Abstract
Psoriasis is a complex chronic immune-mediated inflammatory cutaneous disease associated with the development of inflammatory plaques on the skin. Studies proved that the disease results from a deregulated interplay between skin keratinocytes, immune cells and the environment leading to a persisting inflammatory process modulated by pro-inflammatory cytokines and activation of T cells. However, a major hindrance to study the pathogenesis of psoriasis more in depth and subsequent development of novel therapies is the lack of suitable pre-clinical models mimicking the complex phenotype of this skin disorder. Recent advances in and optimization of three-dimensional skin equivalent models have made them attractive and promising alternatives to the simplistic monolayer cultures, immunological different in vivo models and scarce ex vivo skin explants. Moreover, human skin equivalents are increasing in complexity level to match human biology as closely as possible. Here, we critically review the different types of three-dimensional skin models of psoriasis with relevance to their application potential and advantages over other models. This will guide researchers in choosing the most suitable psoriasis skin model for therapeutic drug testing (including gene therapy via siRNA molecules), or to examine biological features contributing to the pathology of psoriasis. However, the addition of T cells (as recently applied to a de-epidermized dermis-based psoriatic skin model) or other immune cells would make them even more attractive models and broaden their application potential. Eventually, the ultimate goal would be to substitute animal models by three-dimensional psoriatic skin models in the pre-clinical phases of anti-psoriasis candidate drugs.
Impact statement
The continuous development of novel in vitro models mimicking the psoriasis phenotype is important in the field of psoriasis research, as currently no model exists that completely matches the in vivo psoriasis skin or the disease pathology. This work provides a complete overview of the different available in vitro psoriasis models and suggests improvements for future models. Moreover, a focus was given to psoriatic skin equivalent models, as they offer several advantages over the other models, including commercial availability and validity. The potential and reported applicability of these models in psoriasis pre-clinical research is extensively discussed. As such, this work offers a guide to researchers in their choice of pre-clinical psoriasis model depending on their type of research question.
Keywords: Dermatology, psoriasis, keratinocytes, skin equivalents, tissue engineering, drug testing
Introduction
Psoriasis is a complex chronic immune-mediated inflammatory cutaneous disease that can affect skin and nails. It affects 2–3% of the population, with the most common clinical variant being psoriasis vulgaris, affecting approximately 85–90% of all psoriasis patients.1,2 The disease is usually manifested as raised, well-demarcated, erythematous oval plaques with adherent silvery scales. The scales are a result of a hyperproliferative epidermis with premature maturation of keratinocytes (KC). In psoriatic lesions, the granular layer of the epidermis is greatly reduced or absent. As a result, the stratum corneum is formed from incomplete cornified KCs with retention of nuclei (parakeratosis). The mitotic rate of the basal KCs is increased as compared to normal skin, giving rise to a thickened epidermis (acanthosis) with elongated rete ridges. Due to the acceleration of keratinization combined with premature cell death, psoriasis has an unique keratinization process resulting in the disappearance of late differentiation markers such as profilaggrin and loricrin.2–4 Moreover, keratin (K) 1 and K10 are reduced in the psoriatic involved epidermis, whereas proteins absent in healthy skin are expressed in psoriasis, such as SKALP/elafin, K6, K16 and K17.3,5 In addition, inflammatory cells infiltrate the dermis and epidermis. This inflammatory infiltrate consists mainly of dendritic cells (DC), macrophages, and T cells in the dermis, and neutrophils with some T cells in the epidermis.2 The redness of psoriatic lesions can be attributed to the increased number of tortuous capillaries that reach the skin surface through a markedly thinned epithelium adjacent to the acanthotic retes.2
The infiltration of inflammatory immune cells plays an influential role in the disease pathophysiology. They trigger the inflammatory cascade by interaction with and activation of KC, resulting in disease progression. Psoriasis was initially considered to be a Th1 cell-mediated disease due to the presence of large numbers of CD4 + Th1 and CD8 + cytotoxic T cells type 1 (Tc1) and elevated levels of interferon (IFN)-γ, tumor necrosis factor (TNF)-α and IL-12. In addition, interaction of T cells with DCs leads to the secretion of Th1 type cytokines, thus creating a ‘type 1’ inflammatory environment.6–9 Further research, however, demonstrated the added role of IL-17-producing Th17 cells in the pathogenesis of psoriasis, and their downstream effector molecules such as IL-17A, IL-17F, IL-22, IL-21 and TNF-α.10–13 A third subset of Th cells has been implicated in the pathogenesis of psoriasis, namely the Th22 cells, due to their abundant secretion of IL-22.14 It is clear that the pathogenesis of psoriasis is very complex involving multiple interactions between different cell types. Each step towards a better understanding of the mechanisms of psoriasis disease would contribute to the development of new therapeutic agents and improved patient outcomes. In order to study the biology of psoriasis more in depth and to search for novel therapeutic drug candidates, many experimental models of psoriasis have been developed over the years. These can be divided into in vitro, ex vivo, and in vivo models. The in vivo psoriasis mouse models have been summarized in detail by Jean and Pouliot15 and were critically reviewed by Wagner et al.16 Our review will report on existing in vitro psoriasis models, followed by their usability. In addition, a focus was given on psoriatic skin equivalent models as interesting tools to gain further insights in the psoriasis pathology, and to examine potential novel psoriasis drugs.
Monolayer models
Single-cell model
Culturing of psoriasis-derived KC is hindered by the difficulties in sourcing, lack of reproducibility, loss of psoriasis-associated gene expression and difficulties in growth.17 Therefore, groups have investigated the possibility of inducing psoriasis-associated features in normal human epidermal keratinocytes (NHEK) by altering their culture medium. Psoriatic differentiation was first induced by supplementing the culture medium with fetal calf serum (FCS).18 This model, with increased SKALP/elafin, K16 and psoriasin (S100A7) expression, was later used to study the effect of anti-psoriasis drugs.19,20 Recently, our group developed a novel in vitro psoriasis model where the psoriatic phenotype is induced by the addition of FCS and a mix of pro-inflammatory cytokines (IL-1α, IL-17A, IL-6 and TNF-α).17 This model was shown to express a large panel of genes and miRNAs relevant to the pathogenesis of psoriasis. Moreover, it is a robust, easy and highly reproducible model that allows fast screening of therapeutics. Since its development, the model has been successfully used to evaluate the efficiency of RNAi therapeutics in the treatment of psoriasis and to study the effect of Tofacitinib on the activation of Janus kinase (JAK) family members.17,21,22
Co-culture model
To mimic the in vivo environment more closely, a co-culture model of KC and fibroblasts could be established. Different combinations have been evaluated using normal or involved (lesional) and uninvolved (non-lesional) psoriatic cells. Psoriatic fibroblasts were shown to induce hyperproliferation of normal KC, while psoriatic KC retain their high proliferation rate even in combination with normal fibroblasts.23 Recently, Martin et al.24 evaluated the co-culturing of healthy T lymphocytes with normal, healthy KCs versus psoriatic KCs. Beside their previous observation that psoriatic KCs are more sufficient in enhancing T lymphocyte survival, they now showed that T lymphocytes cooperate with psoriatic but not normal KCs to overproduce pro-inflammatory cytokines. Moreover, they evidenced that crosstalk requires direct cell-to-cell contact, confirming previous reports.25,26 Additionally, it was shown that the secretion of one cytokine can influence the expression of others, resulting in a feedback loop as seen in psoriasis skin.25 The effect on cytokine secretion seemed also to depend on how the immune cells were stimulated.24–26 The use of co-cultures allowed in this manner to examine the specific roles of different cell types and to unravel the mechanisms of cell–cell interactions.
