Abstract
Central nervous system diseases are among the most disabling in the world. Neuroprotection and brain recovery from either acute or chronic neurodegeneration still represent a challenge in neurology and neurorehabilitation as pharmacology treatments are often insufficiently effective. Conditioning the central nervous system has been proposed as a potential non-pharmacological neuro-therapeutic. Conditioning refers to a procedure by which a potentially deleterious stimulus is applied near to but below the threshold of damage to the organism to increase resistance to the same or even different noxious stimuli given above the threshold of damage. Hypoxic conditioning has been investigated in several cellular and preclinical models and is now recognized as inducing endogenous mechanisms of neuroprotection. Ischemic, traumatic, or chronic neurodegenerative diseases can benefit from hypoxic conditioning strategies aiming at preventing the deleterious consequences or reducing the severity of the pathological condition (preconditioning) or aiming at inducing neuroplasticity and recovery (postconditioning) following central nervous system injury. Hypoxic conditioning can consist in single (sustained) or cyclical (intermittent, interspersed by short period of normoxia) hypoxia stimuli which duration range from few minutes to several hours and that can be repeated over several days or weeks. This mini-review addresses the existing evidence regarding the use of hypoxic conditioning as a potential innovating neuro-therapeutic modality to induce neuroprotection, neuroplasticity and brain recovery. This mini-review also emphasizes issues which remain to be clarified and future researches to be performed in the field.
Impact statement
Neuroprotection and brain recovery from either acute or chronic neurodegeneration still represent a challenge in neurology and neurorehabilitation. Hypoxic conditioning may represent a harmless and efficient non-pharmacological new therapeutic modality in the field of neuroprotection and neuroplasticity, as supported by many preclinical data. Animal studies provide clear evidence for neuroprotection and neuroplasticity induced by hypoxic conditioning in several models of neurological disorders. These studies show improved functional outcomes when hypoxic conditioning is applied and provides important information to translate this intervention to clinical practice. Some studies in humans provide encouraging data regarding the tolerance and therapeutic effects of hypoxic conditioning strategies. The main issues to address in future research include the definition of the appropriate hypoxic dose and pattern of exposure, the determination of relevant physiological biomarkers to assess the effects of the treatment and the evaluation of combined strategies involving hypoxic conditioning and other pharmacological or non-pharmacological treatments.
Keywords: Central nervous system, conditioning, hypoxia, neurobiology, neuroprotection, physiology
Introduction
Central nervous system (CNS) diseases are among the most disabling in the world, as they induce a broad range of chronic deficiencies, encompassing motor, sensitive, cognitive, or speech impairments. The functional and social consequences of these pathologies significantly impact the quality of life of the patients. Neuroprotection and brain recovery from either acute brain injury (i.e. stroke) or chronic neurodegeneration (i.e. dementia) still represent a challenge in neurology and neurorehabilitation as pharmacology treatments are often insufficiently effective.
It is now well established that conditioning the central nervous system can trigger endogenous mechanisms of neuroprotection. Conditioning refers to a procedure by which a potentially deleterious stimulus is applied near to but below the threshold of damage to the organism.1–3 As mechanisms of cell injury, death and repair overlap, inducing endogenous resistance or tolerance to injury or promoting recovery can be induced by several distinct stimuli4 including ischemia, hypothermia, pharmacological agents or hypoxia.2
Preconditioning is defined as the exposure of a system or an organ to the conditioning stimulus before injury onset, to induce tolerance or resistance to the subsequent injury. Postconditioning refers to the application of the conditioning stimulus after injury or damage, to stimulate tissue reparation or neuroplasticity.
The first experimentations of hypoxic preconditioning targeting the brain were performed in the early 60 s. Dahl et al.5 showed that pre-exposure to hypoxia could prolong anoxic survival by preserving brain metabolism. In a study done by Schurr et al.,6 rat hippocampal slices were exposed to a short 5-min anoxia period; 30 min thereafter, the slices were challenged by a longer period of anoxia (10 min). They found that electrical activity was maintained in these slices as opposed to slices without the 5-min anoxia pre-exposure. Although these in vitro data obtained on hippocampal slices suggest that brain tissue could increase its resistance to anoxia, this kind of anoxic stimulus can induce permanent damage and represent a potentially lethal stimulus in vivo for the tissue. Since these seminal experimentations, different modalities of exposure to the hypoxic stimulus have been tested. Sustained hypoxic conditioning refers to exposure to a constant level of hypoxia (for one or several hours for instance), whereas intermittent hypoxic conditioning (IHC) corresponds to cyclical exposure to alternating bouts of hypoxia and normoxia, which duration ranges from a few seconds to several minutes each. Hypoxic conditioning (HC) sessions (either sustained or intermittent) can be repeated over several days or weeks to extend the neuroprotection period.2 The HC stimulus can be either normobaric (by inhaling a gas mixture with reduced dioxygen fraction, i.e. reduced FiO2) or hypobaric (by reducing the atmospheric pressure within specific hypobaric chamber, i.e. simulated altitude).
