Abstract
A growing body of evidence indicates that circular RNAs are not simply a side product of splicing but a new class of noncoding RNAs in higher eukaryotes. The progression for the studies of circular RNAs is accelerated by combination of several advanced technologies such as next generation sequencing, gene silencing (small interfering RNAs) and editing (CRISPR/Cas9). More and more studies showed that dysregulated expression of circular RNAs plays critical roles during the development of several human diseases. Herein, we review the current advance of circular RNAs for their biosynthesis, molecular functions, and implications in human diseases.
Impact statement
The accumulating evidence indicate that circular RNA (circRNA) is a novel class of noncoding RNA with diverse molecular functions. Our review summarizes the current hypotheses for the models of circRNA biosynthesis including the direct interaction between upstream and downstream introns and lariat-driven circularization. In addition, molecular functions such as a decoy of microRNA (miRNA) termed miRNA sponge, transcriptional regulator, and protein-like modulator are also discussed. Finally, we reviewed the potential roles of circRNAs in neural system, cardiovascular system as well as cancers. These should provide insightful information for studying the regulation and functions of circRNA in other model of human diseases.
Keywords: Circular RNA, miRNA sponge, molecular adaptor, cardiovascular disease, neuronal disease, cancer
Introduction
Noncoding RNA, a group of functional RNA not encoding proteins such as housing-keeping ribosomal RNAs, tRNA and small nucleolar RNAs, has been known for decades. Twenty years ago, the discovery of small interfering RNAs and microRNAs broadened our horizons about the complexed regulatory networks of RNA-mediated gene expression. Nevertheless, the recent progression on investigating the roles of long noncoding RNAs in the regulation of DNA methylation, histone modification and change of chromatin conformation extended the toolbox of the noncoding RNAs. Likewise, the current advance in studying circular RNAs should have opened the gate to another arena of regulatory RNAs.
Circular RNA, consisting of a circular configuration through a typical 5′ to 3′-phosphodiester bond, is recently recognized as a new class of functional molecule. It consists no 5′ or 3′ free terminus, and is much more stable in the cells. The discovery of RNA molecules with circular configuration tracked back to four decades ago.1,2 Early studies found some transcripts with non-colinear or shuffled order and implied that it might be a byproduct resulted from the process of mis-splicing.3,4 Later, accumulating evidence consolidated the existence of circular configuration RNA molecules such as transcripts of mouse Sry, human ETS1, and DCC.4–6 Although these pioneer studies have drafted a blueprint for the current circRNA research such as the requirement of complementary sequences in the flanking introns, the large flanking introns, and high stability, the lack of biological functions and comprehensive analysis halted the progression of circRNA research. In the past few years, the advancement of next generation sequencing technology enabled scientists to perform genome-wide analysis on the expression of circRNAs, and to characterize the diverse origins and composition of circRNAs. In addition, the well-established roles of miRNAs and the theory of competitive endogenous RNA facilitate the big leap of circRNA research.
The biosynthesis of circular RNAs
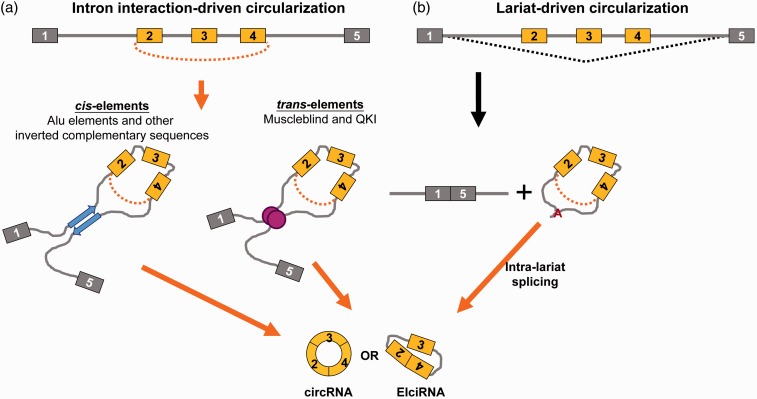
Early studies indicated that circRNAs arise from both exonic and intronic regions.2,5,7,8 Along with the availability of genome-wide analysis, the global and comprehensive studies revealed that circRNAs can be generated from intergenic, intronic, coding region, 5′- or 3′-untranslational regions.9,10 Up to date, there are several proposed models for the biosynthesis of circRNA. Most circRNAs arise from the backsplicing event, in which a downstream 5′ splice donor joins an upstream 3′ splice acceptor11 and have no U2 or U12 spliceosome preference.12 It has been reported that the circularized regions are flanked by large introns.6,13–15 The repetitive sequences in the flanking intron facilitate to make a loop structure that dissolves the opposite direction and discontinuity problems between the downstream 5′ splice site and upstream 3′ splice site (Figure 1(a), left)14 where efficient pairing is achieved by complementary nature of Alu sequences, a short stretch of DNA repeats originally characterized by the action of the Arthrobacter luteus (Alu) restriction enzyme.16
Figure 1.
