Abstract
Background:
The “500 rule” has been used extensively to find the insulin to carbohydrate ratio (ICR) for carbohydrate counting (CC). Duration of insulin action (DIA) is often recommended to be set to 4 hours. Data are lacking on validating these routines in young children.
Methods:
ICR was calculated by dividing carbohydrate grams by insulin units. Insulin sensitivity factor (ISF) was defined by the 100 rule (100 divided by total daily insulin dose [TDD]). DIA was set to 3 hours. ICR, ISF, and DIA were adjusted continuously. Data for this retrospective analysis were taken from pump downloads at a routine visit. ICR and ISF were recalculated to rules (ICR/ISF multiplied by TDD).
Results:
A total of 21 prepubertal children aged 7.0 ± 2.3 (mean ± SD), range 2-10 years, with diabetes duration 3.0 ± 1.9, range 0.5-7.7 years, used the pump bolus calculator for CC. HbA1c IFCC (NGSP) was 53 ± 6 mmol/mol (7.0 ± 0.5%). None had experienced severe hypoglycemia (unconsciousness/seizures) since diabetes diagnosis. TDD was 0.7 ± 0.1 U/kg/24 h (range 0.5-1.0), and the percentage basal insulin 38 ± 11%. Median breakfast rule was 211 (Q, quartiles 162;310), and for other meals 434 (Q 301;496). Median ISF rule was 113 (Q 100;128) in the morning, and 120 (Q 104;134) during the rest of the day. DIA was 2.6 ± 0.5 h (range 2-3) and target BG 5.3 ± 0.4 mmol/l (range 5.0-6.0).
Conclusions:
Prepubertal children seem to need more bolus insulin for meals than calculated from the 500 rule, especially at breakfast, but less insulin for corrections than calculated from the 100 rule. Two to 3 hours seems to be the appropriate range for DIA in this age group.
Keywords: type 1 diabetes, children, insulin, insulin pump, carbohydrate counting, HbA1c
The use of continuous subcutaneous insulin infusion (CSII) in young children is increasing in many countries, and is in Sweden currently 60% in preschool children.1 An international consensus group has recommended CSII in the young age group.2 The bolus calculator in the pump gives the individual the opportunity to use features that simplify the calculation of bolus doses, correcting for the current glucose level. Although a meta-analysis from 2013 found limited evidence for the use of carbohydrate counting in adults,3 recent pediatric studies have found possible beneficial effects in decreasing HbA1c, both with CSII4,5 and multiple daily injections (MDI),6 and increasing the percentage of postmeal glucose readings within target.7 Furthermore, learning about carbohydrates and carbohydrate counting can also result in overall increased knowledge regarding other components of the diet, which can lead to other benefits.
The pharmacodynamic effect of 0.2 U/kg of subcutaneously injected insulin aspart was found to last approximately 4 hours in pubertal and 5 hours in prepubertal children in 1 study,8 and similar results were found in another study using 0.15 U/kg.9 If anything, these clamp studies lengthen the duration of insulin action, since the patient is resting in a bed, and the given dose will often have supplied both the basal and bolus insulin effect. To account for this, Buckingham et al kept the basal rate of the pump throughout the study, and they found that there is persistent insulin activity from a 0.1 U/kg bolus of insulin aspart for at least 5 hours following a bolus in children less than 7 years old, similar to adult studies using larger doses of insulin.10 With CSII it is possible to set the duration of insulin action (DIA), which determines how many hours the bolus calculator will regard that a previous dose still will have some blood glucose lowering effect. This remaining amount of insulin (insulin on board, IOB) will be subtracted by the bolus calculator when giving a dose recommendation.
DIA has been recommended to be set to 4-6 hours in adults, with reference to published data.11 However, data on children are lacking.