In this mini-review, the preclinical biochemical and genomic aspects of HC targeting the CNS and their link with brain morphological and functional changes will be first briefly presented and discussed, the reader being referred to previous recent reviews for more detailed information regarding the mechanisms of hypoxic conditioning.7–9 Then, the available clinical data in the field will be specifically highlighted, as a continuum to preclinical data, to address the current evidences for clinical application of HC in patients. From an anatomical and functional point of view, this review will distinguish the effect of HC on the brain and on the brainstem and spinal cord apart. Perspectives for future research in the field will also be highlighted, especially to confirm the clinical applicability of HC strategies for neurological diseases.
Mechanisms of neuroprotection and plasticity induced by hypoxic conditioning: from genomic reprograming to system adaptations
While hypoxia is well recognized as a common underlying mechanism of many pathological conditions, experimental data indicate that exposure to specific doses of hypoxia can trigger endogenous mechanisms of neuroprotection and neuroplasticity in the CNS.1,2,4,7,10
The biochemical and genomic mechanisms of cerebral tolerance and resistance induced by HC have been recently reviewed either for hypoxic preconditioning7,8 or postconditioning.9 A decrease in oxygen supply to the CNS represents the central and key trigger of the mechanisms of adaptation to hypoxia, which are sequentially organized in two distinct phases, depending on their delay of onset regarding exposure to the hypoxic stimulus. The first phase or immediate phase of adaptation to hypoxia occurs within the first few minutes to a few hours following the exposure to hypoxia. This phase results in a neuroprotective state that lasts for a short duration.7,8 The mechanisms underlying this transient neuroprotective state are alterations in ion channel permeability, protein phosphorylation and post-translational modifications.8 Of note, this phase is mainly characterized by an elevation of the intracellular content and stabilization of the transcription factor hypoxia-inducible factor 1 (HIF-1), and more precisely its α-subunit (HIF-1α).7,11 HIF-1α is thus considered as a key regulator of cellular oxygen homeostasis and plays a pivotal role in the occurrence and triggering of the second phase (long term) adaptation to hypoxia through its targeted pro-adaptive genes.7,12,13
The second phase or delayed HC phase requires gene activation and de novo protein synthesis and occurs after a few hours to days following exposure to the hypoxic stimulus.7,8 At this phase, neuroprotection occurs as a result of an inhibition of injury mechanisms and an increase in mechanisms underlying survival and repair.14 The outbreak of this phase is driven by third messengers either inducible (c-Fos, NGFI-A, HIF-1) or ubiquitous (pCREB, NF-κB) acting as transcription factors and implied in the regulation of genome transcription.15,16 The targeted genes are neurotrophins (and mostly the brain-derived neurotrophic factor BDNF), mitochondrial and cytosolic antioxydant enzymes, antiapoptotic factors, erythropoietin (EPO, which has direct neuroprotective effects) and through the up-regulation of the vascular endothelial growth factor (VEGF, implied in neurovascular remodeling).7,9
The precise mechanisms of HC remain not fully understood and some questions remain unresolved, such as the adequate timing of appliance before or after injury onset, and also the optimal hypoxic regimen (dose, sustained/intermittent hypoxia, number of sessions) limiting their applicability and translation from bench to bedside. Moreover, inducing a prolonged state of protection (i.e. beyond the acute protection phase, which occurs immediately following the exposure to the hypoxic stimulus and lasts for some hours or days) is a major concern in HC strategies.
Hypoxic conditioning for brain or spinal cord injuries: lessons from animal models
Preconditioning the brain to focal ischemia
Whether hypoxic preconditioning could achieve neuroprotection to subsequent ischemia and could prevent at least partly structural and functional damages have been studied in rodent models of stroke. Miller et al.17 by exposing adult mice to sustained hypoxia (2 h, FiO2 = 11%) 48 h before a focal ischemia induced by a transient 90-min period of middle cerebral artery occlusion showed that infarct volume was reduced in animals subjected to hypoxia compared to controls (2 h normoxia, FiO2 = 21%). These results suggest a structural neuroprotective effect of sustained hypoxic preconditioning. Bernaudin et al.18 exposed mice either to sustained normobaric hypoxia for different durations (1 h, 3 h or 6 h, FiO2 = 8 %) or normoxia 24 h or 72 h before a focal permanent ischemia. A significantly reduced infarct volume was found (−31%, P < 0.01) compared to the normoxia group when conditioning was performed for a 6-h duration, 24 h before injury. Difference did not reach significance when preconditioning was performed 72 h before injury. This study provided two important findings: (i) 1 h of exposure to a hypoxic stimulus seems to be sufficient to induce tolerance, as no improvement in neuroprotection with an extended exposure to hypoxia (3 or 6 h) was shown and (ii) the delay between exposure to the conditioning stimulus and the occurrence of the subsequent injury is a determining factor, as the neuroprotective effects observed 24 h following exposure to the conditioning stimulus disappeared after a 72-h delay. Interestingly, HC induced an increase in HIF-1α transcription factor expression compared to control animals. This increase started as early as 1 h after the beginning of HC, was maximal at 3 h and maintained after 6 h of hypoxia. Although reduced following reoxygenation, HIF-1α transcription factor expression increase lasted up to 24 h after exposition to hypoxia. In this study, mRNA levels of erythropoietin (EPO) and vascular endothelial growth factor (VEGF), two targeted genes of HIF-1α, were also increased compared to controls until 6 h of hypoxia but decreased or disappeared after 12 or 24 h of reoxygenation, whereas protein levels of EPO and VEGF were still elevated 24 h after reoxygenation. EPO is well recognized as a potent protective agent against ischemic injury since its binding to EPO receptors activates numerous protective signaling pathways such as Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway and the MAPK pathway, all known to confer cytoprotection and neuroprotection against ischemic injury in vitro19 and in vivo.20 VEGF has also proved in vivo and in vitro protective effects against cerebral ischemia due to its angiogenic effect (endothelial cell mitogen, vascular growth and permeability factor) and a direct neuronal action through neurotrophic effects, such as axonal outgrowth and cell survival.21 Taken together, these results show that sustained hypoxic preconditioning can induce structural neuroprotection in the adult mice brain against either transient or permanent focal ischemia, even with only 1 h of preconditioning. This neuroprotection seems induced by modifications in gene expression patterns which then result in de novo protein synthesis. Interestingly, hypoxic conditioning induced changes in protein levels which persisted longer than changes in mRNA levels, suggesting the involvement of mechanisms in addition to changes in gene expression, such as epigenetic adaptations to HC.22 However, neuroprotection was not maintained over time following this type of preconditioning as the effect vanished 72 h after exposure.