3D skin equivalent models
Two-dimensional cell cultures are not suitable for all research questions, for example transdermal drug delivery experiments, as they do not correspond to normal skin structure. The use of ex vivo human skin biopsies would be more appropriate, as they represent the interactions and mechanisms present in whole skin. This model is, however, limited by skin donor availability and variability.27,28 Therefore, bioengineered human skin equivalents (HSEs) have been developed. HSEs are composed of primary human cells (KC, fibroblasts and/or stem cells) and components of the extracellular matrix (ECM).29 These models are more desirable than monolayer models, as they provide a three-dimensional (3D) microenvironment. Two different kind of skin substitutes as in vitro test system can be engineered: epidermal equivalents containing only a multilayered epidermis, also known as reconstructed human epidermis (RHE) models, and full thickness (FT) skin equivalents containing both an epidermal and dermal skin compartment.
Development of epidermal skin equivalents: From normal to psoriatic models
The simplest version of a matrix used to create epidermal equivalents consists of a plain microporous membrane on which second passage KC can form an epidermis (Figure 1(a)). Rosdy and Clauss30 compared the use of de-epidermized dermis (DED), acetate cellulose filters and polycarbonate films as substrate for NHEKs to form a normal epidermis. Using a high-calcium-defined medium (MCDB 153, 1.15 mM calcium, 5 µg/ml insulin, 10 ng/ml epidermal growth factor (EGF), and 5 × 10−7 M hydrocortisone), multilayered epithelium was formed after 14 days of culture at the air–liquid interface, independent of the substrates used. These data show that KC are able to form an epithelium of normal architecture, with the expression of essential terminal differentiation markers such as K10, involucrin and filaggrin, in the absence of any dermal or undefined serum component. In the above paper, already from 1990, only secondary but not primary or third-passage KC were able to differentiate and to form a stratum corneum. As medium compositions evolved in time, this is presently not an issue anymore. However, it is recommended to use early passage KCs, as keratinocyte quality is critical for epidermis formation.31
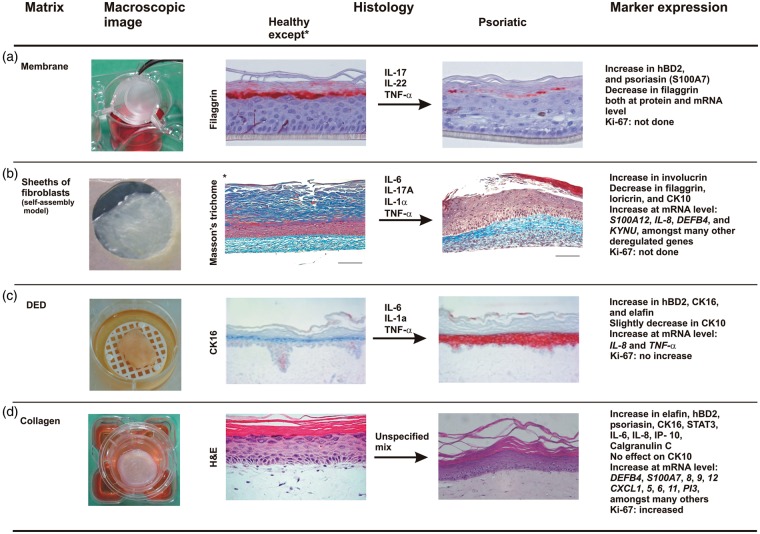
Figure 1.
Overview of the four different psoriatic skin equivalents created by cytokine stimulation of healthy skin equivalents, except for the self-assembly model. Macroscopic images are showing the different matrix types followed by histological pictures or stainings of differentiation markers such as filaggrin in the synthetic membrane model (A)41 and CK16 in the DED-based model (C)61 before and after stimulation with indicated cytokines. In the self-assembly model (B), psoriatic fibroblasts and psoriatic keratinocytes were used to generate a psoriatic full-thickness (FT) 3D skin model.78 Addition of cytokines to this model compensated for the lack of immune cells.94 Psoriatic fibroblasts, but healthy KCs, were used by MatTek to establish their collagen-based FT 3D psoriatic model (D). Finally, proteins and genes known to be differentially expressed in the psoriatic skin models versus healthy controls (except in B, deregulated genes are described after comparison of psoriatic substitutes treated or not with cytokines) are summarized. Models B to D represent FT models, whereas model A contains only a differentiated epidermal part. CK16: Keratin 16; DED: de-epidermized dermis. Scale bar: 100 µm. (Histological images and image of self-assembly model (see referred papers) are reprinted with permission from Elsevier and Future Medicine Ltd)
The role and importance of calcium in keratinocyte and epidermal differentiation were recently reviewed by Bikle et al.32 In low calcium concentrations, KC proliferate but are unable to differentiate into a stratified layer. From concentrations above 0.1 mM, differentiation is initiated and terminal differentiation markers are being expressed. As source of KC, different approaches have been described: KC isolated from human skin,30,33 KC derived from human embryonic stem cells (hESC)34 or induced pluripotent stem cell (iPSC) teratoma-derived KC.35 Moreover, it has been shown that the Rho kinase inhibitor Y-27632 is able to prolong the life span of adult NHEK while maintaining their ability to form HSEs. This indication could aid in generating large quantities of KC required to meet the increasing need for HSEs,36 as the immortalized keratinocyte cell line HaCaT has been found inadequate to form a proper epidermis in skin equivalents.37–39
In contrast to the many available normal RHE models (especially commercial ones such as SkinEthic RHE (Episkin, Lyon, France), EpiDerm (MatTek Corporation, Ashland, USA), Biomimiq’s Leiden epidermal skin model (LEM), (Biomimiq, Leiden, The Netherlands), StratiCell RHE/001 (StratiCELL, Les Isnes, Belgium) etc.), psoriatic epidermal models are limited. However, with the addition of a cytokine cocktail, normal RHE can adapt a psoriasis-like phenotype and gene expression profile. IL-22 was shown to trigger hyperplasia in the SkinEthic RHE model, with a reduced expression of differentiation-related genes and upregulation of psoriasis markers.40 Next, the same group treated RHEs with a mixture of IL-17, IL-22 and TNF-α which resulted in destabilization of the epidermis, parakeratosis and hypogranulosis, resembling partially the lesional psoriasis phenotype. Moreover, filaggrin expression was decreased, whereas S100A7 and hBD-2 expression were overexpressed both at the mRNA and protein level (Figure 1(a)).41 Similarly, the MatTek’s EpiDerm model was found to express psoriasis-like features, such as expression of S100A7, K16 and Stat3 activation with nuclear localization, after the addition of IL-20 subfamily cytokines (IL-19, 20, 22, 24 and 26). IL-22 had the most pronounced effects on KC proliferation (based on increased expression of the hyperproliferative marker K16) and differentiation, including hypogranulosis.42 A parallel approach, addition of cytokines to normal RHEs, was used by companies as well in order to offer epidermal psoriasis models to the scientific community. An overview of commercial psoriatic RHEs is presented in Table 1.
Table 1.
Commercially available psoriasis 3D skin models for in vitro applications
| Brand name | Company | Epidermis | Dermis/matrix | Stimulation | |
|---|---|---|---|---|---|
| Ex vivo | – | Biopta | Lesional psoriatic skin (2 mm diameter punch biopsy) | None | |
| – | Biopta | Healthy human skin (2 mm diameter punch biopsy) | Unspecified cytokine mix | ||
| RHE | PSO RHE | Atera Labs | NHEK | Synthetic carrier membrane | Unspecified psoriasis inducer |
| Custom-made RHE | StratiCell | NHEK or PHEK | Inert polycarbonate filter | Unspecified | |
| EPIBA-0005 | BIOalternatives | NHEK | Culture inserts | OSM | |
| EPIBA-0006, 7, 57, 58 | BIOalternatives | NHEK | Culture inserts | IL-17, OSM, TNF-α | |
| EPIBA-0008, 9 | BIOalternatives | NHEK | Culture inserts | Psoriatic T cell medium | |
| FT | Psoriasis | MatTek | NHEK (from neonatal foreskin) | PHDF in collagen (from adult psoriatic explants) | Unspecified cytokine mix |
NHEK: normal human epidermal keratinocytes; OSM: oncostatin-M; PHDF: psoriatic human dermal fibroblasts; PHEK: psoriatic human epidermal keratinocytes; RHE: reconstructed human epidermis; FT: full-thickness; – stand for unknown.
