Abstract
Objective:
Flash glucose monitoring is a new glucose sensing technique that measures interstitial glucose levels for up to 14 days and does not require any calibration. The aim of this study is to evaluate the performance of the new system in Chinese patients with diabetes.
Methods:
A multicenter, prospective, masked study was performed in a total of 45 subjects with diabetes. Subjects wore 2 sensors at the same time, for up to 14 days. The accuracy was evaluated against capillary blood glucose (BG) and venous Yellow Springs Instrument (YSI; Yellow Springs, OH) measurements. During all 14 days, subjects were asked to perform at least 8 capillary BG tests per day. Each subject attended 3 days of 8-hour clinic sessions to measure YSI and sensor readings every 15 minutes.
Results:
Forty subjects had evaluable glucose readings, with 6687 of 6696 (99.9%) sensor and capillary BG pairs within consensus error grid zones A and B, including 5824 (87.0%) in zone A. The 6969 sensor and venous YSI pairs resulted in 6965 (99.9%) pairs within zones A and B, including 5755 (82.6%) in zone A. The sensor pairs with BG and YSI result in mean absolute relative difference (MARD) of 10.0% and 10.7%, respectively. Overall between-sensor coefficient of variation (CV) was 8.0%, and the mean lag time was 3.1 (95% confidence interval 2.54 to 4.29) minutes.
Conclusions:
The system works well for people with diabetes in China, and it is easy to wear and use.
Keywords: flash glucose monitoring, FreeStyle Libre, sensor accuracy, performance, usability
The challenge of managing diabetes therapy, particularly multidose insulin, includes a lack of understanding of the daily fluctuations of glucose levels and exposure to hypoglycemic levels. HbA1c identifies a patient’s risk of long-term complications, but HbA1c does not accurately reflect the underlying glucose variability or hypoglycemia. Recent research has shown that HbA1c is not a predictor of hypoglycemia,1 with the risk of hypoglycemic episodes significantly increasing for patients both with HbA1c <7.0% (<53 mmol/mol) and >7.5% (>58 mmol/mol) compared to those in the central range of 7.0% to 7.5% (53 to 58 mmol/mol). Continuous glucose monitors have been shown to improve measures of glucose control when reviewed by clinicians for therapy interventions. A study by Weber et al2 of type 2 diabetes patients both with and without insulin therapy showed a high degree of hypoglycemia, particularly overnight, that was reduced after clinical consultation. In a study by Riveline et al3 the periodic review of sensor glucose led to improved control in a group of type 1 diabetes patients. In patients with type 2 diabetes not on insulin, studies have shown improved glucose after periodic sensor use at 6 months that continued at 12 months.4,5 There is growing consensus in the United States and Europe that review of the complete glycemic profile is an integral to successful diabetes management. Bergenstal and colleagues6 proposed the use of the ambulatory glucose profile (AGP) to characterize the underlying abnormal glucose patterns, and this tool has been further supported by expert groups in Europe.7,8 The challenge to date has been the difficulty and expense of acquiring the glycemic profile with continuous glucose monitors. A new method, flash glucose monitoring, promises to reduce these challenges and support clinical review of glucose patterns.
The FreeStyle Libre (FL; Abbott Diabetes Care, Alameda, CA) flash glucose monitoring system monitors interstitial glucose via disposable electronics and subcutaneous sensor filament adhered to the skin. The sensor is put in place by a single-use applicator, and automatically measures glucose every minute for up to 14 days without calibration by self-monitored blood glucose. A quick wireless scan of the sensor by the reader collects the glucose and trend at that minute plus up to 8 hours of prior readings every 15 minutes. The reader includes a FreeStyle Precision strip port that allows capillary BG and ketone testing.
The FL system has been available in Europe since October 2014, supported by the performance demonstrated by Bailey and colleagues.9 In that study, 72 participants with type 1 and type 2 diabetes wore 2 sensors to compare the measurements to capillary BG and venous YSI measurements over the 14-day study period. The FL system compared to capillary BG reference values had 86.7% of the results in the consensus error grid zone A, and the overall mean absolute relative difference (MARD) of 11.4%. The accuracy was not affected by patient characteristics or sensor production lot. The purpose of this study was to evaluate the performance of the FL system against capillary blood glucose and venous blood glucose in Chinese patients with diabetes in a multicenter, prospective study that masked the sensor and blood glucose readings during the trial.