The proposed models of circRNA biosynthesis. (a) The inverted complementary repeat sequences (left) or RNA-binding proteins which modulate the interaction between the flanking long introns (right) facilitate the joining of upstream splice acceptor and downstream donor via 3′,5′-phosphodiester bond (denoted by the orange curve). (b) Alternatively, for certain genes which generate exon-skipped transcripts, the upstream splice acceptor and downstream donor can be brought to the vicinity through the lariat structure, and the secondary splice or intra-lariat splice will occur to generate circRNAs. A: branch point. (A color version of this figure is available in the online journal.)
Alternatively, it has been reported that complementary sequences other than Alu are capable to promote backsplice (Figure 1(a), left).17,18 Several studies using either the cloned intronic sequence-based assay or CRISPR/Cas9-mediated intronic sequence deletion consolidate the importance of regulatory roles of intronic sequences in circRNA biosynthesis.16,18,19 In addition to the complementary sequences, RNA-binding proteins such as Muscleblind (MBL), Quaking (QKI) and RNA binding motif protein 20 (RBM20)20–22 are reported to promote the interaction between upstream and downstream introns and facilitate backsplice (Figure 1(a), right).
In addition to the model of intron interaction-driven circularization, lariat-driven circularization is an alternative mechanism to produce circular RNAs (Figure 1(b)). The original model describes that the skipped exons in the lariat structure undergo secondary splicing and are joined by typical 5′-3′ phosphodiester bond23 (Figure 1(b)). In sum, these models have generally elucidated the potential mechanisms of circRNAs biosynthesis.
Molecular functions of circRNAs
MicroRNA sponge
The journey to explore the world of circRNA functions has just begun. The pioneer study conducted by Hansen and his colleagues demonstrated that ciRS-7 have highly enriched miRNA binding site for miR-7, one of the miRNAs identified on ciRS-7, and discovered that long-known circRNA from mouse Sry gene works as a decoy, or a molecular sponge for miR-13824 (Figure 2(a)). The miRNA binding sites on the ciRS-7 are mismatched on the central part of the miRNA/circRNA duplex, which is distinct from the complete complementary binding of miRNAs to typical 3′-UTR sequences, to prevent the circRNA from miRNA/AGO2-mediated RNA cleavage. This unique nature makes the circRNAs a molecular sponge to sequester miRNA molecules and prevent the targeted mRNA from being degraded by miRNA. In contrast to ciRS-7, ours and other studies found that circRNAs harbor binding sites of multiple miRNAs,19,25,26 suggesting the capability of circRNAs to modulate the activities of multiple miRNAs in parallel.
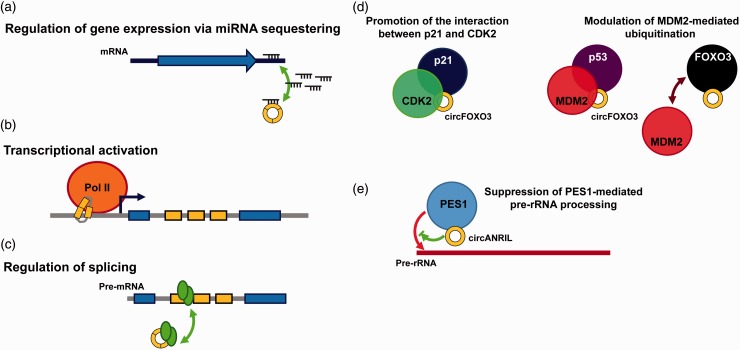
Figure 2.