Note: Further information is available on the company’s website: www.biopta.com; www.ateralabs.com; www.straticell.com; www.bioalternatives.com; www.mattek.com.
Development of FT skin equivalents
Generation of normal FT skin equivalents by using different matrices
In order to represent the major features of skin biology, 3D organotypic co-cultures have been developed. These FT equivalents feature a functional dermal matrix, resembling the native ECM, and thereby stimulating the development of a fully differentiated epidermis by the KC. The effects of fibroblast-keratinocyte crosstalk in skin equivalents have been demonstrated previously, including influences in cell proliferation and secretion of pro-inflammatory cytokines.43–47 Moreover, dermal fibroblasts have been found to increase the resistance of KC to toxic compounds.48 As a consequence, FT models are more frequently and adequately used in toxicological studies compared to epidermal models.
In order to generate FT skin equivalents, four different matrices can be used. The different matrix types as well as their usage in the creation of normal FT skin equivalents are discussed below.
Firstly, similar to epidermal equivalents, porous membranes can be used to co-culture fibroblasts and KC in a 3D manner, each on opposite sides of the membrane.43,49
Secondly, fibroblast-containing protein scaffolds can be used as dermal compartment.29 Dermal matrixes consisting of contracted collagen with incorporated dermal fibroblasts have been used in the past to generate skin grafts.50,51 Later on, fibroblast-containing collagen gels functioned as dermal equivalents to create skin equivalent tissue of FT.52,53
This collagen model offers advantages in biocompatibility and cell adhesion; however, collagen possesses poor mechanical strength.54 Moreover, collagen-based models have a limited lifespan.55 More stable dermal equivalents can be obtained by mechanical stabilization using synthetic polymers or scaffold enforcement.56,57 For the latter, the commercial Hyalograft-3D has been used previously, a fleece-like nonwoven fibrous scaffold containing modified hyaluronic acid fibers. By introducing fibroblasts as fibrin gel suspension into the scaffold, a dermis-type matrix is created on which KC can proliferate and differentiate for at least 12 weeks.55,58
Thirdly, a dead DED is able to provide a dermal matrix for KC to form a morphologically normal stratified and keratinized epidermis.59,60 DED is generally formed using abdominal skin from donors who had undergone cosmetic reduction surgery. Skin is exposed to high temperature to kill the cells, after which the epidermis is removed and the dermal part is used as dermal substitutes in FT models. A DED-based model allows cell culture for four to five weeks at air exposure.61 Although this model lacks living fibroblasts and is thus deficient in producing ECM components and growth factors, no principal morphological differences were reported when using DED compared to collagen-based models.62,63 A possible explanation for this observation could be that essential growth factors present in the enriched culture medium overpowers the endogenous production by dermal fibroblasts.64 The influence of some relevant growth factors on the formation of normal epithelium was evaluated using adult NHEK seeded onto DED in chemically defined medium, free of serum or bovine pituitary extract. A combination of 5 ng/ml of KGF (keratinocyte growth factor) and 2 ng/ml EGF in the medium during air-liquid exposure was optimal to obtain a normal morphology of the epithelium, associated with normal expression of K10 and no expression of the psoriasis-associated proteins SKALP/elafin, hBD-2 or K16.61 In parallel, the use of adult or foreskin NHEK was compared. Under the conditions as described above, substitutes containing foreskin KC displayed parakeratosis and expressed SKALP/elafin. The faster proliferation rate of neonatal foreskin KCs, as compared to adult KCs, might explain partially this phenomenon. Therefore, in this particular normal DED model, adult KC were preferred over foreskin KC to generate constructs with a stratification and protein expression profile similar to normal human epidermis.
Fleischmajer et al.65 introduced a different methodology which is based on the capacity of fibroblasts to synthesize, secrete, and organize their own connective tissue matrix in vitro. Briefly, cell sheets are formed in vitro by fibroblasts that have created their own ECM. A dermal tissue is obtained by stacking two sheets together upon which KC can be seeded to eventually form an epidermal layer by exposure to air. This tissue-engineering procedure formed the basis for the self-assembly model which was successfully used to generate normal reconstructed skin with good epidermal morphogenesis and differentiation.66–69 In addition, this model was also shown to be useful in examining drug permeation and metabolism.66,70 Pictures illustrating the four different matrices, as described above, are provided in Figure 1.
Analogous to KC, it has recently become clear that the morphogenesis of skin substitutes is dependent on the age, source and passage of fibroblasts.71,72 Cells derived from young donors are preferred, although imperfections in (epi-)dermal structure and composition when using over aged cells could be improved by nutrient supplementation of the medium.72 In order to improve fibroblast survival, FT models are mainly cultured in the presence of serum. Serum is, however, a natural product that can differ in composition and contains bioactive factors that could interfere with physiological interactions, and thus alter the biological outcome of the model. Defined medium conditions, eliminating ill-defined medium supplements, are therefore desired and sought after.53,73,74 CELLnTEC is such a company that develops defined growth media eliminating serum and even antibiotics.75
Generation of psoriatic FT skin equivalents
By using the simple membrane model, Krueger and Jorgensen43 were the first to assess the influence of psoriatic fibroblasts both from involved and uninvolved sites, on normal KC. Interestingly, fibroblast from psoriatic patients could induce a psoriasiform phenotype in normal KC via the secretion of soluble factors. The ability of psoriatic (involved and uninvolved) fibroblasts to influence epidermal differentiation and cytokine expression was later confirmed in the collagen model.76 Barker et al.77 characterized a collagen-based model of pathological skin substitutes, combining both psoriatic (involved and uninvolved) KC and fibroblasts.77 The model was compared to substitutes made of normal cells. Between all cultures, regardless of cell source, no obvious differences were observed in epidermal thickness, and none of them showed the psoriasiform morphology. However, psoriatic models, both involved and uninvolved, showed higher proliferation rates (based on increased Ki-67 positive cells after counting) and increased expression of the pro-inflammatory cytokines TNF-α, IL-8 and IFN-γ. These results indicate that intrinsic psoriasis characteristics can partially be preserved in culture. A similar approach was used by Jean et al.78 Using the self-assembly method, four different skin models were produced by combining healthy and/or psoriatic cells. Data indicated, among other things, an increased epidermal thickness and cell proliferation (i.e. elevated Ki-67 positive cells in the basal layer), overexpression of involucrin, and underexpression of filaggrin, loricrin and K10 when using psoriatic KC (Figure 1(b)).
Although diseased cells have the intrinsic capacity to form the psoriasis phenotype, their use is limited by the scarcity and heterogeneity of patient-derived cells. Therefore, studies are focusing on the direction of healthy cells to a diseased state using inflammatory cytokines, similar to the in vitro monolayer stimulation. Cytokines previously identified as players in the pathology of psoriasis have been proven to influence the development of skin equivalents.
For example, various combinations of IL-1α, TNF-α and IL-6 were used to stimulate normal KC in a DED set-up.61 Treatment of the skin constructs with any of the stimuli induced the expression of SKALP/elafin and/or hBD-2. Combining all three cytokines provided the highest induction of these markers, together with an increased expression of K16 and slightly decreased K10 expression (Figure 1(c)). Moreover, the effect of IL-22 on the skin equivalents was examined. IL-22 generates a dose-dependent induction of hBD-2 protein expression, without notable changes on the epidermal morphology or cellular proliferation.61 IL-22 was also used in an attempt to modify the phenotype of the Phenion FT and Labskin (Innovenn) FT model from normal to lesional psoriatic skin.79,80 In both cases, the models were validated by the use of an anti-psoriasis drug (see Table 2).
Table 2.