The 500 rule (500 divided by total daily insulin dose [TDD] of insulin) is often used to find a starting point for the insulin to carbohydrate ratio (ICR), that is, how many grams of carbohydrate 1 unit of insulin covers, and this has been validated in children.7 However, other authors have found this rule to be too conservative in young children.12 The 500 rule may need to be individually adjusted to allow more insulin for breakfast (lower number) and less insulin for a meal in connection with exercise (higher number). For adults, it has been suggested to base the ICR on body weight (BW) and TDD (ICR = 2.8 × BW [lb]/TDD = 6.2 × BW [kg]/TDD).13 The 100 rule (1800 rule for mg/dl) has been used to find the insulin sensitivity factor (ISF), that is, how many mmol/l (or mg/dl) 1 unit of insulin lowers the blood glucose level. ISF equals 100 divided by TDD (1800 divided by TDD for mg/dl). The ISF needs to be individually adapted, for example to allow a larger correction dose at breakfast and a lower during and post-exercise.
From clinical experience, we hypothesized that younger children need more insulin in relation to the amount of carbohydrates in a meal, that is, a lower ICR than calculated from the 500 rule, and that DIA should be shorter than 4 hours.
The aim of this retrospective study was to investigate the DIA setting of prepubertal pumpers, and to determine which ICR and ISF settings that are feasible in well-controlled prepubertal children. Since we evaluated clinical routine practice, and the titration of settings in the bolus calculator were not part of a study protocol, ethical approval was not deemed necessary.
Methods
With the available pharmacodynamics studies in mind, and recommendations from adult pump use,14 we began with 4 h as default setting for DIA in the pumps, but then changed to 3 h as families experienced the bolus calculator not giving enough insulin when IOB was present. ICR, ISF, and DIA were adjusted as needed at each visit. DIA was adjusted according to parents’ experience of the correction effect of a bolus when IOB was present.
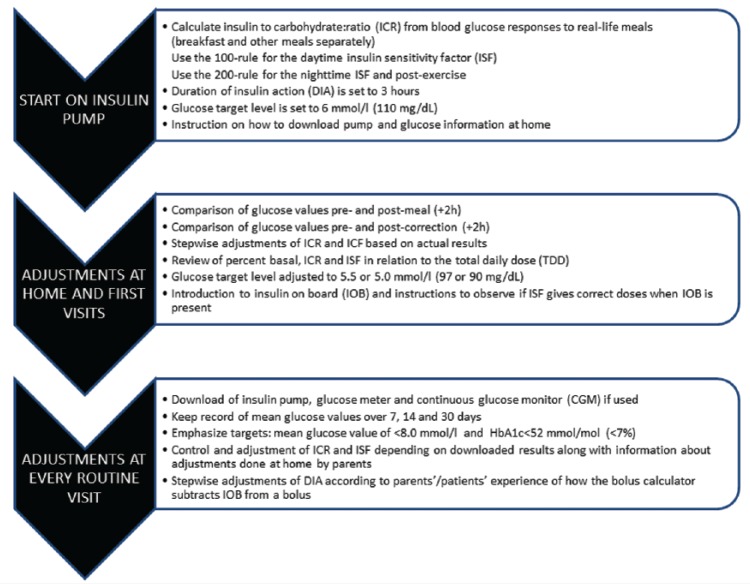
Patients weighed the food and used the carbohydrate content for calculating bolus doses. Fat and protein content were used to determine the glycemic effect of the food, that is, the use of an extended or combined bolus, but not for adding extra insulin to the calculation. ICR was initially calculated from blood glucose responses to real-life meals (breakfast and other meals separately), and ISF from the 100 rule; both were then adjusted according to glucose responses. For the night, we estimated that their insulin sensitivity was increased by 50%, which corresponds to a 200 rule. Families were instructed to give a correction bolus if the glucose level was > 8 mmol/l (145 mg/dl) ≥2 hours after a meal, or in the night, using the bolus calculator to subtract any IOB. The target level was initially set to 6 mmol/l (110 mg/dl), and then lowered to 5.5 or 5.0 mmol/l (97 or 90 mg/dl) as the family got comfortable with the bolus calculator. The ICR and ISF settings were initially adjusted at regular visits by the diabetes team, and later by the families themselves at home. Basal rates were titrated according to glucose levels at night and > 2 hours postprandially during the day. We did not test the basal rates systematically by withdrawing meals, as this is quite difficult to perform in this age group. However, when a meal for some reason was missed or the child slept longer in the morning, we encouraged parents to pay extra attention to the glucose levels and adjust the basal rates accordingly. See Figure 1 for routine procedures. The target HbA1c for all age groups at the clinic was <52 mmol/mol (<7.0%).