The molecular functions of circRNAs. (a) The circRNAs function as molecular sponge to protect 3′-UTR from miRNA attacks. (b) CircRNAs regulates the expression of their parental genes through association with chromatin and/or the promoters of their parental genes to activate the transcription. (c) CircRNAs regulate the expression of their parental genes by competing the splicing factors. (d) CircFOXO3 brings CDK2 and p21 together to suppress the activity of CDK2 (left). In a similar fashion, circFOXO3 promotes the interaction between p53 and MDM2, leading to p53 ubiquitination and subsequent degradation. Furthermore, circFOXO3 also weakly interacts with FOXO3 and prevents it from MDM2-mediated ubiquitination (right). (e) CircANRIL, which has similar secondary structure to pre-rRNA, binds PES1 and inhibits the exonuclease-mediated pre-rRNA processing and maturation. (A color version of this figure is available in the online journal.)
Regulators of transcription and splicing
A growing body of evidence supports the role of non-coding RNA in the regulation of transcriptional activation or silencing through recruitment of epigenetic modulators or transcription factors.27–29 Shan and his colleagues reported that a group of intron-retained circRNA (termed exon-intron circRNAs or EIciRNAs) generated through canonical splice signal was found to be remained in the nuclei.18 While those circRNAs function as miRNA decoy and interact with AGO2, the EIciRNAs are localized to the promoter of their parental genes and associates with RNA polymerase II to enhance the transcription efficiency through the interactions between U1 snRNA/U1A/U1C complex and the 5′-splice site remained in the intron. This pioneer study explored the functions of circRNA on the transcriptional regulation of their parental genes (Figure 2(b)).
In addition to the regulation on the transcription of their parental genes, it also has been observed that the splicing efficiency of certain genes negatively correlated to the level of circRNA, implying the competition of splicing machinery between processing of linear and circular transcripts20 (Figure 2(c)). These studies exemplified that the circRNA can exert both negative and positive regulation on their parental genes at different levels. However, the possibility whether circRNA may interact with other epigenetic modulators or transcription factors to achieve gene-specific regulation (other than parental genes) or whether the competition of splicing is regulatable on other genes is warranted for further investigation.
Adaptor for protein–protein interaction
Compared to the regulatory roles on the transcription, it has been reported that circRNA can work as an adaptor to modulate protein–protein interaction and their activities. A recent study indicates that circFOXO3 functions as an adaptor, bridging the cyclin-dependent kinase 2 (CDK2) and p2130 (Figure 2(d), left). The mitogenic effect of EGF was partially mediated by the downregulation of circFOXO3, which leads to the release of CDK2 from p21 and allow CDK2 to phosphorylate cyclin E and cyclin A for cell cycle progression. In a parallel study, the circFOXO3 has similar bridging function to bring the MDM2 and p53 together, and facilitate the degradation of p53. Interestingly, through weak interaction with FOXO3, circFOXO3 prevents FOXO3 from MDM2-mediated poly-ubiquitination and proteasome degradation31 (Figure 2(d), right).
Ribosomal RNA processing
The study of circANRIL found another novel function of circRNA, which is to inhibit the maturation of ribosomal RNA32 (Figure 2(e)). Through the proteomic analysis of circANRIL-bound proteins, a group of proteins involved in biogenesis and assembly of ribosome and RNA splicing was identified. Further investigation revealed that circANRIL directly interacts with pescadillo zebrafish homologue 1 (PES1), a key component in the complex regulating large 60S ribosome subunit biogenesis. Through interaction with the C-terminal domain of PES1, circANRIL suppresses the exonuclease-mediated rRNA maturation, inducing nucleolar stress and eliciting the p53 stabilization. These studies extended our knowledge about the molecular functions of circRNA from sequence pairing-based regulators (miRNA sponge and splicing competitor) to structure-based protein-like modulators.
Biological functions and implications in human diseases
Regulation of neuronal diseases
The potent pathological role of circRNAs was first implicated in the neuronal tissues. The region- and time-specific changes of protein levels in neuronal cells are particularly important and achieved by a regulated asymmetric distribution of mRNAs mediated by dendritic translocation of specific mRNAs.33 MiR-7, a key miRNA playing a crucial role on the expression of α-synuclein during the pathogenesis of Parkinson’s disease, is found to coexpress and colocalize with ciRS-7 in dendrite, implying the potential regulatory function of ciRS-7 on α-synuclein and brain development (Figure 3(a)).