Overview of applications using psoriasis(-induced) skin substitutes
| Matrix | Cell type | Cytokine treatment | Usage | Ref |
|---|---|---|---|---|
| A. Commercial | ||||
| Membranes | ||||
| • SkinEthic RHE from Episkin | NHEK | IL-22 (20 ng/ml) | Studying the role of IL-22 in inflammatory skin processes | 40 |
| • RHE from BIOalternatives | NHEK | mix of IL-17, IL-22 and TNF-α (3 ng/ml each) | Studying the role of IL-22, IL-17 or TNF-α in inflammatory skin processes | 41 |
| • EpiDerm from MatTek | NHEK | IL-20 subfamily members (20 ng/ml each) | Studying the role of IL-20 subfamily members in the immunopathology of psoriasis | 42 |
| • EpiScreen from CYTOO | NHEK | EpiScreen cytokine mix | Psoriasis β-defensin-2 rescue by all-trans retinoic acid or tazarotene | 81 |
| Collagen | ||||
| • Phenion FT | NHEK + NHDF | IL-22 (10 ng/ml) | Drug testing: Calcipotriol | 79 |
| • MatTek FT | NHEK + NHDF | IL-17 or IL-22 (200 ng/ml each) | Inflammatory effect of Th17 cytokines | 82 |
| IL-17 or IL-22 (200 ng/ml) or IFN- γ (20 ng/ml) | Mimicking T cells useful for testing anti-IL-17 agents | 83 | ||
| • MatTek FT | NHEK + PHDF | MatTek cytokine mix | Drug testing: PTTC, calcipotriol, IL-4, coal tar, psoriasin gel, delphinidin | 84–88 |
| MatTek cytokine mix | Gene therapy: siRNA against DEFB4 or IL-6 | 22,89 | ||
| IL-17 and IFN-γ | Drug testing: determine surrogate markers for anti-IL17 drugs | 90 | ||
| Fibrin | ||||
| • Labskin from Innovenn | NHEK + NHDF | IL-22 (10 ng/ml) | Drug testing: acetretin and analysis by MALDI-MSI | 80 |
| B. In-house. | ||||
| Matrix |
Cell type |
Pre-treatment |
Usage/drug tested |
Ref |
| Membrane | NHEK + PHDF | None | Role of fibroblasts in the creation of epidermal psoriatic features | 43 |
| Collagen | NHEK + PHDF | None None | Role of fibroblasts in epidermal differentiation and cytokine expression Role of NGF in skin regeneration, BM formation and nerve migration | 76 91 |
| DED | NHEK | IL-1α (10 ng/ml), TNF-α (5 ng/ml) and IL-6 (5 ng/ml) | Drug testing: retinoic acid Modeling of in vivo epidermal β-defensin-2 concentrations | 61 92 |
| NHEK + NHDF | Putrescine (2 mM) | Epithelial induction of early-stage neovascularization | 93 | |
| Self-assembly | PHEK + PHDF | TNF-α (5 ng/ml), IL-1α (10 ng/ml), IL6 (5 ng/ml) and IL17A (10 ng/ml) | Study of psoriasis transcriptome and mimicking an immune effect (T cells) useful for testing of anti-psoriasis drugs | 94 |
| PHEK + N or PHDF | None | Drug testing: retinoic acid | 95 | |
BM: basal membrane; MALDI-MSI: Matrix-assisted laser desorption ionization mass spectrometry imaging; NGF: nerve growth factor; NHDF: normal human dermal fibroblasts; NHEK: normal human epidermal keratinocytes; PHDF: psoriatic human dermal fibroblasts; PTTC: 3,5-di-tert-butyl-4-hydroxyhydrocinnamate
Although the commercial Labskin model was used, one should be aware of the fact that the epidermal morphology of the model (±IL-22), as shown in the above-cited paper, was of poor quality and their report of acanthosis was solely based on epidermal and stratum corneum thickness. Stainings for the proliferation marker Ki-67 were lacking. This was unfortunately also the case for the IL-22-treated Phenion FT model. Next, the EpiDermFT model of MatTek was used by Nograles et al.82 to study the effect of IL-17 and IL-22 on genes expressed by KC and on skin morphology. For an unspecified reason, the authors used a high concentration of both cytokines, namely 200 ng/ml, which might put into question the biological relevance of their usage. In contrast to IL-17, the authors claimed that IL-22 treatment resulted not only in epidermal hyperplasia and hypogranulosis but also in parakeratosis, downward epidermal projections and acanthosis within four days of treatment. However, no Ki-67 stainings were performed in order to prove the presence of hyperproliferation, a typical hallmark of psoriasis but very often lacking in psoriasis reconstructed skins. If the described acanthosis in this model is mainly caused by hyperplasia (a phenomenon often seen with IL-22) then it is not physiologically relevant for psoriasis as in this disease, the acanthosis is a consequence of the hyperproliferation.
Interestingly, addition of a panel of inflammatory cytokines (although not specified which ones) was also successfully applied by MatTek in order to generate a commercially-available 3D model of psoriasis.96 Advantages of this psoriasis model are the full inspection on its quality and the increased expression, both at the mRNA and protein level, of many psoriasis-associated markers (Figure 1(d)). In addition, this psoriasis tissue model is characterized by increased basal cell proliferation as evidenced by (enhanced) Ki-67 positivity and shows an elevated release of psoriasis-specific cytokines as compared to normal skin models. As such, the MatTek psoriasis model is of high value to study the efficacy and safety of therapeutic compounds, and to gain more insights into biological processes associated with psoriasis ontology.
Generation of psoriatic skin equivalents containing or mimicking immune cells
With the discovery that cytokines have the ability to influence skin substitutes made from healthy KC and fibroblasts, incorporation of immune cells in 3D skin equivalents became a subject of intense study. Few hurdles are, however, hindering immune cell incorporated models, including the uniform application of the cells, their migration in the ECM, and the medium requirements for co-culture.64 The first model of HSE populated with immune cells was presented by van den Bogaard et al.97 Briefly, allogeneic CD4 + T cells were stimulated using anti-CD3/CD28 mAb-coated beads and were seeded underneath fully developed DED skin equivalents. After two days, activated CD4 + T cells migrated towards the epidermis and pro-inflammatory cytokine and chemokine production was observed, including IL-6, IL-8, IL-23 and CXCL10. Moreover, the expression of psoriasis-associated proteins (i.e. hBD-2, K16, SKALP/elafin) was induced and terminal differentiation process was disturbed. However, no signs of keratinocyte hyperproliferation (based on Ki-67 expression at the mRNA and protein level), acanthosis or hyperkeratosis were observed. Similar induction of the psoriasis phenotype was observed when using Th1- and Th17-polarized T cells. In contrast to the monolayer co-culture, the authors showed that direct contact is not absolutely necessary for crosstalk between the T cells and KC, as indirect contact induced the activation of KC to produce chemokines.97
It is obvious that 3D skin equivalents containing immune cells would be very interesting to study the functions of different T cell subsets and cytokines in psoriasis. However, making such models is very difficult and challenging as some technical aspects (such as those described above) need to be overcome. To avoid this complexity, a few groups investigated the addition of cytokine supplements to existing skin models in order to compensate for the absence of immune cells in these models. For example, Chiricozzi et al.83 added IL-17 to MatTek’s EpiDermFT model and examined its effect on gene expression level. As the obtained gene expression profile resembled very closely the one of psoriasis, the authors concluded that this model could be useful to investigate the effects of anti-IL-17 agents.
The group of Pouliot exposed their psoriatic self-assembly model to a mix of cytokines (IL-1a, IL-6, IL-17A and TNF-α).94 Gene expression analysis of this model versus the non-treated psoriatic skin model revealed similar transcriptome alterations to those found in psoriatic skin in vivo. Therefore, this model might be regarded as an interesting biological tool to study the pathophysiology of psoriasis more in depth, without the need of incorporating T cells.
Obviously, the addition of pro-inflammatory cytokines to reconstructed skin models aiming to mimic as much as possible the physiological conditions of in vivo psoriatic skin remains after all an artificial set-up. Consequently, the biological relevance of such models and the obtained scientific results might be disputed. It is up to the researcher to critically interpret the data and, where possible, observations or outcome of drugs should be further explored in clinical trials.