As a single sustained hypoxic preconditioning session does not trigger a sustained state of protection, recent studies have been investigating the protective effects of repeated exposures to normobaric hypoxia. Hence, Lin et al.23 exposed female rats either to repeated hypobaric hypoxia (two to four weeks, 15 h/day, barometric pressure of 380 mmHg corresponding to an altitude of 5800 m) or normoxia. Two weeks of preconditioning induced a significant reduction of infarct volume following a transient ischemic occlusion of the right middle cerebral artery and bilateral common carotid arteries. Four weeks of preconditioning potentiated these effects, suggesting a duration-dependent effect of preconditioning. However, the neuroprotective effect of a four-week preconditioning period was limited in time: if the protection was still effective after one week, the effect was abolished three weeks after cessation of hypoxic preconditioning. This reduction in infarct size was paralleled by limited elevation in lipid peroxidation, a biological marker of free radicals’ cell injury. The authors suggested that the reduced lipid peroxidation was a result of a combination of a reduction in free radical formation and an enhancement of antioxydative systems. Pursuing the search for the optimal dose of hypoxia able to induce a sustained state of neuroprotection, Stowe et al.24 showed that HC by repeated hypoxia exposures (nine sessions over two weeks, 2 or 4 h, FiO2 = 8 or 11%) protects against transient focal stroke for eight weeks after the end of the HC intervention, together with reduced post-ischemic inflammation. A series of daily repetitive HC stimuli (12 days, 2 h, FiO2 = 8 or 11%) can induce neuroprotection in the retina that lasts up to four weeks.25 Repetitive HC may therefore be an attractive option to induce a prolonged state of neuroprotection. This seems to be achieved through a change in inflammatory phenotypes, a reduced oxidative stress and an enhancement of antioxydative factors. As hypoxic exposure duration and severity vary between studies, the optimal dose of hypoxia to induce a prolonged state of neuroprotection remains to be determined.
Following brain ischemia: Hypoxic postconditioning induced neuroplasticity
As stroke is an unpredictable event, translating hypoxic preconditioning to clinical practice seems difficult. However, and as stroke is the most disabling medical condition in western countries, developing postconditioning strategies to enhance brain recovery and neuroplasticity is of clinical relevance. One of the potential mechanisms underpinning neuroplasticity induced by hypoxic postconditioning is an increased neurogenesis. Hypoxic postconditioning (two weeks, 4 h a day, hypobaric hypoxia with a simulated altitude of 3000 m and 5000 m) increased neurogenesis in the subventricular zone and dentate gyrus of adult rats, compared to controls exposed to normoxia for the same duration.26 Interestingly, in a rat model of ischemic stroke, hypoxic postconditioning for seven days with moderately reduced inspiratory oxygen fraction (FIO2 = 12%, 4 h per day) has been shown to enhance hippocampal neurogenesis and to reverse spatial learning and memory deficiencies induced by stroke.27 These changes were paralleled by an increased in cFOS, a transcription factor implicated in memory formation. The increased cFOS expression along with changes in neurogenesis seemed to have been mediated by the phosphorylated mitogen-activated protein kinase (pMAPK), a second messenger implicated in learning and memory, activated by an overexpression of HIF-1α in response to hypoxic exposure. These results were supported by a recent study28 where 20 mice were randomized, following a three-vessel occlusion-induced brain ischemia, either to a hypoxia group (n = 10, seven days, 4 h a day, hypobaric hypoxia with a simulated altitude of 5000 m) or a normoxia group (n = 10). Mice exposed to hypoxia showed an enhancement of cognitive functional recovery, in association with an increase of neuronal salvage. Interestingly, and in accordance with the results of preconditioning studies,24 the authors suggested that IHC can accelerate cognitive function recovery by attenuating neuro-inflammation. Thus, lower levels of the inducible isoform of nitric oxide synthase (iNOS), an inflammation factor, were found in the hypoxia group in the border between infarct and normal tissues of the hippocampus.28 Along with changes in the expression of transcription factors and reduced inflammation, the structural and functional effects of hypoxic postconditioning are underpinned by changes in the expression of neurotrophic factors. In rodents, intermittent hypoxic postconditioning (seven days, 4 h a day, FIO2 = 12%), initiated seven days after transient mild cerebral artery occlusion significantly improved functional outcomes regarding spatial learning and memory.29 This was associated with hippocampal neurogenesis and synaptogenesis through an increased BDNF expression and the BDNF/PI3K/AKT pathway. Finally, and as for preconditioning, EPO has been shown to play a pivotal role in the cerebral infarct size reduction provided by chronic intermittent hypoxia postconditioning in mice.30
Hypoxic conditioning to prevent brain functional alterations and chronic neurodegeneration
If acute neurodegeneration induced by brain ischemia is the most studied model in the field of hypoxic conditioning, other studies have focused on the benefits of HC in rodent models of chronic neurodegeneration, psychiatric disease or seizures.