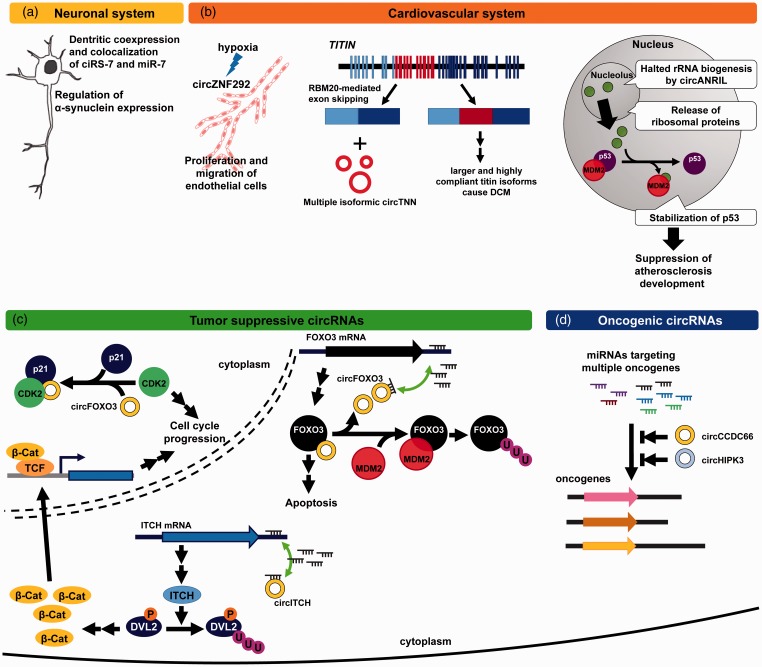
Figure 3.
The implications of circRNAs in human diseases. (a) MiR-7 is an important regulator for the expression of α-synuclein, a critical player in the pathogenesis of Parkinson’s disease, and is coexpressed and colocalized with ciRS-7 in the dendrites. (b) CircZNF292 is induced by hypoxia and required for migration and proliferation of endothelial cells, suggesting that circZNF292 might play a role in angiogenesis-related diseases (left). Expression of circTTN is correlated to RMB20, a crucial regulator for TITIN alternative splicing (the gene structure of TITIN is not drawn to scale). Loss of RMB20 leads to the expression of larger TITIN isoforms and contributes to the pathogenesis of DCM (middle). CircANRIL inhibits ribosomal RNA maturation, triggers nucleolar stress, and causes p53 accumulation, which may further prevent the development of atherosclerosis (right). (c) CircITCH suppresses WNT/β-Catenin pathway through increased expression of ITCH. In the nucleus, circFOXO3 inhibits cell cycle progression through promoting p21/CDK2 interaction. In contrast, circFOXO3 prolongs the half-life of FOXO3 mRNA and protects FOXO3 protein from MDM2-mediated ubiquitination in cytoplasm. The increased FOXO3 protein leads to apoptosis. (d) CircHIPK3 and circCCDC66 protect multiple oncogenes from miRNA attack. (A color version of this figure is available in the online journal.)
Regulation of cardiovascular disease
In addition to the role in neural system, circRNA is also implicated in the pathogenesis of vascular system. Through genome-wide analysis, it has been reported that a specific subgroup of circRNA in endothelial cells is upregulated by hypoxic stress.34 Among these circRNAs, circZNF292 was found to be induced by hypoxia in a HIF-1α independent manner, implying the involvement of other HIF members or indirect mediators. Depletion of circZNF292 suppresses the proliferation and sprouting of endothelial cells, both of which are critical steps for the development of vascular networks (Figure 3(b), left). Nevertheless, the molecular mechanisms underlying the circZNF292 for promoting the cell cycle progression and migration remain unclear and require further investigation.
In contrast, the human TITIN gene (TTN), which encodes a molecular spring responsible for the elasticity of muscle fibers, was found to differentially generate several isoforms of circTTN in tissues from dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy compared to normal tissues.22 The expression of DCM-specific circTTNs positively correlates to RBM20, a known splice regulator facilitating the exon skipping of N-terminal I-band of TITIN.35,36 Mutation of RBM20 causes exon retention in the region of I-band and leads to expression of larger and highly compliant titin isoforms, presumably causing DCM. RBM20 facilitates the removal of exons in the region of I-band, and the skipped exons were further processed to circTTNs presumably through the lariat-typed secondary splicing35 (Figure 3(b), middle).