Usage of HSE
Since the early 1990s, commercial RHEs of normal skin have been developed in addition to healthy FT skin equivalents such as EpiDermFT (MatTek), StrataTest (Stratatech), Phenion FT (Phenion), Labskin (Innovenn) and Biomimiq’s fibroblast-derived matrix (FDM) and full-thickness model (FTM). The offer in commercial psoriasis 3D skin models is however limited (Table 1). Interestingly, psoriatic RHE’s as well as MatTek’s FT psoriasis model can be used in general to test the efficacy of pharmaceutical or cosmetic formulations for the treatment of psoriatic symptoms, e.g. hydration level, anti-inflammatory activity or in some cases hyperproliferation of KC. The models also provide the opportunity to apply a wide range of liquid and solid substances topically onto the skin equivalents. Additionally, test substances can be applied to the culture medium simulating a systemic application. As such they are ideal models for anti-psoriasis drug screening. Some drugs that have been tested in these models are, but not limited to, calcipotriol and delphinidin (see also Table 2(a)).79,80,84–88 As mentioned before, psoriasis skin equivalents are often generated by the addition of specific cytokines making them ideal models to study the role of these cytokines in the immunopathology of psoriasis.40–42,82 Additionally, other groups use cytokines, such as IL-17 or cytokine combinations, to substitute for T cell subsets making them suitable models to test anti-IL17 agents83,90 or anti-psoriasis drugs in general.94
Interestingly, our group and Depieri et al.89 used the MatTek’s FT psoriasis model successfully to target respectively the DEFB4 and IL-6 gene by topical delivery of siRNA molecules.22 Recently, a new (commercial) in vitro psoriatic skin model (EpiScreen model) was developed by the French company CYTOO.81 Although this model could offer great perspectives towards the screening for novel anti-psoriasis drugs at high throughput level, further validation is warranted.
In addition to these commercially available models, some researchers used in-house developed models to study for example the role of fibroblasts in the development of epidermal psoriatic features43,76 or the role of nerve growth factor on skin regeneration, basement membrane formation and nerve migration.91 Jean et al.95 used their psoriasis self-assembly model to study the effects of retinoic acid on keratinocyte proliferation and differentiation. However, the shown immunofluorescence images have to be interpret with caution as they differ partially in quality and light intensity, especially since no software-based quantification of the staining intensities was performed. Cell proliferation, however, was analyzed by counting Ki-67 positive cells (no images shown). These applications, among others, are summarized in Table 2(b).
Discussion
For decades, researchers have put efforts in the development of disease models, such as those for psoriasis, as they are of priceless value for testing novel candidate therapeutic drugs. One of the most commonly used models for psoriasis is the mouse model, which holds advantages over in vitro cultured cells, short-term ex vivo skin explants and in vitro reconstructed skins. An overview of the pros and cons of each model is given in Table 3. However, none of the existing mouse models shows all the features of psoriasis.98 Taking this into account together with ethical considerations of animal experiments, the need for different models, that mimic the human psoriasis pathology more closely, was obvious. Based on the knowledge of existing normal (healthy) reconstructed skin models, several psoriasis skin equivalents were created. These models were histologically examined and very often the expression profile of differentiation markers and their metabolic activity was examined. Epidermal psoriasis models (RHE) are mostly generated by companies after cytokine stimulation of NHEK cultured on synthetic membranes (Table 1). This inflammatory cytokine mix is, however, often unspecified. Although these models were promoted to test the efficacy of pharmaceutical compounds (topical and systemic) for the treatment of psoriasis, their usage is limited in practice. This could be due to the rather poor histological characterization of these models, complete lack of information about possible hyperproliferation of KC and/or the absence of a dermal part.
Table 3.
Overview of the different types of psoriasis models with pros and cons, and their most current applications
| Model | Usage/application | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Monolayers • Pso-induced NHEK | To investigate the role of normal or pathological HEK Drug screening Gene silencing | Large number of cells available High-throughput screening Downscaled | Not fully validated Not all psoriasis markers present No cell–cell interactions No skin morphology | 17,18,21,22 |
| Co-cultures • KCs + FBs • PHEK + T-cells | To validate the specific roles of the different cell types Deciphering the mechanisms of cell interactions Cytokine/chemokine production studies | Cell-cell interaction | No skin morphology | 23,24 |
| Ex vivo skin explants | Drug screening Transdermal drug delivery To study biological processes or disease states Migration of different cell types As co-culture model to study cell-cell interactions | Complete skin structure and pathophysiological profile at harvest | Limited skin availability and donor variability Stimulation may be required to maintain the disease activity Loss of vascular and nervous system Short term culture | 99 |
| Psoriasis mouse models • Spontaneous • Xenotransplantation • Transgenic | Studies of local pathogenic events Systemic drug responses | Many well-characterized models exist Relevant to study the role of different cell types. Study of systemic responses | Presence of immune cell/reaction is model dependent – limited psoriasiform phenotype High costs Ethical considerations | 15 |
| RHE skin equivalents | Therapeutic use Drug screening Cytokine studies | Validated models available High degree of quality control | Incomplete skin morphology Inferior barrier function Optimized for skin irritation and corrosion assays No systemic response | 99,100 |
| FT skin equivalents | Skin physiology and biology studies Drug screening Transdermal drug delivery Therapeutic use Gene silencing Cytokine studies | Validated models available Controllable cellular composition Improved barrier function compared to RHE Cell-cell interaction DED: incorporation of immune cells possible Prolonged culturing time compared to ex vivo skin explants | Collagen-based FT: expensive to buy or make No systemic response Often lack of hyperproliferation | 22,99,101 |
Psoriasis skin equivalents composed of two (or more) different cell types became more interesting as they allow communication between different cell types and thereby mimic the in vivo situation more closely (Table 3). The use of normal versus psoriatic (involved and uninvolved) fibroblasts and/or KC in FT equivalents influences the epidermal differentiation and cytokine expression, as shown in FTs using collagen as matrix77 or using the self-assembly method.78 Both systems revealed that cells derived from psoriatic patients could partially preserve their psoriatic characteristics in culture and establish an in vitro FT psoriasis skin equivalent even with enhanced cell proliferation, as shown by Ki-67 positivity. However, this approach was not copied by other research groups probably by the scarcity, limited size and heterogeneity of psoriasis skin biopsies. As a consequence, researchers introduced the creation of psoriasis skin models by cytokine stimulation of normal DED-based FT skin equivalents.61 This in-house DED model seemed also not further used by other research groups. This might be explained by the lack of typical histological psoriatic features and absence of hyperproliferation, despite the expression of typical psoriasis markers.
