Abstract
Advances in insulin pump and continuous glucose monitoring technology have primarily focused on optimizing glycemic control for people with type 1 diabetes. There remains a need to identify ways to minimize the physical burden of this technology. A unified platform with closely positioned or colocalized interstitial fluid glucose sensing and hormone delivery components is a potential solution. Present challenges to combining these components are interference of glucose sensing from proximate insulin delivery and the large discrepancy between the life span of current insulin infusion sets and glucose sensors. Addressing these concerns is of importance given that the future physical burden of this technology is likely to be even greater with the ongoing development of the artificial pancreas, potentially incorporating multiple hormone delivery, glucose sensing redundancy, and sensing of other clinically relevant nonglucose biochemical inputs.
Keywords: unified platform, colocalization, insulin delivery, continuous glucose monitoring, closed loop, artificial pancreas
Replicating the ability of the healthy pancreas to maintain glucose homeostasis for people with type 1 diabetes requires accurate and rapid measurement of glucose levels, and adjustment of exogenous insulin dosing accordingly. Over the past 80 years, type 1 diabetes management has evolved from subcutaneous injection of relatively impure animal insulin using glass syringes in conjunction with urinary glucose measurements for assessing glycemia, to subcutaneous rapid-acting insulin analog delivery via pumps combined with real-time continuous glucose monitoring (CGM) incorporating ‘smart’ features such as a low-glucose suspend (LGS) or predictive low-glucose suspend (PLGS). The ultimate goal is a fully-automated ‘artificial pancreas’. Technological advances over the past 10–15 years have prioritized and achieved significant improvements in automated glycemic control, with lower HbA1c levels, improved time-in-target glucose range and less hypoglycemia.1-3
Progress has been made minimizing the technology-associated physical burden. However, this has not received the same attention as advances automating insulin delivery. Original insulin infusion sets included uncomfortable metal cannulas, large external adhesives, and infusion lines fixed to the pump. These have evolved, with lower-profile infusion sets incorporating detachable lines, smaller integrated adhesives, and flexible cannulas. The CGM transmitter too has been miniaturized. The incorporation of a CGM display monitor into insulin pumps in 2006 was a step forward.4 Reduction in the size of the sensors and insulin pumps has been less marked.
Consequently, while each generation of computerized insulin delivery devices and glucose sensors have provided a more sophisticated platform than the last, the device footprint has remained largely unchanged. In the future greater demands are likely to be placed upon skin insertion sites, with closed-loop systems infusing more than one hormone, redundant glucose sensors, and the potential requirement for sensors measuring molecules other than glucose.5-7
The burden associated with CGM wear is evident in both clinical practice and clinical trials, where individuals with type 1 diabetes frequently wear their glucose sensors much less than 100% of the time.8 Minimizing the physical presence and increasing sensor accuracy may increase patient acceptance and utilization of such systems.9 We aim to explore issues influencing a unified platform with closely positioned glucose sensing and insulin delivery functions relevant to a closed-loop setting, as well as potential innovations to improve the technology’s reliability, convenience and efficacy.
Combining Insulin Delivery and Glucose Sensing Components
In current clinical practice, people with diabetes who elect to use an insulin pump in conjunction with a glucose sensor need to insert the insulin delivery cannula and glucose sensing element separately. To our knowledge, all subcutaneous tissue-based closed-loop system studies to date have also involved separate insertions.
Interference of Insulin Delivery With Colocalized Glucose Sensing
When combining insulin delivery and glucose sensing functions into a single platform, it is of paramount importance to determine whether interstitial fluid glucose can be measured accurately and reliably in close proximity to exogenous insulin delivery. Commercially-available CGM sensors utilize glucose oxidase methodology to measure interstitial fluid glucose levels. Initial feasibility studies performed in dogs combined insulin delivery and glucose sensing components into a single subcutaneous element. Reported findings were interference of insulin delivery with the function of the glucose sensor, with artifact spikes in output observed after insulin bolus delivery (Gayane Voskanyan, personal communication). The hypothesized mechanism behind this interference is direct oxidation of the phenol and meta-cresol preservatives in the insulin formulation when in contact with the sensor electrode. These preservatives belong to a class of phenolic compounds which includes recognized glucose sensor interferents. A common example, acetaminophen, contains a free phenolic hydroxyl group which is considered responsible for acetaminophen oxidation resulting in interference of sensor electrodes.10,11 Such interference would significantly compromise performance and safety of a closed-loop system where algorithms rely on sensor inputs.