In an experimental rat model of Alzheimer disease, repeated HC (14 days, 4 h per day, simulated altitude of 4000 m) protected cognitive functions by blocking memory degradation assessed 14 days after β-amyloid injection.31 The authors reported that the slowing of memory degradation could be achieved by limited increase in oxidative stress, restricted overproduction of brain nitrite oxide and the absence of injured neurons with pathomorphological changes or dead neurons. If hypoxic conditioning can protect the brain from chronic neurodegeneration, it could also be efficient against psychiatric disease such as depression32,33 or post-stress depressive episodes.34,35 The effect of hypoxic conditioning in epilepsy, a chronic brain dysfunction disease, has also been tested. In a rat model of Kainic acid-induced seizures, either hypoxic pre- (three days, 90 min a day, FIO2 = 9%) or postconditioning (three days, 90 min a day, FIO2 = 9%) was efficient in reducing the number of apoptotic hippocampal cells and in improving cognitive function.36 Interestingly, the combination of pre- and postconditioning was more protective than either pre- or postconditioning alone.
Hence, in addition to acute neurodegeneration induced by ischemic stroke, HC seems to be efficient in inducing neuroprotection in several chronic cerebral pathologies encompassing dementia, psychiatric diseases or seizures. Although HC appears to be a promising nonpharmacological intervention to prevent or treat chronic neurodegeneration, further preclinical studies are required to confirm these preliminary data and to extend our understanding of the neuroprotection mechanisms triggered by HC in the context of chronic cerebral pathologies.
Hypoxic conditioning in animal models of brainstem and spinal cord injuries
The most studied model of HC in brainstem and spinal cord is spinal cord injury (SCI). IHC (7 days, 10 cycles per day of 5 min hypoxia (FIO2 = 10.5%) – 5 min normoxia (FIO2 = 21%)) initiated seven days after a spinal cord hemisection (level C2) improved respiratory motor function (assessed seven days after the hypoxic conditioning intervention) and forelimb motor function (assessed four weeks after the intervention). These improvements in respiratory motor function were achieved through neuroplasticity in uninjured cross-phrenic pathways along with neurochemical changes in motor nuclei (increased levels of BDNF and TrkB). The same neuroplasticity was found for non-respiratory motor pathways.37 Interestingly, the authors also reported safety outcomes, with no hippocampal injury in the mice subjected to daily IHC. Results regarding forelimb motor function improvement are contrasting since another study reported that task-specific training in addition to HC may be required to improve motor function in spinal cord injured rats, introducing the concept of combined therapy.38 This could be the condition to induce non-respiratory motor plasticity in SCI and provides important information for potential clinical applications.
If the precise mechanisms implied in spinal cord plasticity following exposure to intermittent hypoxia remain to be determined,39,40 these preclinical results are encouraging and support their translation in clinical studies to induce respiratory and motor plasticity in humans.
Brain hypoxic conditioning in humans
Stroke
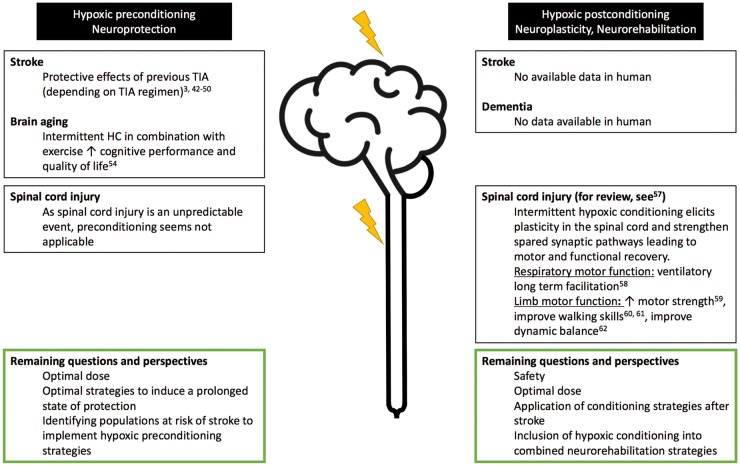
In humans, remarkable clinical data support the ability of HC to enhance brain tolerance to subsequent ischemia (preconditioning), while evidences in favor of brain postconditioning to enhance recovery following stroke are scarce (Figure 1). When considering the potential relevance of repetitive exposure to hypoxia in order to promote endogenous neuroprotection, the clinical model of transient ischemic attacks (TIAs) is of particular interest. Induced by a transient, reversible disruption of cerebral blood flow, TIAs are defined as a brief, transient episode of neurologic dysfunction caused by focal ischemia without acute infarction.41 Although TIAs are now considered as a risk factor for a subsequent stroke,41 converging data suggest their role in preconditioning the brain to subsequent infarction.3 TIAs occurrence may lessen brain injury and enhance recovery following a constituted stroke42–47 and improve thrombolysis efficacy by promoting faster recanalization.44,46 Despite some discrepancies,48–50 these findings suggest an endogenous ischemic neuroprotective preconditioning effect triggered by TIAs. Neuroprotection induced by TIAs is time-dependent (TIAs must be recent, i.e. must occur within a week before stroke onset), duration dependent (TIAs must be brief, < 20 min) and must occur repeatedly over time, to some extent (≤2–3 episodes).3 Moreover, this preconditioning effect seems to be age-dependent as TIAs do not induce protection in the elderly brain (≥65 years). Again, this clinical model of ischemic preconditioning illustrates that neuroprotection mainly relies on TIA regimen, in other words, that conditioning is a matter of dose.