Methods
This prospective, single-arm, masked clinical study was conducted at 3 Chinese clinical sites. The protocol and informed consent were approved by the ethics committee of each site consistent with the Declaration of Helsinki, and all subjects provided written informed consent before enrollment.
Study inclusion criteria were age at least 18 years and diagnosed type 1 or type 2 diabetes requiring insulin therapy. The insulin could be administered by pump and/or injections, and the therapy had to be stable at least 6 months prior to and throughout the study. Furthermore, participants had to read and understand Chinese, perform all study visits and tasks, and be able to follow study instructions. Exclusion criteria included known allergy to medical grade adhesive or isopropyl alcohol used to prepare the skin, pregnancy or attempting to conceive, skin lesions, scarring, redness, infection, or edema at the sensor application sites. Participants could not be enrolled in another clinical study, have donated blood recently, have a concomitant medical condition or planned procedure that could interfere or present a risk to the participant.
Study participants wore a sensor on the back of each upper arm, 1 inserted by site staff, and 1 inserted by the subject, without any over-bandage, for up to 14 days while going about normal daily activities. At least 8 capillary BG tests, using the BG meter built into the reader, were required to be performed on each day of the sensor wear. The preferred testing was upon waking, before each meal, an hour after each meal, and at bedtime. Immediately after each BG test, participants scanned the sensor with the reader to obtain a confirmation of a successful sensor scan. Both the BG and sensor readings were masked to site staff and participants, who were asked to maintain their established diabetes management plan. There was no study-prescribed manipulation of the glucose levels of the participants other than their normal meals, insulin doses and physical activities.
There were 3 scheduled in-clinic visits during the 14-day sensor wear period, where venous blood samples were collected every 15 minutes over an 8-hour period for YSI analyzer reference tests. The first in-clinic visit was between day 1 and day 3, the second between day 4 and day 9, and the third between day 10 and day 14. All 40 participants attended the first and second in-clinic visits, 36 attended the third visit, and all participants had at least a 1-day interval between in-clinic visits. Sensor scans were performed each time a venous blood sample was taken, and BG tests were performed as close to a time of venous blood sample as possible. Sensors that were dislodged before or during the second in-clinic visit were replaced, while those after the second in-clinic visit were not replaced. Participants were offered at least 2 meals and provided access to drinks and snacks during in-clinic visits. Of the 114 of 117 successful sensor insertion attempts, 109 sensors produced glucose readings and 108 sensors produced at least 1 paired reading.
Performance evaluation was conducted by analysis of the consensus error grid,10 Clarke error grid,11 continuous glucose error grid,12 and summary difference and regression statistics between the sensor and reference measurements. The lag between the FL and YSI reference was evaluated by performing least square linear regression of the difference between the sensor glucose and YSI versus the sensor rate of change. Between-sensor precision was assessed by calculation of the coefficient of variation of the simultaneous sensor readings provided by each participant. Questionnaire responses and adverse events were tabulated. Analyses were carried out using SAS version 9.2 software (SAS Institute, Cary, NC, USA).
Results
Forty of 45 study participants were included in the sensor performance evaluation, and an additional 6 preplanned, prospectively recruited participants during the site training phase were included in the safety evaluation. Five participants withdrew consent due to time limitations to attend to study procedures. The evaluable participants had a large age range (range 23 to 72, mean 53.1, standard deviation 12.5 years), a large HbA1c range (range 5.6 to 13.4%, mean 8.6%, standard deviation 1.6%), were primarily Han Chinese with Type 2 diabetes and managed with multiple daily injections of insulin (Table 1 and Table 2).
Table 1.
Baseline Characteristics of Evaluable Study Participants (n = 40).
| Mean ± SD | Median | Range | |
|---|---|---|---|
| Age (years) | 53.1 ± 12.5 | 58.0 | 23-72 |
| Height (cm) | 167.6 ± 8.9 | 169.5 | 152.8-186.0 |
| Weight (kg) | 74.9 ± 15.0 | 70.8 | 54.0-118.6 |
| BMI (kg/m2)a | 26.5 ± 3.9 | 26.3 | 18.0-36.5 |
| HbA1c (%) | 8.60 ± 1.6 | 8.35 | 5.6-13.4 |
| HbA1c (mmol/mol) | 70 ± 18 | 68 | 38-123 |
| Years since diagnosis | 14.1 ± 6.9 | 13.3 | 2.3-29.0 |
| Number of insulin injections per day | 2.6 ± 1.0 | 2.0 | 1-4 |
| Total daily insulin dose (units) | 37.0 ± 16.6 | 36.0 | 6-74 |
BMI = weight (kg)/height2(m2).