Figure 1.
Algorithms of settings used in the bolus calculator at pump start and subsequent follow-up.
After 6-12 months of experience of adjusting ICR, ISF, and DIA incrementally by titration, pumps, glucose meters, and continuous glucose monitors (CGM) were downloaded once at a routine visit, and HbA1c was recorded. ICR and ISF were recalculated to rules (ICR/ISF multiplied by TDD). All downloads were done within a 3-month interval.
HbA1c was measured with DCA Vantage by Siemens (Princeton, NJ, USA), calibrated to IFCC units.
Results
At the time of the study, 41 prepubertal children (age < 13 years) were seen at regular visits. Thirty-five (85%) used CSII and the remainder MDI. Five were excluded due to diabetes duration < 6 months, and 9 did not fully use carbohydrate counting. Twenty-one children aged 7.0 ± 2.3 (mean ± SD) (range 2-10) years and with diabetes duration of 3.0 ± 1.9 (0.5-7.7) years used the pump bolus calculator for carbohydrate counting (CC) and correction boluses. Twelve patients used Animas Vibe and 9 Medtronic Veo (530 G in the United States). Fifteen out of 21 (71%) started pumps and 7/21 (33%) started CC from the onset of diabetes. Their HbA1c was 53 ± 6 mmol/mol (7.0 ± 0.5%), and none had experienced any episodes of diabetic ketoacidosis (DKA) or severe hypoglycemia (SH, with unconsciousness or seizures) since diagnosis of diabetes (data from 64 patient years). Six (29%) used continuous glucose monitoring (CGM) with a mean glucose of 8.9 ± 3.8 mmol/l (160 ± 68 mg/dl) over the last month, the others took 10.1 ± 2.0 plasma glucose (PG) tests/day with a mean PG of 9.1 ± 4.4 mmol/l (164 ± 79 mg/dl). Their TDD was 0.7 ± 0.1 U/kg/24 h (range 0.5-1.0), and their percentage basal insulin was 38 ± 11% (range 17-65%). Thirteen had their highest basal rate between 9 PM and midnight (159 ± 39% of mean total hourly rate), 4 between 6 and 9 PM.
The ICR was 13-42 in the 2-4 y olds (n = 4, median 30), 17-33 in the 5-7 (n = 9, median 20) and 10-60 in the 8- to 10-year-olds (n = 8, median 16). Eighteen (86%) had a lower ICR in the morning (ie, more insulin), and mean ICR was significantly lower both in the morning (13.7 ± 5.4 vs 24.7 ± 12.4, P < .01) and evening (19.5 ± 9.4 vs 24.7 ± 12.4, P < .01) compared to the day (lunch and dinner). The ISF was 8-20 in the 2-4 y olds (median 13.3), 4-10 in the 5-7 (median 6.5) and 3-9 in the 8- to 10-year-olds (median 5). Eighteen out of 21 children had a higher ISF during the night (ie, less insulin).
There was a significant correlation between TDD and both ICR (r = –.48, P = .028 for breakfast, r = –.51, P = .039 for other meals) and ISF (r = –.72, P < .001 in the morning, r = –.72, P < .001 during the day).
When ICR and ISF were recalculated to “rules” (Figure 2), the median breakfast rule was 211 (Q, quartiles 162;310), and for other meals 435 (Q 301;496). The median ISF rule was 113 (Q 100;128), 2034 (1800;2304 for mg/dl) in the morning, 120 (Q 104;134) during the rest of the day and 200 (Q 146;233), 3600 (Q 2628;4194 for mg/dl) during the night (Figure 3).
Figure 2.