A few years later, this cytokine stimulation approach was also applied to protein-based models.79,80,82,83 Remarkably, researchers did not use in-house reconstructed skin models but applied this approach (so far) only to commercially available normal collagen-based FT skin models.82,83 In parallel, a fully commercially available full-thickness psoriasis skin model (SOR-300-FT) was established by MatTek.96 While all these models expressed multiple psoriatic disease markers such as hBD-2, SKALP/elafin and/or S100A7, and demonstrated typical psoriatic characteristics, such as epidermal hyperplasia and hypogranulosis, it was only in the psoriasis MatTek model that hyperproliferation of the keratinocyte layer (leading to acanthosis) was clearly present, although with absence of elongated rete ridges typically for psoriasis. The value of commercial full-thickness psoriasis skin models is illustrated by its applications, namely used in drug testing, cytokine studies and gene therapy by applying siRNA molecules (Table 2(a)). As MatTek models are fully validated and standardized, they circumvent the lack of reproducibility and variability often observed with in-house made models, aspects which are often minimalized in published literature. On the other hand, these models are very expensive and do not offer the possibility to incorporate genetically altered cells in order to investigate the role of a single gene in the psoriasis pathology. Also, immune cells are lacking in commercial FT psoriasis skin models. Researchers are trying to adapt their existing psoriasis skin models in order to include immune cells or mimic their function, which would allow examining the role of different T cell subsets or cytokines in the psoriasis pathology. The current lack of immune cells in almost all published models hindered the testing of therapeutic drugs known or assumed to work solely on T cells. This might explain the still limited number of applications of psoriasis(-induced) skin substitutes (Table 2). Therefore, it is worthwhile to further explore the incorporation of immune cells (and/or other cells) in human psoriatic skin equivalents as they will match even more psoriasis biology. Indeed, any (psoriasis-specific) cell type102 that is absent in a certain model can influence the disease in a great extent. Here as well, one could learn from previously performed experiments with healthy skin equivalents. In 2012 for example, a tri-layer model was developed by incorporating a viable layer of subcutaneous adipose tissue.103 Characterization of the skin part of this model revealed that the proliferation and differentiation capacity of the epidermal layer was highest through day 9, then decreased in time to remain stable at days 11 to 18. Additionally, endothelial cells incorporated in the adipose tissue were no longer detectable at the end of the study. Recently, the incorporation of microvascular and lymphatic endothelial cells in skin substitutes was successfully performed with the creation of blood and lymphatic capillaries.104,105
To conclude, it is clear that different types of psoriasis skin equivalents (commercial or in-house) exist having an epidermal compartment combined or not with a dermal part. The present review demonstrates that none of them match in vivo psoriatic skin lesions completely. Some of the published (or commercially available) in vitro psoriatic skin models are even poorly histologically characterized and describe often the presence of acanthosis (based on hyperplasia), while the real cause of it in psoriasis, namely hyperproliferation (or the proof of hyperproliferation based on Ki-67 stainings) is lacking. As a consequence, the MatTek FT psoriasis model is in our opinion the best characterized model, and therefore mostly used at present (see also Table 2(a)).
However, choosing the right psoriasis skin equivalent for a certain experiment may also depend on the type of research questions and/or therapeutic drugs to be tested (for example toxicity tests versus permeability tests or examination of drugs targeting mainly KC or immune cells). Although the developed psoriasis skin equivalents have been proven to be interesting biological tools for anti-psoriasis drug screening and to elucidate the psoriasis pathology, the road for further improvement is still open. Major (technical) challenges lie in the incorporation of immune cell types, such as specific T cell subtypes or DC, or even in the addition of a hypodermis. Eventually, this could lead to a more physiological relevant psoriasis model augmenting its usage, and perhaps discarding animal testing.
Acknowledgments
This work was funded by a TGO (transformationeel geneeskundig onderzoek) grant with numbers 120828 and 120829 from VLAIO (Flanders Innovation & Entrepreneurship), Belgium).
Authors’ contributions
All authors participated in review of the manuscript, and ED, AR and MVG wrote the manuscript. MVG verified that all individuals who made contributions to this manuscript are included as authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet 1998; 7: 1537–45. [DOI] [PubMed] [Google Scholar]
- 2.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496–509. [DOI] [PubMed] [Google Scholar]
- 3.Iizuka H, Takahashi H, Honma M, Ishida-Yamamoto A. Unique keratinization process in psoriasis: late differentiation markers are abolished because of the premature cell death. J Dermatol 2004; 31: 271–6. [DOI] [PubMed] [Google Scholar]
- 4.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature 2007; 445: 866–73. [DOI] [PubMed] [Google Scholar]
- 5.Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol 1995; 133: 501–11. [DOI] [PubMed] [Google Scholar]
- 6.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol 1999; 113: 752–9. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res 2000; 292: 519–21. [DOI] [PubMed] [Google Scholar]
- 8.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol 2004; 25: 295–305. [DOI] [PubMed] [Google Scholar]
- 9.Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, Meyer zum Buschenfelde KH, Fleischer B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol 1994; 102: 145–9. [DOI] [PubMed] [Google Scholar]
- 10.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, Lecron JC, Morel F. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol 2007; 150: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, Giustizieri ML, Pacciani V, Mazzotta A, Campione E, Macdonald TT, Chimenti S, Pallone F, Costanzo A, Monteleone G. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med 2009; 15: 1013–5. [DOI] [PubMed] [Google Scholar]
- 12.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010; 130: 1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008; 128: 1207–11. [DOI] [PubMed] [Google Scholar]
- 14.Luan L, Ding Y, Han S, Zhang Z, Liu X. An increased proportion of circulating Th22 and Tc22 cells in psoriasis. Cell Immunol 2014; 290: 196–200. [DOI] [PubMed] [Google Scholar]
- 15.Jean J, Pouliot R. In vivo and in vitro models of psoriasis. In: Eberli D. (ed). Tissue engineering and regenerative medicine: tissue engineering, Croatia: InTech, 2010. [Google Scholar]
- 16.Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol 2010; 6: 704–14. [DOI] [PubMed] [Google Scholar]
- 17.Bracke S, Desmet E, Guerrero-Aspizua S, Tjabringa SG, Schalkwijk J, Van Gele M, Carretero M, Lambert J. Identifying targets for topical RNAi therapeutics in psoriasis: assessment of a new in vitro psoriasis model. Arch Dermatol Res 2013; 305: 501–12. [DOI] [PubMed] [Google Scholar]
- 18.van Ruissen F, de Jongh GJ, Zeeuwen PLJM, van Erp PEJ, Madsen P, Schalkwijk J. Induction of normal and psoriatic phenotypes in submerged keratinocyte cultures. J Cell Physio 1996; 168: 442–52. [DOI] [PubMed] [Google Scholar]
- 19.Amigo M, Schalkwijk J, Olthuis D, De Rosa S, Paya M, Terencio MC, Lamme E. Identification of avarol derivatives as potential antipsoriatic drugs using an in vitro model for keratinocyte growth and differentiation. Life Sci 2006; 79: 2395–404. [DOI] [PubMed] [Google Scholar]
- 20.Pol A, Bergers M, van Ruissen F, Pfundt R, Schalkwijk J. A simple technique for high-throughput screening of drugs that modulate normal and psoriasis-like differentiation in cultured human keratinocytes. Skin Pharmacol Appl Skin Physiol 2002; 15: 252–61. [DOI] [PubMed] [Google Scholar]
- 21.Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One 2016; 11: e0164080–e0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmet E, Bracke S, Forier K, Taevernier L, Stuart MCA, De Spiegeleer B, Raemdonck K, Van Gele M, Lambert J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int J Pharm 2016; 500: 268–74. [DOI] [PubMed] [Google Scholar]
- 23.Saiag P, Coulomb B, Lebreton C, Bell E, Dubertret L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science 1985; 230: 669–72. [DOI] [PubMed] [Google Scholar]
- 24.Martin G, Guerard S, Fortin MM, Rusu D, Soucy J, Poubelle PE, Pouliot R. Pathological crosstalk in vitro between T lymphocytes and lesional keratinocytes in psoriasis: necessity of direct cell-to-cell contact. Lab Invest 2012; 92: 1058–70. [DOI] [PubMed] [Google Scholar]