Table 2.
Demographic Characteristics of Evaluable Study Participants (n = 40).
| Characteristics | n (%) |
|---|---|
| Female | 16 (40) |
| Han ethnicity | 36 (90) |
| Education | |
| Grade school | 8 (20) |
| High school | 18 (45) |
| College | 14 (35) |
| Diabetes type | |
| Type 1 | 6 (15) |
| Type 2 | 34 (85) |
| Insulin administrationa | |
| MDI | 40 (100) |
| CSII | 1 (2.5) |
One subject indicated both MDI and CSII for insulin administration.
The real-time sensor glucose reading was available for 99.1% (13 578/13 701) of scans. The first glucose result was available within 1 hour 10 minutes after insertion for 108 of 109 sensors (99.1%). A total of 6696 capillary BG and 6969 venous YSI results were paired with sensor glucose results. Adherence to perform at least 8 capillary BG tests per day was high, as the median tests per day was 8.0 (mean = 7.3, SD = 1.3). Four BG reference glucose values were excluded because they were beyond the BG system’s dynamic range (1.1 to 27.8 mmol/L), and 46 real-time sensor glucose readings were excluded because the sensor result was beyond the sensor’s dynamic range (2.2 to 27.8 mmol/L). Of 3735 YSI data recorded, 46 (1.2%) YSI readings were excluded. Of these, 26 (56.5%) were tested after 15 minutes of IV draw, 4 (8.7%) were excluded because YSI duplicate results were greater than 4% or 0.22 mmol/L apart, and 16 (34.8%) results were incomplete for other technical reasons. The percentage of results in zone A of the consensus and Clarke error grids were 87.0% and 88.8% respectively when compared to capillary BG readings, as shown in Figure 1. When compared to venous YSI results, the zone A percentages were 82.6% and 85.1% for the Consensus and Clarke Error Grids, respectively. For combined zones A and B of the consensus and Clarke error grids, the percentages were 99.9% and 99.5% compared to capillary BG readings, and 99.9% and 99.1% compared to venous YSI measurements. The percentage of sensor results within ± 1.11 mmol/L or ± 20% of capillary BG and venous YSI reference were 89.7% and 86.0%, respectively.
Figure 1.
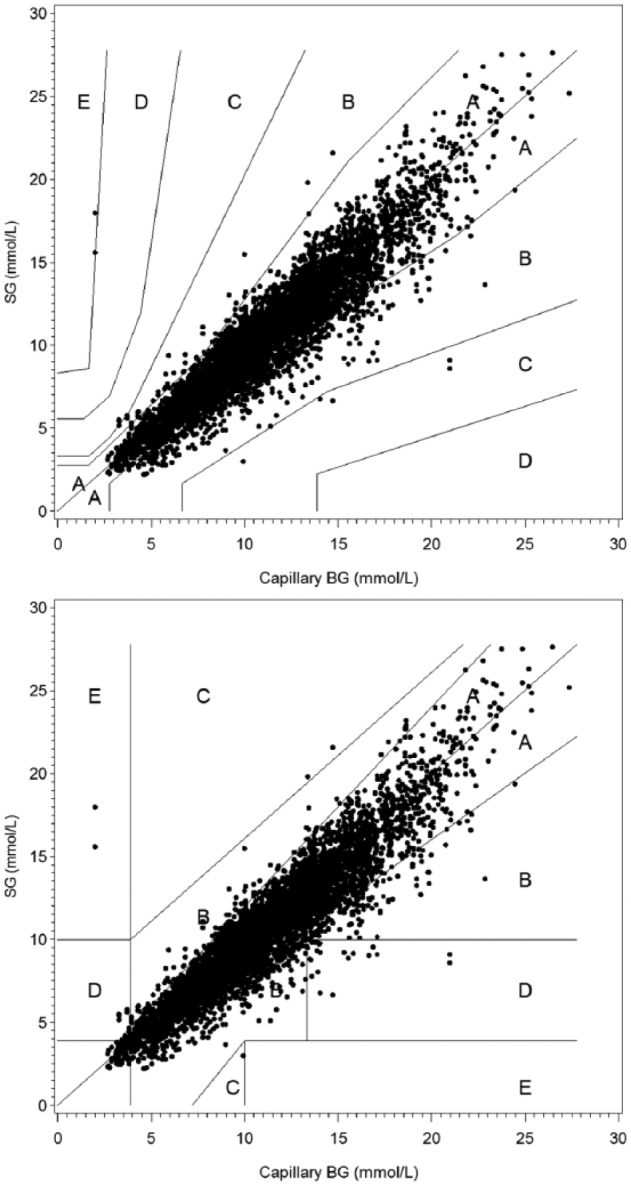
Consensus and Clarke error grid analysis between real-time sensor glucose (SG) and capillary blood glucose (BG).