In addition to RBM20-regulated circTTNs in cardiomyocytes, expression of circANRIL was found in atherosclerotic plaques, aneurysm and several primary cells including peripheral monocytes, endothelial cells, adventitial fibroblast and smooth muscle cells.32 CircANRIL suppresses the cleavage of pre-ribosomal RNA, causes the nucleolar stress, and further induces the stabilization and accumulation of p53 which may play a protective role on the development of atherosclerosis (Figure 3(b), right).
Regulation of cancer progression
The multifaceted roles of circRNAs in cancer development and progression have not been fully understood. Early studies showed that circRNAs are differentially expressed in many cancerous tissues and cell lines. Cancerous cell lines are prone to express a more diverse pattern of circRNAs compared to non-cancerous cell lines.37 It has been reported that the expression of circRNAs were globally downregulated in colorectal and ovarian cancers, and were negatively correlated with the disease status and proliferation.38 Two recent studies are in line with these early studies, supporting the tumor suppressive effect of certain circRNAs to protect parental genes of tumor suppressor from miRNA attacks. In esophageal squamous cell carcinoma, circITCH functions as miRNA sponge to protect its parental transcript which encodes Itchy E3 Ubiquitin Protein Ligase (ITCH). Thus the tumor suppressive effect of circITCH is achieved by ITCH-mediated ubiquitination of hyperphosphorylated Dishevelled, one of the key components in the canonical Wnt signaling pathway39 (Figure 3(c)).
In parallel, multiple roles of circFOXO3 in tumor suppression were reported in a series of comprehensive studies.25,30,31 The level of circFOXO3 is lower in the breast tumor specimens compared to adjacent benign tissues. CircFOXO3 works as a miRNA sponge protecting Foxo3 mRNA from miRNA attack, as an adaptor to bridge p21 and CDK2 inhibiting the cell cycle progression, and as a steric blocker preventing FOXO3 from MDM2-mediated ubiquitination and further proteasome degradation in breast cancer (Figure 3(c)). These studies greatly exemplify the tumor suppressive roles of circRNAs and how the loss of these circRNAs may contribute to the development and progression of tumor.
In contrast to the tumor suppressive circRNAs, the levels of several circRNAs were elevated during the process of epithelial–mesenchymal transition or positively correlated with tumor development,21 suggesting the presence of oncogenic circRNAs. More recent studies comprehensively identify differentially expressed circRNAs from many types of adjacent non-tumor and tumor specimens including bladder urothelial carcinoma, breast cancer, colorectal cancer, gastric cancer, hepatocellular carcinoma, clear cell carcinoma, prostate cancer, and ovarian cancer.19,26,40,41 Among these identified circRNAs, circHIPK3 derived from exon 2 of HIPK3 gene was an abundantly expressed circRNA with high backsplice efficiency.19 Through the screening of a library containing 424 miRNAs, nine miRNAs show great suppressive ability on the HIPK3 exon 2-based reporter system. Intriguingly, these nine miRNAs all harbor tumor suppressive activities. Expression of two known proliferation-promoting genes, IL6R and DLX2, targeted by miR-124 (one of the identified miRNAs) was found to be elevated by the overexpression of circHIPK3, implying that the oncogenic activity of circHIPK3 is mediated via blocking the tumor suppressive effect of miR-124. On the other hand, our recent study also indicated that circCCDC66, a circRNA upregulated in pre-cancerous and cancerous tissues from colorectum, carries multiple miRNAs sites and these predicted that circCCDC66-bound miRNAs target a group of oncogenes.26 Thus circCCDC66 preferentially protects oncogenes from attacks of multiple miRNAs. In vivo study using xenograft and orthotopic models of mouse demonstrated that knockdown of circCCDC66 suppresses tumor growth and metastasis (Figure 3(d)). These studies demonstrated that the expression of circRNAs is dynamically regulated in different statuses of human diseases, and exerts their regulatory functions via multiple routes.