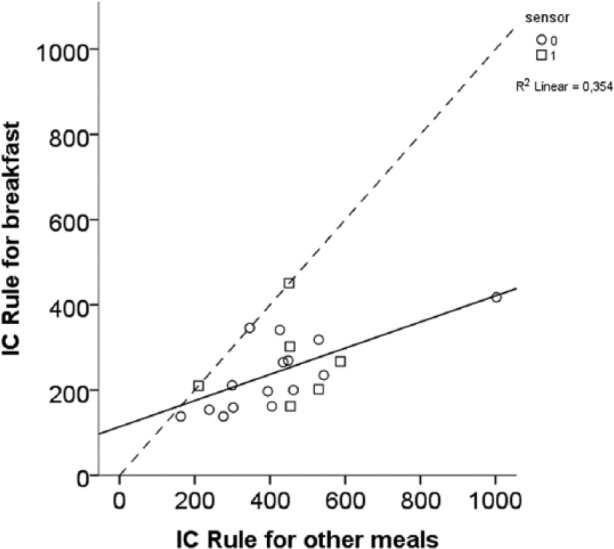
Insulin to carbohydrate ratio (ICR) recalculated to rules. Most children needed more insulin than derived from the 500 and 330 rules, respectively, for both breakfast and other meals (ie, lower ICR). The dashed line represents equal rules for breakfast and other meals.
Figure 3.
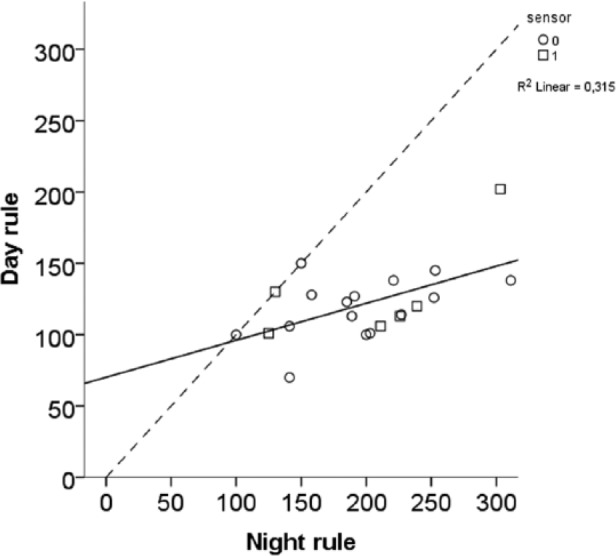
Less insulin than derived from the 100 rule was needed for both the day and night (ie, higher insulin sensitivity factor [ISF]). The dashed line represents equal rules for day and night.
When using BW to account for differences in insulin resistance, there was a significant correlation between calculated ICR and the actual ICR in the pumps (r = .58, P = .06), but the calculated ICR was in all children lower than the actual ICR, on average by a factor of 0.42.
DIA was set to 2.6 ± 0.5 h (range 2-3) and target BG to 5.3 ± 0.4 mmol/l (range 5.0-6.0). DIA was slightly shorter with lower TDD, but the correlation was not statistically significant (r = .40, P = .07).
Discussion
Contrary to the adult recommendation of not using DIA times < 4 hours, we find that families find it beneficial to keep DIA in the interval of 2-3 hours, and there is a need to have increments of 30 minutes, perhaps even 15 minutes, for a better adaptation of DIA to the individual child. Some pumps have only 1 h increments which could be seen as a limitation. The factors entered in a bolus calculator all depend on each other, meaning that a certain setting of DIA can be the result of other settings, rather than be the explanation for how the other settings are derived. Appropriate settings are in our mind those setting that lead to a low HbA1c, and a low rate of disabling and SH. The settings used in our practice may seem to lead to a risk of insulin stacking, and thus carry a hypoglycemia risk in case of repeated meals taken within a short time frame, which is common in the younger age group. However, our rate of SH compares favorably to that found in preschool children in Germany (1.6%) and the United States (2.7%).15
Unless educated, and actively encouraged to do so, neither clinicians nor patients/parents will in our experience change the default DIA setting. Pump autopsy data will therefore be relevant only if patients/parents have been properly educated. Correlating DIA with HbA1c without sufficient education on how DIA and changes in DIA functions may lead to incorrect conclusions on clinically relevant DIA settings.