The overall MARD was 10.0% for sensor results compared to capillary BG results, and 10.7% compared to venous YSI readings. Comparison statistics are shown in Table 3 across glucose ranges, finding accurate readings of 94.0%, 98.3%, and 94.5% for hypoglycemic, euglycemic, and hyperglycemic ranges and 96.1% overall by continuous glucose error grid analysis. There was no difference in MARD across the 3 clinical sites for either capillary BG reference (range 9.5% to 11.3%) or venous YSI reference (range 10.0% to 11.3%). Moreover, there was no effect of body mass index (BMI) on sensor performance as there was no significant relationship between BMI and MARD per subject.
Table 3.
Difference and Continuous Glucose Error Grid Analysis.
| CG-EGA |
|||||
|---|---|---|---|---|---|
| Glucose range | Capillary BG | Venous YSI | Accurate | Benign | Erroneous |
| ≤3.9 mmol/L | 94.0% | 0% | 6.0% | ||
| MAD (mmol/L) | 0.8 | 0.4 | |||
| n | 101 | 69 | 63 | 0 | 4 |
| 4.0 to 10.0 mmol/L | 98.3% | 1.5% | 0.1% | ||
| MARD (%) | 10.0 | 11.5 | |||
| n | 3060 | 2765 | 2661 | 41 | 4 |
| > 10.0 mmol/L | 94.5% | 3.1% | 2.4% | ||
| MARD (%) | 9.5 | 10.1 | |||
| n | 3535 | 4135 | 3738 | 123 | 93 |
| Overall | 96.1% | 2.4% | 1.5% | ||
| MARD (%) | 10.0 | 10.7 | |||
| n | 6696 | 6969 | 6462 | 164 | 101 |
CG-EGA, continuous glucose error grid analysis; MAD, mean absolute difference; MARD, mean absolute relative difference. In this study venous YSI during the in-clinic periods and number of comparisons are reduced due to needing sequential YSI readings spaced by 15 minutes.
Regression analysis resulted in high agreement between the sensor glucose results compared to both capillary BG and venous YSI readings, with slopes of 1.01 and 0.95, intercepts of −0.63 and −0.41 mmol/L, and correlation coefficients of .94 and .95, respectively
The mean lag time between the sensor and YSI reference was 3.1 minutes, with a 95% confidence interval of 2.5 to 4.3 minutes. The between-sensor coefficient of variation when the patients wore 2 sensors simultaneously was 8.0%.
The accuracy of the sensor was stable across the 2-week evaluation period, with no statistically-significant difference found between the first and second week of sensor wear. When compared to capillary BG, the MARD of weeks 1 and 2 were 9.2% and 11.5%, respectively. For venous YSI, the MARD of weeks 1 and 2 were 11.1% and 9.8%, respectively. For the first day, the capillary BG MARD was 11.5% (n = 755), higher than days 2-5 (MARD = 8.9%, n = 2525), but in agreement with the MARD of week 2. For participants with an in-clinic session during the first 9 hours after sensor insertion, the venous YSI MARD was 17.2% (n = 1325), higher than days 2-5 (MARD = 8.7%, n = 2564) and week 2.
The study was not intended to directly assess the wear duration, since in a real scenario only 1 sensor would be worn, which is easier to protect from dislodgement. Furthermore, there was no familiarization period to become accustomed to wear the sensor and participants had no other prior sensor wear experience. With those limitations, participants left the clinic with 80 sensors in place, which had median duration of 269.0 hours. Thirty-one sensors on 20 participants were in place for the entire 14 days. Forty-two sensors detached due to participant activity or adhesive loss, and 7 stopped early due to the sensor electronics.