Challenges and perspectives
In the past five years, the world of circRNAs in its biosynthesis and molecular function has been greatly explored. However, there are more awaiting for further investigation. Several independent lines of evidence support the theory of miRNA sponge, either for single miRNA or multiple ones. How the tripartite interaction among the target genes, miRNAs, and circRNA is coordinated to facilitate the development of human diseases warrants more comprehensive investigation. The regulation of circRNA expression or degradation in the specific disease context is another important issue that needs further investigation. In addition, the protein-like properties of circRNAs to modulate the molecular interaction open up a whole new avenue. How to predict the tertiary structure of circRNA and their potential interactors requires more systematic and genome-wide survey and analysis. Taken together, circRNA as a novel class of regulatory molecules has become new therapeutic and diagnostic targets.
Acknowledgements
The authors would like to acknowledge the financial support by the Ministry of Science and Technology of Taiwan (MOST 104-2320-B-006-036-MY3), the National Health Research Institute (NHRI-EX102-10244BI) and the Top University Grant of National Cheng Kung University (grant # D103-35A17).
Authors’ contributions
KYH, HSS, and SJT discussed and wrote the draft of manuscript. SJT coordinated and wrote the final draft of manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 1979; 280: 339–40. [DOI] [PubMed] [Google Scholar]
- 2.Halbreich A, Pajot P, Foucher M, Grandchamp C, Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular RNA. Cell 1980; 19: 321–9. [DOI] [PubMed] [Google Scholar]
- 3.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell 1991;64:607–13. [DOI] [PubMed]
- 4.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J 1993; 7: 155–60. [DOI] [PubMed] [Google Scholar]
- 5.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, et al. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993; 73: 1019–30. [DOI] [PubMed] [Google Scholar]
- 6.Cocquerelle C, Daubersies P, Majerus MA, Kerckaert JP, Bailleul B. Splicing with inverted order of exons occurs proximal to large introns. EMBO J 1992; 11: 1095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasman Z, Been MD, Garcia-Blanco MA. Exon circularization in mammalian nuclear extracts. RNA 1996; 2: 603–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Braun S, Domdey H, Wiebauer K. Inverse splicing of a discontinuous pre-mRNA intron generates a circular exon in a HeLa cell nuclear extract. Nucleic Acids Res 1996; 24: 4152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495: 333–8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell 2013; 51: 792–806. [DOI] [PubMed] [Google Scholar]
- 11.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19: 141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 2014; 15: 409–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westholm Jakub O, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, et al. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 2014; 9: 1966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19: 141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 2012; 7: e30733–e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev 2014; 28: 2233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell 2014; 159: 134–47. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22: 256–64. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 2016; 7: 11215–11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 2014; 56: 55–66. [DOI] [PubMed] [Google Scholar]
- 21.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015; 160: 1125–34. [DOI] [PubMed] [Google Scholar]
- 22.Khan MA, Reckman YJ, Aufiero S, van den Hoogenhof MM, van der Made I, Beqqali A, et al. RBM20 regulates circular RNA production from the titin gene. Circ Res 2016; 119: 996–1003. [DOI] [PubMed] [Google Scholar]
- 23.Zaphiropoulos PG. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A 1996; 93: 6536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495: 384–8. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Du WW, Li X, Yee AJ, Yang BB. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene 2016; 35: 3919–31. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res 2017; 77: 2339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010; 329: 689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohmdorfer G, Wierzbicki AT. Control of chromatin structure by long noncoding RNA. Trends Cell Biol 2015; 25: 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 2013; 503: 371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 2016; 44: 2846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ 2016; 24: 357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 2016; 7: 12429–12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci 2007; 8: 776–89. [DOI] [PubMed] [Google Scholar]
- 34.Boeckel JN, Jae N, Heumuller AW, Chen W, Boon RA, Stellos K, et al. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res 2015; 117: 884–90. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Guo W, Dewey CN, Greaser ML. Rbm20 regulates titin alternative splicing as a splicing repressor. Nucleic Acids Res 2013; 41: 2659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med 2012; 18: 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol 2015; 16: 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, et al. Correlation of circular RNA abundance with proliferation exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep 2015; 5: 8057–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F, Zhang L, Li W, Deng J, Zheng J, An M, et al. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget 2015; 6: 6001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed I, Karedath T, Andrews SS, Al-Azwani IK, Mohamoud YA, Querleu D, et al. Altered expression pattern of circular RNAs in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget 2016; 7: 36366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep 2016; 6: 30919–30919. [DOI] [PMC free article] [PubMed] [Google Scholar]