Why do we find at a shorter DIA compared to published scientific data? Both pumps count IOB from meal boluses (with carbohydrates entered into the bolus calculator) and correction boluses, but will subtract IOB only from a correction bolus when later correcting for a high glucose level. When adding an extra meal with carbohydrates within the DIA time, the pump will show the total IOB (from correction + carb boluses), but will not subtract IOB from a previous correction or meal bolus, allowing insulin to be given to cover the extra carbohydrate intake.
The fat content of the meal slows down the emptying of the stomach, thereby causing an increase in insulin requirements for a longer time after meals. The time for emptying of the stomach after a meal is dependent on the fat content,16 and in adolescents the gastric emptying time has been shown to be prolonged when the fat content of a meal was increased.17 Age does not seem to affect the gastric emptying time in a meta-analysis including the whole age span from neonates to adults.18 Available data show that preschool children with type 1 diabetes have a significantly lower intake of carbohydrate than a healthy control group (47 vs 54%), in spite of having received advice from a dietitian on the recommended intake being the same as for the general pediatric population.19 It may be that parents voluntarily, although not recommended by diabetes teams, restrict the amount of carbohydrate in the food, thereby increasing the fat content and increasing the gastric empting time. Further studies are needed to shed light on this issue. With a higher fat content and thereby slower emptying of the stomach, the correction dose after 2-3 hours would need to be higher to account for the carbohydrate content of the food still remaining in the stomach. A prolonged time of gastric emptying, seen in adults with gastroparesis as a complication to diabetes, would create the need of longer bolus duration, but this is unlikely to be the situation in prepubertal children.
Some centers use fat protein units (FPU) with extra insulin to cover fat and protein being delivered as an extended bolus. The amount of insulin needed for 10 g of carbohydrates is equal to that used for 100 kcal of fat or protein.20,21 A higher carbohydrate content in the diet would create a higher but shorter glucose response, which would promote a lower ICR, that is, a higher bolus dose. The shorter glucose peak will lead to a lower need for correction insulin (higher ISF) if there is an increased blood glucose after 2-4 hours.
A slower delivery of the bolus dose (ie, the use of extended and combined boluses) should theoretically drive toward a longer DIA since the insulin action will last longer. Any endogenous insulin production should lead to a lower need of basal and corrective insulin, which could lead the user to set a longer DIA.
Walsh et al suggest a rigorous research protocol for a study to determine the true DIA.11 This would need to be repeated in children of different age groups (preschool and school children) and adolescents (13-18 years of age). This also needs to be tested in different insulin pumps as their way of calculating IOB differ. Another more practical approach they mention is the pump autopsy method, that is, reading the pump memory,22 previously also used by Davidson et al, who developed the first rules for bolus calculators,13 and by Walsh and Roberts for pump settings in adults.14 We used this method to determine which settings active families have found functional after proper education on how DIA and IOB work in their respective pump. After finding a correct ISF when no IOB was present, families were introduced to the concept of DIA. They were encouraged to always keep an eye on what the calculator said about the amount of IOB, and to evaluate if the pump suggested a correct amount of insulin for correction when IOB was present, that is, subtracted from the calculated dose. At the next visit, all parents had a clear idea of how IOB worked, and if it needed to be changed. We used the default of 3 hours, and the only change suggested by parents was in the direction of lowering it, that is, they felt that the suggested correction dose was too low when IOB was present.
A shorter DIA is supposed to drive toward lower basal rates, which is in line with our findings and match international recommendations and other authors with a mean basal percentage around 40%.2,22 We found that the number in the ICR rule often was lower than 500, that is, young children need relatively higher meal boluses, similar to the results of Alemzadeh et al.12 However, in contrast to their findings we found that a clear majority of the young children needed more insulin for breakfast (ie, a lower ICR) compared to other meals. Although we found wide individual variations in ICR, there was an age effect in that younger children had a higher median ICR. The lower ICR in the morning reflects the well-known morning insulin resistance, while the lower ICR in the evening reflects the reversed dawn phenomenon often found in young children.23,24 Calculating ICR based on BW gives too low numbers, that is, would result in even higher bolus doses. The glycemic control in this study compares favorably with published data where the mean HbA1c in 4- to 10-year-old children was 7.9% (63 mmol/mol).25
Limitations of this study include the retrospective design, the small number of patients, and the lack of a control group with longer DIA. However, the DIA in the pumps were adjusted after careful consideration of the experience of skillful parents, so a control group would not be within the scope of this study. Furthermore, we did not ask for food recalls, or check the accuracy of the carbohydrate counting performed at home. We are also missing the number and size of boluses taken per day, the percentage of bolus given as corrections and the hour-by-hour pattern of the basal rates. A strength is that all prepubertal children using pumps and CC at the clinic were included. The pumps used during this study calculate the IOB in a similar way, but the results on IOB may not be applicable to other pump models. Otherwise, differences between pumps and the way active insulin is calculated might have an impact on the adjustments on IOB. In this study, no such influence was apparent.