After sensor insertion and sensor removal, study participants completed study-specific questionnaires to rate their experience with the system. The statements were worded such that agreement indicated a positive experience or acceptable attribute of the system and the responses were selected from strongly agree, agree, neither agree or disagree, disagree, and strongly disagree. Twelve ratings were given by 40 participants, resulting in 480 total responses, of which strongly agree or agree was indicated for 479 (99.8%) ratings. For example, all 40 respondents agreed with the statements “Applying the Sensor was less painful than a routine fingerprick,” “The sensor was comfortable to wear,” “This sensor did not get in the way of daily activities,” and “Scanning the sensor is easier than pricking my finger.” The only disagree response was for “The sensor was easy to wear due to its small size,” which received the responses of 13 (32.5%) strongly agree, 26 (65%) agree, and 1 (2.5%) disagree. Pain assessments were recorded for 117 sensor insertions and participants reported no pain for 28 (23.9%) sensors, slight pain for 88 (75.2%) sensors, and mild pain for 1 (0.9%) sensor.
There were 11 adverse events reported by 8 of the 51 participants. There were no severe adverse events. Six participants experienced 9 anticipated adverse device effects that were all rated as mild (4 erythema, 3 bruising, and 2 pain). Two subjects each had an unanticipated adverse event that was not related to the device or study procedures, one that was mild and the other moderate (a fracture not related to the study device or procedures). Signs and symptoms observed on the skin at the sensor application site recorded 125 of 129 observations (96.9%) with no erythema and 100% with no edema. Two (1.6%) of the erythema observations were a slight pink color at the application site, and 1 (0.8%) produced well-defined redness.
Discussion
The FreeStyle® Libre™ flash glucose monitoring system is a novel glucose measurement device for people with diabetes and this study evaluated its performance and usability when used by patients in China. The system uses a subcutaneous sensor, which incorporates wired enzyme glucose sensing technology13-16 to monitor glucose levels in interstitial fluid. Wired enzyme technology was first introduced in the FreeStyle Navigator CGM system,17 and has evolved to support factory calibration—not needing capillary BGs to calibrate the sensor readings. This has been achieved by producing sensor batches with extremely low variation in glucose sensitivity. The technology has demonstrated stable performance, during both storage and the sensor monitoring period.13,14 Therefore, no additional calibrations are needed from the time of manufacture.
The primary performance evaluation compares the sensor results to capillary BG results since these are used by patients on a day-to-day basis that the sensor is intended to replace. Second, the capillary BGs during the study reflected real-life settings and diabetes management activities during the entire 14-day period of use. As the sensor is factory-calibrated, there is no reliance on the capillary BGs for the sensor readings.
The results of this study agree very closely with the evaluation of this same sensor in the United States,9 which had similar study methods but different patient populations, notably this trial having participants being 90% Han Chinese ethnicity. The consensus error grid zone A percentages when compared to capillary BG was 86.7% compared to 87.0% in the present study. Both studies agreed with regard to not having any discernable effect of BMI on sensor performance, unlike prior studies of earlier versions of sensors.17
A prior study by Zhou and colleagues18 evaluated the performance a nonwired enzyme interstitial glucose sensor in a similar Chinese population of adults with diabetes (44 of 48 with type 2 diabetes). The study established the performance in the euglycemic and hyperglycemic ranges, but was unable to confirm clinically acceptable performance in the hypoglycemic range. There were only 26 paired reference-sensor readings with the sensor value below 4.44 mmol/L, and only 57.7% of these were found within the combined Clarke error grid zones A and B, and they found only 50% of the sensor readings within 1.11 mmol/L of the venous YSI reference during hypoglycemia. In the current study, below 3.9 mmol/L there were 64 of 67 (94.0%) accurate readings by continuous error grid analysis. This suggests clinically acceptable performance of the sensor in the current study across all glucose ranges. While the results do not meet ISO15197:2013 criteria, that standard does not apply as it defines the accuracy criteria for blood glucose monitoring systems for self-testing and uses capillary blood samples tested both on a blood glucose monitoring system and a reference method. For sensor-based technologies, difference and error grid analyses are typically used to evaluate the accuracy of the system.
The sensors were dislodged more than expected, however this study was intended to evaluate the accuracy performance and not intended to directly assess the wear duration. All participants had no prior sensor wear experience, and there was no familiarization period to become accustomed to wear the sensor. In a real scenario, only 1 sensor would be worn, which is easier to protect against external impact or dislodgement.