Figure 1.
Hypoxic pre- and post-conditioning in central nervous system injury in humans: available proofs and remaining questions and perspectives (A color version of this figure is available in the online journal)
As previously emphasized, the unpredictable nature of stroke restrains the clinical applicability of hypoxic preconditioning. However, identifying populations at risk of stroke could be helpful to develop and implement hypoxic preconditioning strategies. The efficiency of remote ischemic conditioning (consisting in brief ischemic episodes induced in the lower or upper limb by cyclic cuff inflation and deflation) in the prevention of stroke recurrence in patients with symptomatic intracranial arterial stenosis has been tested with supportive results.51,52
Postconditioning might be a useful paradigm in a rehabilitative perspective but remains to be evaluated in humans. Results of remote ischemic postconditioning in patients at an acute phase of ischemic stroke, alone or as an adjunct therapy to conventional fibrinolysis, are encouraging.53 Since HC seems to be harmless and relatively easy to implement at bedside, it may represent a promising adjunct therapy in stroke patients.
Alzheimer and dementia
The effect of HC in the field of cognitive performances has also been explored in healthy older individuals. In a recent randomized controlled trial,54 34 retired healthy subjects (60–70 years) not physically active and cognitively preserved (MMSE > 27/30), were assigned either to a normoxic group (control group, n = 17, SpO2 = 94–98%) or to a HC group (n = 17, cycles of 10 min hypoxia – 5 min of normoxia for 1 h per session, FiO2 in hypoxia was adjusted to reach a SpO2 = 90% the first two weeks, 85% the third week and 80% in the last three weeks). Both groups performed three 1-h sessions per week for six weeks; 30 min strength-endurance training followed each normoxic/hypoxic session. Combining IHC and exercise training enhanced cognitive performance and quality of life to a greater extent than exercise training alone.54
Based on the promising results in animal models of Alzheimer’s disease submitted to HC,31 further research is needed to evaluate the effect of HC strategies in neurological conditions and its potential to become a preventive or therapeutic tool for these diseases (Figure 1). As physical activity, by triggering beneficial neurovascular adaptations55 and modifying vascular risk factors, HC may be an attractive therapeutic strategy to prevent or slow down brain ageing.56
Hypoxic conditioning in humans with SCI
While HC has not yet been tested in the field of ischemic brain injury, several studies have evaluated its potential interest for SCI (Figure 1). The benefits of IHC have been previously reviewed from a rehabilitative point of view.57 In SCI, IHC elicits plasticity in the spinal cord and strengthen spared synaptic pathways leading to motor and functional recovery in two main motor domains: respiratory motor function and limb motor function.
Respiratory function and respiratory motor plasticity
In cervical SCI, patients can suffer from impaired respiratory motor function. As mentioned above in animal models, IHC could promote recovery of respiratory motor function by promoting ventilatory long-term facilitation. In a controlled trial, 8 individuals with either cervical (n = 6) or thoracic (n = 2) incomplete SCI were exposed to intermittent hypoxia (eight cycles of 2-min hypoxia (FiO2 = 8%) – 2-min normoxia (FiO2 = 21%)) for 10 consecutive days, with controlled end-tidal CO2 level (2 mmHg above resting values).58 A subset of four participants received sham exposure (room air). After each single hypoxic conditioning session, an increase in minute ventilation for 30 min was observed, whereas no change was observed following sham exposure to room air. These results occurred within the first two days of HC and persisted throughout the 10 days of the intervention. The ventilatory long-term facilitation remained present up to 10 days after the intervention in some (n = 2) but not all participants.