- 25.Muhr P, Renne J, Schaefer V, Werfel T, Wittmann M. Primary human keratinocytes efficiently induce IL-1-dependent IL-17 in CCR6 + T cells. Exp Dermatol 2010; 19: 1105–7. [DOI] [PubMed] [Google Scholar]
- 26.Renne J, Schafer V, Werfel T, Wittmann M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br J Dermatol 2010; 162: 1198–205. [DOI] [PubMed] [Google Scholar]
- 27.Reus AA, Usta M, Krul CA. The use of ex vivo human skin tissue for genotoxicity testing. Toxicol Appl Pharmacol 2012; 261: 154–63. [DOI] [PubMed] [Google Scholar]
- 28.Sidgwick GP, McGeorge D, Bayat A. Functional testing of topical skin formulations using an optimised ex vivo skin organ culture model. Arch Dermatol Res 2016; 308: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Michniak-Kohn BB. Tissue engineered human skin equivalents. Pharmaceutics 2012; 4: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosdy M, Clauss LC. Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J Invest Dermatol 1990; 95: 409–14. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp 2011; 54: e2937–e2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 2012; 7: 461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975; 6: 331–43. [DOI] [PubMed] [Google Scholar]
- 34.Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, Del Rio M, Barrault CC, Bernard FX, Peschanski M, Baldeschi C, Waksman G. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet 2009; 374: 1745–53. [DOI] [PubMed] [Google Scholar]
- 35.Garcia M, Quintana-Bustamante O, Segovia JC, Bueren J, Martinez-Santamaria L, Guerrero-Aspizua S, Escamez MJ, Del Rio M, Larcher F. Long-term skin regeneration in xenografts from iPSC teratoma-derived human keratinocytes. Exp Dermatol 2016; 25: 736–8. [DOI] [PubMed] [Google Scholar]
- 36.van den Bogaard EH, Rodijk-Olthuis D, Jansen PA, van Vlijmen-Willems IM, van Erp PE, Joosten I, Zeeuwen PL, Schalkwijk J. Rho kinase inhibitor Y-27632 prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng Part A 2012; 18: 1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boelsma E, Verhoeven MC, Ponec M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). J Invest Dermatol 1999; 112: 489–98. [DOI] [PubMed] [Google Scholar]
- 38.Kehe K, Abend M, Kehe K, Ridi R, Peter RU, van Beuningen D. Tissue engineering with HaCaT cells and a fibroblast cell line. Arch Dermatol Res 1999; 291: 600–5. [DOI] [PubMed] [Google Scholar]
- 39.Schoop VM, Mirancea N, Fusenig NE. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol 1999; 112: 343–53. [DOI] [PubMed] [Google Scholar]
- 40.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174: 3695–702. [DOI] [PubMed] [Google Scholar]
- 41.Bernard FX, Morel F, Camus M, Pedretti N, Barrault C, Garnier J, Lecron JC. Keratinocytes under fire of proinflammatory cytokines: bona fide innate immune cells involved in the physiopathology of chronic atopic dermatitis and psoriasis. J Allergy 2012; 2012: 718725–718725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 2007; 178: 2229–40. [DOI] [PubMed] [Google Scholar]
- 43.Krueger GG, Jorgensen CM. Experimental models for psoriasis. J Invest Dermat 1990; 95: S56–8. [DOI] [PubMed] [Google Scholar]
- 44.Konstantinova NV, Duvic M. Effects of fibroblast-keratinocyte interactions on the secretion of interleukin-8. Bull Exp Biol Med 1996; 121: 167–70. [Google Scholar]
- 45.Krueger GG, Jorgensen CM. Defined system to assess the in vitro induction of a psoriasis phenotype on normal keratinocytes by fibroblasts from psoriatic subjects. J Cutaneous Med Surg 1997; 2: 20–5. [Google Scholar]
- 46.Boehnke K, Mirancea N, Pavesio A, Fusenig NE, Boukamp P, Stark HJ. Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol 2007; 86: 731–46. [DOI] [PubMed] [Google Scholar]
- 47.El Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol 2002; 147: 230–43. [DOI] [PubMed] [Google Scholar]
- 48.Sun T, Jackson S, Haycock JW, MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 2006; 122: 372–81. [DOI] [PubMed] [Google Scholar]
- 49.Limat A, Mauri D, Hunziker T. Successful treatment of chronic leg ulcers with epidermal equivalents generated from cultured autologous outer root sheath cells. J Invest Dermatol 1996; 107: 128–35. [DOI] [PubMed] [Google Scholar]
- 50.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981; 211: 1052–54. [DOI] [PubMed] [Google Scholar]
- 51.Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg 1995; 21: 839–43. [DOI] [PubMed] [Google Scholar]
- 52.Smola H, Stark HJ, Thiekotter G, Mirancea N, Krieg T, Fusenig NE. Dynamics of basement membrane formation by keratinocyte-fibroblast interactions in organotypic skin culture. Exp Cell Res 1998; 239: 399–410. [DOI] [PubMed] [Google Scholar]
- 53.Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol 1999; 112: 681–91. [DOI] [PubMed] [Google Scholar]
- 54.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials 2010; 3: 1863–87. [Google Scholar]
- 55.Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, Boukamp P. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. J Investig Dermatol Symp Proc 2006; 11: 93–105. [DOI] [PubMed] [Google Scholar]
- 56.Dai NT, Yeh MK, Chiang CH, Chen KC, Liu TH, Feng AC, Chao LL, Shih CM, Sytwu HK, Chen SL, Chen TM, Adams EF. Human single-donor composite skin substitutes based on collagen and polycaprolactone copolymer. Biochem Biophys Res Commun 2009; 386: 21–5. [DOI] [PubMed] [Google Scholar]
- 57.Ng KW, Hutmacher DW. Reduced contraction of skin equivalent engineered using cell sheets cultured in 3D matrices. Biomaterials 2006; 27: 4591–8. [DOI] [PubMed] [Google Scholar]
- 58.Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur J Cell Biol 2004; 83: 631–45. [DOI] [PubMed] [Google Scholar]
- 59.Régnier M, Pruniéras M, Woodley D. Growth and differentiation of adult human epidermal cells on dermal substrates. Front Matrix Biol 1981; 9: 4–35. [Google Scholar]
- 60.Régnier M, Schweizer J, Michel S, Bailly C, Pruniéras M. Expression of high molecular weight (67K) keratin in human keratinocytes cultured on dead de-epidermized dermis. Experimental Cell Research 1986; 165: 63–72. [DOI] [PubMed] [Google Scholar]
- 61.Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol 2008; 173: 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rikimaru K, Moles JP, Watt FM. Correlation between hyperproliferation and suprabasal integrin expression in human epidermis reconstituted in culture. Exp Dermatol 1997; 6: 214–21. [DOI] [PubMed] [Google Scholar]
- 63.Ponec CM, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra J, Mommaas AM. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol 1997; 109: 348–55. [DOI] [PubMed] [Google Scholar]
- 64.Soboleva AG, Mezentsev A, Zolotorenko A, Bruskin S, Pirusian E. Three-dimensional skin models of psoriasis. Cells Tissues Organs 2014; 199: 301–10. [DOI] [PubMed] [Google Scholar]
- 65.Fleischmajer R, Contard P, Schwartz E, MacDonald ED, 2nd, Jacobs L, 2nd, Sakai LY. Elastin-associated microfibrils (10 nm) in a three-dimensional fibroblast culture. J Invest Dermatol 1991; 97: 638–43. [DOI] [PubMed] [Google Scholar]
- 66.Contard P, Bartel RL, Jacobs L, 2nd, Perlish JS, MacDonald ED, 2nd, Handler L, Cone D, Fleischmajer R. Culturing keratinocytes and fibroblasts in a three-dimensional mesh results in epidermal differentiation and formation of a basal lamina-anchoring zone. J Invest Dermatol 1993; 100: 35–9. [DOI] [PubMed] [Google Scholar]
- 67.Michel M, L’Heureux N, Pouliot R, Xu W, Auger FA, Germain L. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol Anim 1999; 35: 318–26. [DOI] [PubMed] [Google Scholar]
- 68.Pouliot R, Larouche D, Auger FA, Juhasz J, Xu W, Li H, Germain L. Reconstructed human skin produced in vitro and grafted on athymic mice. Transplantation 2002; 73: 1751–7. [DOI] [PubMed] [Google Scholar]
- 69.El Ghalbzouri A, Commandeur S, Rietveld MH, Mulder AA, Willemze R. Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials 2009; 30: 71–8. [DOI] [PubMed] [Google Scholar]
- 70.Slivka SR, Landeen LK, Zeigler F, Zimber MP, Bartel RL. Characterization, barrier function, and drug metabolism of an in vitro skin model. J Invest Dermatol 1993; 100: 40–6. [DOI] [PubMed] [Google Scholar]
- 71.Janson D, Saintigny G, Mahe C, El Ghalbzouri A. Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp Dermatol 2013; 22: 48–53. [DOI] [PubMed] [Google Scholar]
- 72.Lacroix S, Bouez C, Vidal S, Cenizo V, Reymermier C, Justin V, Vicanova J, Damour O. Supplementation with a complex of active nutrients improved dermal and epidermal characteristics in skin equivalents generated from fibroblasts from young or aged donors. Biogerontology 2007; 8: 97–109. [DOI] [PubMed] [Google Scholar]
- 73.Ng W, Ikeda S. Standardized, defined serum-free culture of a human skin equivalent on fibroblast-populated collagen scaffold. Acta Derm Venereol 2011; 91: 387–91. [DOI] [PubMed] [Google Scholar]
- 74.Jean J, Bernard G, Duque-Fernandez A, Auger FA, Pouliot R. Effects of serum-free culture at the air-liquid interface in a human tissue-engineered skin substitute. Tissue Eng Part A 2010; 17: 877–88. [DOI] [PubMed] [Google Scholar]
- 75.CELLnTEC Advanced Cell Systems. Integrated skin cell culture, cellntec.com/products/skin (accessed 1 September 2016).