The HbA1c target of most Swedish diabetes teams is <7.0% (52 mmol/mol) without problems of SH. In this study, we show that this is quite feasible, and that key factors contributing to success in children using insulin pupms are a DIA of 2-3 h, and individually adjusted ICR and ISF. When setting realistic and ambitious targets for HbA1c, clinically correct numbers for ICR, ISF, and DIA time are important elements.
Conclusions
To our knowledge, this is the first study to examine how settings for DIA in the bolus calculator is efficiently set in young children. Prepubertal children seem to benefit from lower DIA settings than published pharmacodynamic data show. A larger study is needed to determine a possible relationship between DIA and TDD. Half-hour or 15-minute increments of DIA are valuable for fine-tuning. We found that prepubertal children need considerably more bolus insulin for meals than calculated from the 500 rule, especially at breakfast, but with a large individual variation. This warrants the calculation of ICR from real-life meals rather than a specific rule. For corrections, they needed slightly less insulin than calculated from the 100 rule (1800 rule for mg/dl). The 100 rule during daytime can be used as a starting point for ISF, while the lower nighttime, or post exercise insulin resistance, warrants a higher number (150 or 200 rule) when beginning with the bolus calculator. When adjusting the bolus calculator according to downloaded data (glucose readings and pump information), and the parents’ experience of the DIA, a good metabolic control can be achieved with a low rate of severe hypoglycemia.
Abbreviations
CC, carbohydrate counting; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; DIA, duration of insulin action; DKA, diabetic ketoacidosis; FPU, fat protein unit; ICR, insulin to carbohydrate ratio; IOB, insulin on board; ISF, insulin sensitivity factor; MDI, multiple daily injections; TDD, total daily insulin dose.
Acknowledgments
We are grateful to the patients and families for sharing their data, and to our diabetes teams that helped in caring for the children. We thankfully acknowledge support from Fyrbodal Research Institute.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RH has received lecture honoraria from Novo Nordisk, Eli Lilly, Sanofi, Roche, Medtronic, DexCom, Menarini, and Abbott, and participated in Advisory Board for Eli Lilly, Novo Nordisk, Abbott, Sanofi, and Medtronic. PA has received lecture honoraria from Nordisk, Eli Lilly, Sanofi, Roche, Medtronic, DexCom, Menarini, and Abbott, and participated in Advisory Board for Eli Lilly, Abbott, Roche, and Sanofi. PA is one of the founders and a board member of Diasend Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Fyrbodal Research Institute provided financial support.
References
- 1. Samuelsson U, Gudbjörnsdottir S, Hanberger L, et al. Annual report 2014 from the Swedish National Paediatric Diabetes Registry (SWEDIABKIDS). Available at: https://swediabkids.ndr.nu. Accessed November 2015.