Motor function
In a randomized controlled double blind crossover trial,59 13 incomplete SCI subjects performed a single IHC session consisting in 15 cycles of 60–90 s hypoxia (FiO2 = 9) and 60 s of normoxia (FiO2 = 21%). The hypoxic conditioning session was compared to a session in which subjects received sham exposure (i.e. room air). Changes in maximum isometric ankle plantar flexor torque generation were significantly increased by 82 ± 33% immediately after the HC session and were maintained above baseline for more than 90 min, while no change from baseline was observed following the sham session. Increased ankle plantar flexor electromyogram activity was correlated with increased torque (r2 = 0.5, P < 0.001). Muscle strength stayed elevated in some participants for more than 4 h, suggesting that even a single session of HC could promote long-lasting effect, offering the opportunity to implement combined therapy in a rehabilitative perspective. This type of combined intervention has been tested by coupling IHC and gait training. Hayes et al.,60 in a randomized, double blind, placebo-controlled, crossover study, exposed 19 participants with chronic incomplete SCI to either 15 cycles of 90-s hypoxia (FiO2 = 9%) – 60-s normoxia (FiO2 = 21%) or to a sham normoxic exposure (FiO2 = 21%), on five consecutive days, alone or combined with 30 min of over-ground walking 1 h later. In this study, IHC improved both walking speed and endurance, and the impact of HC was enhanced when combined with walking.60 Combined therapy has been further investigated in recent studies. In a randomized, controlled study, incomplete SCI patients (American Spinal Injury Association (ASIA) impairment scale C (less than half of key muscle functions below the single neurological level of injury have a muscle grade ≥ 3) and D (at least half of key muscle functions below the single neurological level of injury having a muscle grade ≥ 3)) were submitted either to IHC (sessions of 15 cycles of 1.5 min hypoxia (FiO2 = 9%) – 1.5 min normoxia (FiO2 = 21%)) or continuous normoxia (FiO2 = 21%, placebo) for five consecutive days, then three sessions per week during three weeks, over the same duration. Both groups benefitted of 45 min body-weight supported gait treadmill training during the five first days of conditioning. Five days of combined therapy increased gait speed and endurance in the HC group, but not timed up and go performance. These improved performances were maintained (gait speed) or enhanced (gait endurance) during the following three weeks of IHC, and up to two weeks after the end of the intervention.61 These results support both the major effect of combined therapy in improving locomotor function and the ability of repeated exposures to intermittent hypoxia to improve the recovery of locomotor function, providing proof of concept for IHC as a novel rehabilitative tool. The same protocol of combined HC improved dynamic, but not standing balance in persons with incomplete SCI and was safe as performances in the domains of visual and verbal memory were intact following the intervention.62 In order to maximize the effects of intermittent hypoxia on motor function recovery, nine adults with chronic incomplete SCI were given in a double blind, single cross-over design, randomized manner, either a single-800 mg dose of ibuprofen or placebo 90 min prior to hypoxic conditioning (one single session of 45 min, cycles of 90-s hypoxia (FiO2 = 9%) interspersed by 60-s normoxia). The authors reported no significant effect of ibuprofen pretreatment, suggesting a strong, independent effect of intermittent hypoxia.63
As no harmful events have been reported in those promising seminal studies, these results support the development of HC strategies to enhance motor and respiratory function in several neurologic diseases.
Perspectives
Although representing a promising therapeutic option in the field of neuroprotection and neuroplasticity, many issues remain to be considered to confirm the protective or therapeutic effects of HC in clinical practice while remaining safe and harmless for the patients (Figure 1). First, the optimal pattern and severity of the hypoxic stimulus (i.e. the hypoxic regimen) to induce neuroprotection and recovery remain to be determined. The respective effects of intermittent versus sustained hypoxic sessions should be clarified.64 While most previous studies defined the level of hypoxia based on the FiO2 or the simulated altitude levels, optimal SpO2 should rather be individually targeted since for a given FiO2 or altitude level, large interindividual differences in SpO2 and therefore in induced neurophysiological responses could be observed. Differences between normobaric and hypobaric hypoxic exposure might also be considered regarding the effect of hypoxic conditioning.65
Second, in order to individualize the optimal hypoxic stimulus which might differ between individuals according to age or pathological conditions for instance, physiological biomarkers need to be determined. Heart rate variability has been recently proposed as a sensitive marker of hypoxic conditioning responses.64 Other biomarkers – either clinical, biological or imaging biomarkers – specific to the CNS responses to HC will need to be determined. Last, the opportunity offered by combined strategies including HC in order to potentiate or extend its effect remained to be explored. While some results suggest that rehabilitative strategies combining HC and physical exercise might be an attractive option, further studies are required to determine the optimal strategy, considering for instance hypoxic exposure and exercise training sequentially or in combination.66 The relevance of combining pharmacological treatment and hypoxic conditioning need also to be considered, aiming at potentiate tolerance and efficiency of treatments.
A promising field in the use of HC in a combined approach is cellular therapies. Preconditioning stem cells may extend the proliferation, survival and induce differentiation of central nervous system precursors.67 As suggested for spinal cord injury,57 combining hypoxic conditioning to transplantation may enhance host-graft integration. The combination of stem cell therapy, an innovative and promising therapy in stroke,68,69 and hypoxic conditioning remains to be explored.
Acknowledgements
We thank the “Fond de Dotation Agir pour les maladies chroniques” for financial support regarding our research in the field of hypoxic conditioning.
Authors’ contributions
All the authors participated in the writing process of the manuscript and revised it critically.
Declaration of Conflicting Interests
The authors wrote this paper within the scope of their medical expertise or academic and affiliated research positions. None of the authors has any conflict of interest to declare.