Limitations of the current study are single body site for sensor glucose measurement and limited venous reference glucose over the 14-day sensor wear period due to practical limitations of collecting blood samples and instrument location. Future studies could evaluate the sensor when applied to different body sites and in different groups, particularly children and during pregnancy. Further research should also be performed to evaluate the clinical value of the system, in terms of both long-term use and health outcome improvement but also for improved diabetes management decisions and workflow efficiency in the clinic.
Conclusions
In this prospective, multicenter study, the performance of the factory-calibrated FL flash glucose monitoring system in Chinese subjects was established by the accuracy of the sensor readings across all clinically important ranges of glucose and demonstrated to be equivalent to accuracy results in other populations. The system performed equally well for both the first and second week of the 14-day sensor wear, and was not affected by subject characteristics. The system was highly accepted by the study participants, indicating the system was easy to operate and wear. The system promises to provide valuable clinical insight to support effective diabetes management in Chinese diabetes patients.
Acknowledgments
We thank the study participants and the 3 hospitals in China for their contributions in this study.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; BG, blood glucose; BMI, body mass index; CG-EGA, continuous glucose error grid analysis; CV, coefficient of variation; FL, FreeStyle Libre; MAD, mean absolute difference; MARD, mean absolute relative difference; SG, sensor glucose; YSI, Yellow Springs Instrument.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JL reports receiving consulting and lecture fee from Abbott, Astrazeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Novartis, Novo Nordisk, Merck, Takeda, Sanofi-Aventis, GlaxoSmithKline, Roche, Johnson & Johnson, Boehinger Ingelheim, and Guangzhou Zhongyi Pharmaceutical.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Abbott Diabetes Care.
References
- 1. Weinstock RS, Xing D, Maahs DM, et al. Severe Hypoglycemia and Diabetic Ketoacidosis in Adults with Type 1 Diabetes: Results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2013;98:3411-3419. [DOI] [PubMed] [Google Scholar]
- 2. Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes. 2007;115:491-494. [DOI] [PubMed] [Google Scholar]
- 3. Riveline JP, Schaepelynck P, Chaillous L, et al. EVADIAC Sensor Study Group. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care. 2012;35:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5:668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7:562-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthaei S, Antuña DeAlaiz R, Bosi E, Evans M, Geelhoed-Duijvestijn N, Joubert M. Consensus recommendations for the use of ambulatory glucose profile in clinical practice. Br J Diabetes Vasc Dis. 2014;14:153-157. [Google Scholar]
- 8. Joubert M, Baillot-Rudoni S, Catargi B, et al. EVAluation dans le Diabète des Implants ACtifs Group (EVADIAC). Indication, organization, practical implementation and interpretation guidelines for retrospective CGM recording: a French position statement. Diabetes Metab. 2015;41:498-508. [DOI] [PubMed] [Google Scholar]
- 9. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143-1148. [DOI] [PubMed] [Google Scholar]
- 11. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622-628. [DOI] [PubMed] [Google Scholar]
- 12. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors. Diabetes Care. 2004;27:1922-1928. [DOI] [PubMed] [Google Scholar]
- 13. Feldman B, Brazg R, Schwartz S, Weinstein R. A continuous glucose sensor based on wired enzyme technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5:769-779. [DOI] [PubMed] [Google Scholar]
- 14. Hoss U, Jeddi I, Schulz M, Budiman E, Bhogal C, McGarraugh G. Continuous glucose monitoring in subcutaneous tissue using factory-calibrated sensors: a pilot study. Diabetes Technol Ther. 2010;12:591-597. [DOI] [PubMed] [Google Scholar]
- 15. Hoss U, Budiman ES, Liu H, Christiansen MP. Continuous glucose monitoring in the subcutaneous tissue over a 14- day sensor wear period. J Diabetes Sci Technol. 2013;7:1210-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoss U, Budiman ES, Liu H, Christiansen MP. Feasibility of factory calibration for subcutaneous glucose sensors in subjects with diabetes. J Diabetes Sci Technol. 2014;8:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-Day FreeStyle Navigator continuous glucose monitoring system. Diabetes Care. 2007;30:1125-1130. [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Lv X, Mu Y, et al. The accuracy and efficacy of real-time continuous glucose monitoring sensor in Chinese diabetes patients: a multicenter study. Diabetes Technol Ther. 2012;14:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]