- 2. Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F. Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653-1662. [DOI] [PubMed] [Google Scholar]
- 3. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014;2:133-140. [DOI] [PubMed] [Google Scholar]
- 4. Marigliano M, Morandi A, Maschio M, et al. Nutritional education and carbohydrate counting in children with type 1 diabetes treated with continuous subcutaneous insulin infusion: the effects on dietary habits, body composition and glycometabolic control. Acta Diabetol. 2013;50:959-964. [DOI] [PubMed] [Google Scholar]
- 5. Goksen D, Atik Altinok Y, Ozen S, Demir G, Darcan S. Effects of carbohydrate counting method on metabolic control in children with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2014;6:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabbone I, Scaramuzza AE, Ignaccolo MG, et al. Carbohydrate counting with an automated bolus calculator helps to improve glycaemic control in children with type 1 diabetes using multiple daily injection therapy: an 18-month observational study. Diabetes Res Clin Pract. 2014;103:388-394. [DOI] [PubMed] [Google Scholar]
- 7. Enander R, Gundevall C, Stromgren A, Chaplin J, Hanas R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatr Diabetes. 2012;13:545-551. [DOI] [PubMed] [Google Scholar]
- 8. Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care. 2008;31:44-46. [DOI] [PubMed] [Google Scholar]
- 9. Mortensen HB, Lindholm A, Olsen BS, Hylleberg B. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr. 2000;159:483-488. [DOI] [PubMed] [Google Scholar]
- 10. Buckingham B, Block J, Wilson D, Rebrin K, Steil G. Novolog pharmacodynamics in toddlers. Diabetes. 2005;54:A454 (poster 1889). [Google Scholar]
- 11. Walsh J, Roberts R, Heinemann L. Confusion regarding duration of insulin action: a potential source for major insulin dose errors by bolus calculators. J Diabetes Sci Technol. 2014;8:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alemzadeh R, Hoffmann RG, Dasgupta M, Parton E. Development of optimal kids insulin dosing system formulas for young children with type 1 diabetes mellitus. Diabetes Technol Ther. 2012;14:418-422. [DOI] [PubMed] [Google Scholar]
- 13. Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14:1095-1101. [DOI] [PubMed] [Google Scholar]
- 14. Walsh J, Roberts R. Pumping Insulin. Everything You Need for Success on an Insulin Pump. 5th ed. San Diego, CA: Torrey Pines; 2012. [Google Scholar]
- 15. Maahs DM, Hermann JM, DuBose SN, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia. 2014;57:1578-1585. [DOI] [PubMed] [Google Scholar]
- 16. Welch IM, Bruce C, Hill SE, Read NW. Duodenal and ileal lipid suppresses postprandial blood glucose and insulin responses in man: possible implications for the dietary management of diabetes mellitus. Clin Sci (Lond). 1987;72:209-216. [DOI] [PubMed] [Google Scholar]
- 17. Lodefalk M, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with type 1 diabetes. Diabet Med. 2008;25:1030-1035. [DOI] [PubMed] [Google Scholar]
- 18. Bonner JJ, Vajjah P, Abduljalil K, et al. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm Drug Dispos. 2015;36:245-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sundberg F, Augustsson M, Forsander G, Cederholm U, Axelsen M. Children under the age of seven with diabetes are increasing their cardiovascular risk by their food choices. Acta Paediatr. 2014;103:404-410. [DOI] [PubMed] [Google Scholar]
- 20. Pankowska E, Blazik M, Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012;14:16-22. [DOI] [PubMed] [Google Scholar]
- 21. Kordonouri O, Hartmann R, Remus K, Blasig S, Sadeghian E, Danne T. Benefit of supplementary fat plus protein counting as compared with conventional carbohydrate counting for insulin bolus calculation in children with pump therapy. Pediatr Diabetes. 2012;13:540-544. [DOI] [PubMed] [Google Scholar]
- 22. Pankowska E, Skorka A, Szypowska A, Lipka M. Memory of insulin pumps and their record as a source of information about insulin therapy in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2005;7:308-314. [DOI] [PubMed] [Google Scholar]
- 23. Nicolajsen T, Samuelsson A, Hanas R. Insulin doses before and one year after pump start: children have a reversed dawn phenomenon. J Diabetes Sci Tech. 2012;6:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conrad SC, McGrath MT, Gitelman SE. Transition from multiple daily injections to continuous subcutaneous insulin infusion in type 1 diabetes mellitus. J Pediatr. 2002;140:235-240. [DOI] [PubMed] [Google Scholar]
- 25. Tansey M, Beck R, Ruedy K, et al. Persistently high glucose levels in young children with type 1 diabetes. Pediatr Diabetes. 2016;17:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]