References
- 1.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 2009; 8: 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S. Hypoxic conditioning as a new therapeutic modality. Front Pediatr 2015; 3: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dezfulian C, Garrett M, Gonzalez NR. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl Stroke Res 2013; 4: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke 2007; 38(2 Suppl): 680–5. [DOI] [PubMed] [Google Scholar]
- 5.Dahl NA, Balfour WM. Prolonged anoxic survival due to anoxia pre-exposure: brain atp, lactate, and pyruvate. Am J Physiol 1964; 207: 452–6. [DOI] [PubMed] [Google Scholar]
- 6.Schurr A, Reid KH, Tseng MT, West C, Rigor BM. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res 1986; 374: 244–8. [DOI] [PubMed] [Google Scholar]
- 7.Rybnikova E, Samoilov M. Current insights into the molecular mechanisms of hypoxic pre- and postconditioning using hypobaric hypoxia. Front Neurosci 2015; 9: 388–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Hafeez A, Noorulla F, Geng X, Shao G, Ren C, Lu G, Zhao H, Ding Y, Ji X. Preconditioning in neuroprotection: from hypoxia to ischemia. Prog Neurobiol. 2017. Epub ahead of print 18 January. DOI: 10.1016/j.pneurobio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetrovoy OV, Rybnikova EA, Samoilov MO. Cerebral mechanisms of hypoxic/ischemic postconditioning. Biochemistry 2017; 82: 392–400. [DOI] [PubMed] [Google Scholar]
- 10.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 2003; 26: 248–54. [DOI] [PubMed] [Google Scholar]
- 11.Lukyanova LD, Sukoyan GV, Kirova YI. Role of proinflammatory factors, nitric oxide, and some parameters of lipid metabolism in the development of immediate adaptation to hypoxia and HIF-1alpha accumulation. Bull Exp Biol Med 2013; 154: 597–601. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009; 24: 97–106. [DOI] [PubMed] [Google Scholar]
- 13.Dengler VL, Galbraith MD, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol 2014; 49: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 2006; 7: 437–48. [DOI] [PubMed] [Google Scholar]
- 15.Rybnikova E, Gluschenko T, Tulkova E, Churilova A, Jaroshevich O, Baranova K, Samoilov M. Preconditioning induces prolonged expression of transcription factors pCREB and NF-kappa B in the neocortex of rats before and following severe hypobaric hypoxia. J Neurochem 2008; 106: 1450–8. [DOI] [PubMed] [Google Scholar]
- 16.Rybnikova E, Glushchenko T, Tyulkova E, Baranova K, Samoilov M. Mild hypobaric hypoxia preconditioning up-regulates expression of transcription factors c-Fos and NGFI-A in rat neocortex and hippocampus. Neurosci Res 2009; 65: 360–6. [DOI] [PubMed] [Google Scholar]
- 17.Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport 2001; 12: 1663–9. [DOI] [PubMed] [Google Scholar]
- 18.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab 2002; 22: 393–403. [DOI] [PubMed] [Google Scholar]
- 19.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci 2002; 22: 10291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 1999; 19: 643–51. [DOI] [PubMed] [Google Scholar]
- 21.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999; 19: 5731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidday JM. Extending injury- and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Front Neurol 2015; 6: 42–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin AM, Dung SW, Chen CF, Chen WH, Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann N Y Acad Sci 2003; 993: 168–78; discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 24.Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol 2011; 69: 975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci 2007; 48: 1735–43. [DOI] [PubMed] [Google Scholar]
- 26.Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 2005; 1055: 1–6. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS One 2011; 6: e24001–e24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao Y, Liu Z, Yan X, Luo C. Effect of intermittent hypoxia on neuro-functional recovery post brain ischemia in mice. J Mol Neurosci 2015; 55: 923–30. [DOI] [PubMed] [Google Scholar]
- 29.Tsai YW, Yang YR, Sun SH, Liang KC, Wang RY. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cereb Blood Flow Metab 2013; 33: 764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, Roussel S, Schumann-Bard P, Bernaudin M. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke 2009; 40: 3349–55. [DOI] [PubMed] [Google Scholar]
- 31.Manukhina EB, Goryacheva AV, Barskov IV, Viktorov IV, Guseva AA, Pshennikova MG, Khomenko IP, Mashina SY, Pokidyshev DA, Malyshev IY. Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer’s disease by adaptation to hypoxia. Neurosci Behav Physiol 2010; 40: 737–43. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 2010; 30: 12653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Vataeva L, et al. Antidepressant-like effects of mild hypoxia preconditioning in the learned helplessness model in rats. Neurosci Lett 2007; 417: 234–9. [DOI] [PubMed] [Google Scholar]
- 34.Rybnikova EA, Samoilov MO, Mironova VI, Tyul’kova EI, Pivina SG, Vataeva LA, Vershinina E, Abritalin E, Kolchev A, Nalivaeva N, Turner AJ, Samoilov M. The possible use of hypoxic preconditioning for the prophylaxis of post-stress depressive episodes. Neurosci Behav Physiol 2008; 38: 721–6. [DOI] [PubMed] [Google Scholar]
- 35.Kushwah N, Jain V, Deep S, Prasad D, Singh SB, Khan N. Neuroprotective role of intermittent hypobaric hypoxia in unpredictable chronic mild stress induced depression in rats. PLoS One 2016; 11: e0149309–e0149309. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Yang Y, Jianhua C, Li L, Yusong G, Jun C, Zhou F, Weiping L. Effect of different mild hypoxia manipulations on kainic acid-induced seizures in the hippocampus of rats. Neurochem Res 2013; 38: 123–32. [DOI] [PubMed] [Google Scholar]