- 76.Konstantinova NV, Duong DM, Remenyik E, Hazarika P, Chuang A, Duvic M. Interleukin-8 is induced in skin equivalents and is highest in those derived from psoriatic fibroblasts. J Invest Dermatol 1996; 107: 615–21. [DOI] [PubMed] [Google Scholar]
- 77.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, Hutchinson PE, Smith GM, Pringle JH. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol 2004; 123: 892–901. [DOI] [PubMed] [Google Scholar]
- 78.Jean J, Lapointe M, Soucy J, Pouliot R. Development of an in vitro psoriatic skin model by tissue engineering. J Dermatol Sci 2009; 53: 19–25. [DOI] [PubMed] [Google Scholar]
- 79.Rüffer C. Psoriatic in vitro epidermis – a human tissue culture model for testing cosmetical and medical skin care products. H&PC Today 2011; 2: 30–22. [Google Scholar]
- 80.Harvey A, Cole LM, Day R, Bartlett M, Warwick J, Bojar R, Smith D, Cross N, Clench MR. MALDI-MSI for the analysis of a 3D tissue-engineered psoriatic skin model. Proteomics 2016; 16: 1718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young J, Fernandes M, Chapuis V, Duchemin-Pelletier E, Poydenot P. EpiScreenTM: a physiological in vitro skin model to develop predictive cell-based assays, Scottsdale, AZ: Society for Investigative Dermatology Meeting, 2016. [Google Scholar]
- 82.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, Pensabene C, Coats I, Novitskaya I, Lowes MA, Krueger JG. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008; 159: 1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculan J, Cardinale I, Bonifacio KM, Gulati N, Mitsui H, Guttman-Yassky E, Suarez-Farinas M, Krueger JG. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One 2014; 9: e90284–e90284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayehunie S, Hedin C, Landry T, Spratt M, Child M, Wang A, Clark R, Kupper T, Klausner M. Three dimensional (3D) reconstructed psoriatic tissue model, Phoenix, AZ: Society for Investigative Dermatology Meeting, 2011. [Google Scholar]
- 85.Chamcheu JC, Pal HC, Siddiqui IA, Adhami VM, Ayehunie S, Boylan BT, Noubissi FK, Khan N, Syed DN, Elmets CA, Wood GS, Afaq F, Mukhtar H. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol Physiol 2015; 28: 177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gujjar M, Arbiser J, Coulon R, Banga AK. Skin irritation and potential efficacy of a novel compound for localized treatment of psoriasis, San Diego, CA: American Association of Pharmaceutical Scientists Meeting and Exposition, 2014. [Google Scholar]
- 87.MatTek Coorporation. Evaluation of psoriasis drug formulations using a human tissue model of psoriasis, www.mattek.com/application/anti-psoriasis-drug-screening (accessed 11 August 2016).
- 88.Pal HC, Sharma S, Ayehunie S, Afaq F. Delphinidin induces epidermal differentiation and inhibits proliferation and inflammation in a three-dimensional reconstructed skin model of psoriasis. Raleigh, NC: Society for Investigative Dermatology Meeting, 2012.
- 89.Depieri LV, Borgheti-Cardoso LN, Campos PM, Otaguiri KK, Vicentini FT, Lopes LB, Fonseca MJ, Bentley MV. RNAi mediated IL-6 in vitro knockdown in psoriasis skin model with topical siRNA delivery system based on liquid crystalline phase. Eur J Pharm Biopharm 2016; 105: 50–8. [DOI] [PubMed] [Google Scholar]
- 90.Ayehunie S, Landry T, Stevens Z, Armento A, Hayden P, Klausner M. Targeting the IL-17 – CCL20 pathway to screen drug candidates in an organotypic psoriasis tissue model, Scottsdale, AZ: Society for Investigative Dermatology Meeting, 2016. [Google Scholar]
- 91.Konstantinova NV, Lemak NA, Duvic M. Nerve growth factor in normal and psoriatic skin equivalent models. Arch Dermatol Res 1998; 290: 610–4. [DOI] [PubMed] [Google Scholar]
- 92.Jansen PA, Rodijk-Olthuis D, Hollox EJ, Kamsteeg M, Tjabringa GS, de Jongh GJ, van Vlijmen-Willems IM, Bergboer JG, van Rossum MM, de Jong EM, den Heijer M, Evers AW, Bergers M, Armour JA, Zeeuwen PL, Schalkwijk J. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One 2009; 4: e4725–e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krajewska E, Lewis C, Staton C, MacGowan A, MacNeil S. New insights into induction of early-stage neovascularization in an improved tissue-engineered model of psoriasis. J Tissue Eng Regen Med 2011; 5: 363–74. [DOI] [PubMed] [Google Scholar]
- 94.Pouliot-Bérubéˆ C, Zaniolo K, Guérin SL, Pouliot R. Tissue-engineered human psoriatic skin supplemented with cytokines as an in vitro model to study plaque psoriasis. Regen Med 2016; 11: 545–57. [DOI] [PubMed] [Google Scholar]
- 95.Jean J, Soucy J, Pouliot R. Effects of retinoic acid on keratinocyte proliferation and differentiation in a psoriatic skin model. Tissue Eng Part A 2011; 17: 1859–68. [DOI] [PubMed] [Google Scholar]
- 96.Ayehunie S, Hedin C, Landry T, Cataldo A, Spratt M, Clark R, Kupper T, Klausner M. Development and characterization of 3D psoriatic tissue model, Raleigh, NC: Society for Investigative Dermatology meeting, 2012. [Google Scholar]
- 97.van den Bogaard EH, Tjabringa GS, Joosten I, Vonk-Bergers M, van Rijssen E, Tijssen HJ, Erkens M, Schalkwijk J, Koenen HJPM. Crosstalk between keratinocytes and T-cells in a 3D microenvironment: a model to study inflammatory skin diseases. J Invest Dermatol 2014; 134: 719–27. [DOI] [PubMed] [Google Scholar]
- 98.Schön MP. Animal models of psoriasis: a critical appraisal. Exp Dermatol 2008; 17: 703–12. [DOI] [PubMed] [Google Scholar]
- 99.Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev 2014; 69-70: 81–102. [DOI] [PubMed] [Google Scholar]
- 100.Dumont C, Prieto P, Asturiol D, Worth A. Review of the availability of in vitro and in silico methods for assessing dermal bioavailability. Appl In Vitro Toxicol 2015; 1: 147–64. [Google Scholar]
- 101.Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering – in vivo and in vitro applications. Adv Drug Deliv Rev 2011; 63: 352–66. [DOI] [PubMed] [Google Scholar]
- 102.Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR, Nair RP. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol 2010; 130: 1213–26. [DOI] [PubMed] [Google Scholar]
- 103.Bellas E, Seiberg M, Garlick J, Kaplan DL. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol Biosci 2012; 12: 1627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayata RE, Chabaud S, Auger M, Pouliot R. Behaviour of endothelial cells in a tridimensional in vitro environment. Biomed Res Int 2015; 2015: 630461–630461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marino D, Luginbuhl J, Scola S, Meuli M, Reichmann E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med 2014; 6: 221ra14–221ra14. [DOI] [PubMed] [Google Scholar]