubiquitylating Frizzleds. Proc Natl Acad Sci U S A 2016; 113: E2945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem BA, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004; 127: 1110–22. [DOI] [PubMed] [Google Scholar]
- 43.Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun 2014; 449: 163–8. [DOI] [PubMed] [Google Scholar]
- 44.Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell 2015; 163: 1225–36. [DOI] [PubMed] [Google Scholar]
- 45.Cervenka I, Valnohova J, Bernatik O, Harnos J, Radsetoulal M, Sedova K, Hanakova K, Potesil D, Sedlackova M, Salasova A, Steinhart Z, Angers S, Schulte G, Hampl A, Zdrahal Z, Bryja V. Dishevelled is a NEK2 kinase substrate controlling dynamics of centrosomal linker proteins. Proc Natl Acad Sci U S A 2016; 113: 9304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin S, Zhou S, Jiang S, Liu X, Wang Y, Zheng X, Zhou H, Li X, Cai X. NEK2 regulates stem-like properties and predicts poor prognosis in hepatocellular carcinoma. Oncol Rep 2016; 36: 853–62. [DOI] [PubMed] [Google Scholar]
- 47.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009; 28: 2569–80. [DOI] [PubMed] [Google Scholar]
- 48.Jiang QF, Tian YW, Shen Q, Xue HZ, Li K. SENP2 regulated the stability of beta-catenin through WWOX in hepatocellular carcinoma cell. Tumour Biol 2014; 35: 9677–82. [DOI] [PubMed] [Google Scholar]
- 49.Park SW, Ludes-Meyers J, Zimonjic DB, Durkin ME, Popescu NC, Aldaz CM. Frequent downregulation and loss of WWOX gene expression in human hepatocellular carcinoma. Br J Cancer 2004; 91: 753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aderca I, Moser CD, Veerasamy M, Bani-Hani AH, Bonilla-Guerrero R, Ahmed K, Shire A, Cazanave SC, Montoya DP, Mettler TA, Burgart LJ, Nagorney DM, Thibodeau SN, Cunningham JM, Lai JP, Roberts LR. The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced by the tumor suppressor WWOX. J Hepatol 2008; 49: 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell 2006; 11: 213–23. [DOI] [PubMed] [Google Scholar]
- 52.Qualmann B, Kessels MM. Endocytosis and the cytoskeleton. Int Rev Cytol 2002; 220: 93–144. [DOI] [PubMed] [Google Scholar]
- 53.Kirchhausen T, Owen D, Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol 2014; 6: a016725–a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 2013; 14: 98–112. [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Shen H, Zhang Y, Zhong F, Liu Y, Qin L, Yang P. CAV1 promotes HCC cell progression and metastasis through Wnt/beta-catenin pathway. PLoS One 2014; 9: e106451–e106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology 2004; 39: 1683–93. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/beta-catenin signaling. Cancer Lett 2011; 300: 162–72. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Wang Z, Tang J, Tang R, Shan X, Zhang W, Chen Q, Zhou F, Chen K, Huang A, Tang N. Hepatitis C virus core protein activates Wnt/beta-catenin signaling through multiple regulation of upstream molecules in the SMMC-7721 cell line. Arch Virol 2011; 156: 1013–23. [DOI] [PubMed] [Google Scholar]
- 59.Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell 2009; 17: 788–99. [DOI] [PubMed] [Google Scholar]
- 60.Acebron SP, Karaulanov E, Berger BS, Huang YL, Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol Cell 2014; 54: 663–74. [DOI] [PubMed] [Google Scholar]
- 61.Kim NG, Xu C, Gumbiner BM. Identification of targets of the Wnt pathway destruction complex in addition to beta-catenin. Proc Natl Acad Sci U S A 2009; 106: 5165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res 2004; 10: 2065–71. [DOI] [PubMed] [Google Scholar]
- 63.Lin SY, Pan HW, Liu SH, Jeng YM, Hu FC, Peng SY, Lai PL, Hsu HC. ASPM is a novel marker for vascular invasion, early recurrence, and poor prognosis of hepatocellular carcinoma. Clin Cancer Res 2008; 14: 4814–20. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Xia H, Zhang X, Karthik S, Pratap SV, Ooi LL, Hong W, Hui KM. ECT2 regulates the Rho/ERK signalling axis to promote early recurrence in human hepatocellular carcinoma. J Hepatol 2015; 62: 1287–95. [DOI] [PubMed] [Google Scholar]
- 65.Dai Y, Liu L, Zeng T, Zhu YH, Li J, Chen L, Li Y, Yuan YF, Ma S, Guan XY. Characterization of the oncogenic function of centromere protein F in hepatocellular carcinoma. Biochem Biophys Res Commun 2013; 436: 711–8. [DOI] [PubMed] [Google Scholar]