- 37.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 2012; 32: 3591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prosser-Loose EJ, Hassan A, Mitchell GS, Muir GD. Delayed intervention with intermittent hypoxia and task training improves forelimb function in a rat model of cervical spinal injury. J Neurotrauma 2015; 32: 1403–12. [DOI] [PubMed] [Google Scholar]
- 39.Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 2015; 266: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komnenov D, Solarewicz JZ, Afzal F, Nantwi KD, Kuhn DM, Mateika JH. Intermittent hypoxia promotes recovery of respiratory motor function in spinal cord-injured mice depleted of serotonin in the central nervous system. J Appl Physiol (1985) 2016; 121: 545–57. [DOI] [PubMed] [Google Scholar]
- 41.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL, American Heart A, American Stroke Association Stroke C, Council on Cardiovascular S, Anesthesia, Council on Cardiovascular R, Intervention Intervention, Council on Cardiovascular N, Interdisciplinary Council on Peripheral Vascular D. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40: 2276–93. [DOI] [PubMed] [Google Scholar]
- 42.Arboix A, Cabeza N, Garcia-Eroles L, Massons J, Oliveres M, Targa C, Balcells M. Relevance of transient ischemic attack to early neurological recovery after nonlacunar ischemic stroke. Cerebrovasc Dis 2004; 18: 304–11. [DOI] [PubMed] [Google Scholar]
- 43.Moncayo J, de, Freitas GR, Bogousslavsky J, Altieri M, van, Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology 2000; 54: 2089–94. [DOI] [PubMed] [Google Scholar]
- 44.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M, Stroke MRIiASSGotGCN. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke 2004; 35: 616–21. [DOI] [PubMed] [Google Scholar]
- 45.Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhaupl KM. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke 1999; 30: 1851–4. [DOI] [PubMed] [Google Scholar]
- 46.Schaller B. Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevance. Neurosci Lett 2005; 377: 206–11. [DOI] [PubMed] [Google Scholar]
- 47.Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, Davalos A. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol 2003; 54: 811–9. [DOI] [PubMed] [Google Scholar]
- 48.Della, Morte D, Abete P, Gallucci F, Scaglione A, D’Ambrosio D, Gargiulo G, De Rosa G, Dave KR, Lin HW, Cacciatore F, Mazzella F, Uomo G, Rundek T, Perez-Pinzon MA, Rengo F. Transient ischemic attack before nonlacunar ischemic stroke in the elderly. J Stroke Cerebrovasc Dis 2008; 17: 257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoshino T, Mizuno S, Shimizu S, Uchiyama S. Clinical features and functional outcome of stroke after transient ischemic attack. J Stroke Cerebrovasc Dis 2013; 22: 260–6. [DOI] [PubMed] [Google Scholar]
- 50.Johnston SC. Ischemic preconditioning from transient ischemic attacks? Data from the Northern California TIA study. Stroke 2004; 35(11 Suppl 1): 2680–2. [DOI] [PubMed] [Google Scholar]
- 51.Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics 2015; 12: 667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012; 79: 1853–61. [DOI] [PubMed] [Google Scholar]
- 53.Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Botker HE, Ostergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014; 45: 159–67. [DOI] [PubMed] [Google Scholar]
- 54.Schega L, Peter B, Torpel A, Mutschler H, Isermann B, Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology 2013; 59: 316–23. [DOI] [PubMed] [Google Scholar]
- 55.Tarumi T, Zhang R. Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 2014; 5: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manukhina EB, Downey HF, Shi X, Mallet RT. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp Biol Med 2016; 241: 1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 2015; 119: 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 2014; 189: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 2012; 26: 163–72. [DOI] [PubMed] [Google Scholar]
- 60.Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 2014; 82: 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma 2016; 34: 1803–12. [DOI] [PubMed] [Google Scholar]
- 62.Navarrete-Opazo A, Alcayaga J, Testa D, Quinteros AL. Intermittent Hypoxia Does not Elicit Memory Impairment in Spinal Cord Injury Patients. Arch Clin Neuropsychol 2016; 31: 332–42. [DOI] [PubMed] [Google Scholar]
- 63.Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: a pilot study. J Spinal Cord Med. 2016. Epub 9 February. doi: 10.1080/10790268.2016.1142137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chacaroun S, Borowik A, Morrison SA, Baillieul S, Flore P, Doutreleau S, Verges S. Physiological responses to two hypoxic conditioning strategies in healthy subjects. Front Physiol 2017; 7: 675–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coppel J, Hennis P, Gilbert-Kawai E, Grocott MP. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extrem Physiol Med 2015; 4: 2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millet GP, Debevec T, Brocherie F, Malatesta D, Girard O. Therapeutic use of exercising in hypoxia: promises and limitations. Front Physiol 2016; 7: 224–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 2000; 20: 7377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borlongan CV, Jolkkonen J, Detante O. The future of stem cell therapy for stroke rehabilitation. Future Neurol 2015; 10: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moisan A, Favre I, Rome C, De, Fraipont F, Grillon E, Coquery N, Mathieu H, Mayan V, Naegele B, Hommel M, Richard MJ, Barbier EL, Remy C, Detante O. Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplant 2016; 25: 2157–71. [DOI] [PubMed] [Google Scholar]