The Freestyle Libre flash glucose monitoring (FGM; Abbott Diabetes Care, Witney, UK) system was introduced in the United Kingdom in 2013. Although similar to conventional continuous glucose monitoring (CGM) systems, a few significant differences exist. FGM sensors are factory calibrated and therefore do not require calibration with blood glucose testing over their 14-day lifespan. FGM is also considerably cheaper than conventional CGM1 but lacks alarm features and connectivity with continuous subcutaneous insulin infusion (CSII) devices, such as low-glucose suspend.2 The accuracy and usability of FGM have been validated in patients with both type 1 and type 2 diabetes.3
We sought to prospectively assess the impact of introducing FGM to patients attending our type 1 diabetes clinic in a university teaching hospital, over a 16-week period. In particular, we assessed the impact on HbA1c, hypoglycemia (recorded and self-reported) and quality of life measures (Diabetes Distress Scale). The only inclusion criteria were a diagnosis of type 1 diabetes and a willingness to upload FGM data at least monthly. Data were analyzed as intention-to-treat.
Of the 25 participants, 13 were men, and the mean age was 39.8 ± 2.0 years. Mean duration of diabetes was 19 ± 2 years. A total of 8 patients were treated with CSII, and 17 used multiple daily injections. Immediately prior to commencement of FGM, the mean HbA1c of participants was 8.0 ± 0.14%, which did not differ from the mean of the previous 4 clinic recorded HbA1c values (8.0 ± 0.2%, P = .833). Mean HbA1c fell from 8.0 ± 0.14% to 7.5 ± 0.14% (–0.48%, P = .001) following 16 weeks of FGM. The number of people with an HbA1c of 7.5% or below more than doubled after FGM use (Figure 1). The mean reduction in HbA1c was greater in those with a baseline HbA1c > 7.5%: –0.59 ± 0.15% compared to −0.2 ± 0.11% in those with HbA1c <7.5% at baseline (P = .005). Female participants had greater mean reduction in HbA1c (–0.74 ± 0.19%) compared to men (–0.23 ± 0.15%, P = .049) despite no significant difference in baseline HbA1c (8.2 ± 0.25% vs 7.8 ± 0.14%, P = .174). Of participants, 24% (6/25) achieved an HbA1c reduction of greater than 1.0%.
Figure 1.
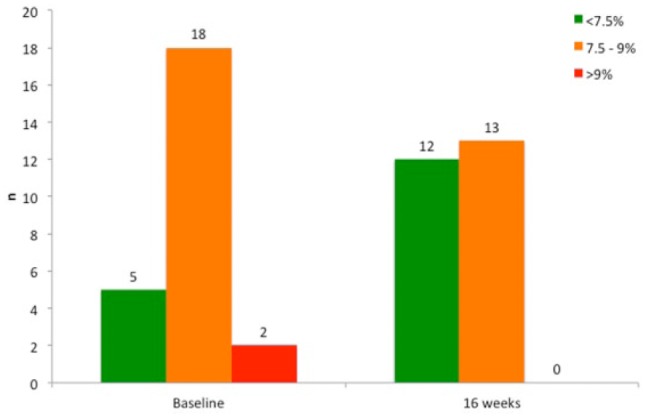
FGM increases the proportion of patients achieving good glycemic control. Data were analyzed using the chi-squared test. P = .015 comparing those below target (<7.5%) at baseline and at end of FGM use.
Episodes of hypoglycemia (glucose <72 mg/dl), as determined from FGM glucose data, reduced from 17 (IQR 10-20) in the first 2 weeks of use to 12 (IQR 8.5-16) in the final 2 weeks (P = .019). Significant reductions were observed in the Diabetes Distress Scale mean score (P = .006), as well as emotional burden (P = .035) and regimen-related distress subscores (P = .005). FGM use was associated with a significant increase in delivering bolus insulin 15-20 minutes in advance of meals (compared to immediately before or after meals), from 16% to 44% (P = .026).
In summary, these results support the wider use of FGM to improve outcomes in people with type 1 diabetes. Benefits are realized across a number of important domains including improved HbA1c, hypoglycemia, and quality of life.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; FGM, flash glucose monitoring; MDI, multiple daily injections.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Freestyle Libre readers and sensors were jointly funded by Abbott Diabetes Care and the Edinburgh Royal Infirmary Diabetes Treatment Trust.
References
- 1. Heinemann L, Freckmann G. CGM versus FGM; or, continuous glucose monitoring is not flash glucose monitoring. J Diabetes Sci Technol. 2015;9(5):947-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. [DOI] [PubMed] [Google Scholar]
- 3. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]