The findings described above suggest that the insulin delivery and glucose sensing elements need to be physically separated to ensure unaffected sensor function. A prototype single-site platform separating glucose sensing and insulin delivery functions by 11 mm was clinically evaluated in a feasibility study, with no compromise of glucose sensor function apparent.12 This was subsequently confirmed by a study utilizing 225 of the commercially-available devices (MiniMed Duo, Medtronic, Northridge, CA) during a brief pilot release.13 Separation distances of less than the 11 mm between components utilized in the feasibility study above remain to be explored.
Microdialysis sensors utilize fine, hollow fibers with a semipermeable wall that are placed subcutaneously allowing interstitial fluid to diffuse up to an apparatus measuring glucose via enzymatic amperometric methodology.14 Interstitial fluid glucose measurement in early studies using subcutaneous microdialysis or microperfusion catheters for simultaneous insulin delivery and glucose sampling has shown viability.15,16 However, in these studies catheter effluent was collected for external analysis rather than utilizing in situ amperometric sensors. In addition, samples were collected at 30–60 minute intervals, potentially missing transient artifacts. Regittnig et al reported accurate glucose measurements on interstitial fluid sampled via a microperfusion cannula during brief interruptions of an insulin infusion.17 A limitation of this approach is that closed-loop function mandates near-continuous glucose sensing and insulin delivery. A single platform with discrete microdialysis sensors and insulin delivery cannulas would address this limitation. In a study of 10 participants with type 1 diabetes, microdialysis CGM sensors retained accuracy when placed 9 mm from an insulin delivery site.18 A reduction in the separation distance between insulin delivery and glucose sensing with a microdialysis configuration may be possible if interfering substances are completely or partially excluded from proximity to the sensing electrode by a semipermeable membrane.
Alternate methods for measuring glucose may circumvent the issue of interference associated with the co-delivery of insulin. Optical sensors utilize the properties of different light frequencies (scattering, absorption and reflection) to determine the glucose concentration.19 Nacht et al placed two optical sensors incorporating near infrared phosphorescent porphyrin dyes into the wall of an insulin catheter. The wavelength output from a glucose biosensor (GOx enzyme layer and an oxygen sensitive layer) and an adjacent reference oxygen sensor was read non-invasively via a flurometer. Correlation between sensor glucose readings taken from the infusion catheter and reference blood glucose levels was observed in pigs.20 However, optical sensor technology has not yet reached maturity, and sensor accuracy needs to be improved significantly before clinical application.
Discrepancy in Lifetimes of Glucose Sensor and Insulin Infusion Set
Differences between the lifespans of the insulin infusion set and the glucose sensor is a significant limiting factor to the wider adoption of a single platform. Despite the sensor lifetime of 6 days, MiniMed Duo users were advised to change both the insulin delivery set and glucose sensor at least every 3 days due to the shorter lifespan of the insulin delivery component.
In addition to the inconvenience and expense associated with replacing a combined platform every 3 days, this practice will also impact glucose sensor accuracy. Day 1 sensor inaccuracy is a recognized limitation of all current generation CGM systems.21,22 Restricting sensor use to the 3-day lifespan of the infusion set decreases the percentage of time with optimal sensor performance. Patients may also be tempted to use the insulin catheter longer than the intended wear duration of 3 days. Evidence suggesting that patients may indeed act in this manner is provided by a survey of 47 patients using the MiniMed Duo who rated a median response of 6, on a scale from 1 (strong disagreement) to 7 (strong agreement), when asked if they would use the platform for longer than recommended.13 As an alternative to changing the set every 3 days patients could utilize the MiniMed Duo as a single insertion platform for only 50% of the time by inserting a separate new insulin delivery line after 3 days while continuing to use the platform solely for CGM for a further 3 days. However, this strategy creates an even greater physical footprint in comparison with separate insertion sites.
A limited body of published evidence supports the recommendation from most pump manufacturers to change insulin infusion sets every 48–72 hours to ensure adequate insulin delivery and to reduce equipment-related dermatological complications. A 6-month randomized, cross-over study comparing the tolerability of 4-day versus 2-day insulin infusion set use in 24 adults reported that 4-day use was associated with increased hyperglycemic events, infusion set complications and injection site reactions.23 Schmid et al studied 12 adults wearing insulin infusion sets for progressively longer periods (up to 7 days) and observed that infusion set and injection site complications increased significantly after day 3 of wear. There was no single specific type of adverse event reported with extended catheter use, but rather a range of events including: cannula occlusion or dislodgement, adhesive failures, equipment leakage and skin changes such as bruising, erythema and swelling. An increase in mean daily blood glucose levels also correlated with increased duration of insulin delivery catheter use.24 This is consistent with findings in a double-blind randomized controlled trial of 20 participants, which demonstrated glycemic deterioration from day 2 to day 5 of wear with the daily average glucose level increase of 34%.25 Another study collecting self-reported blood glucose data via the internet (n = 243 type 1 diabetic participants) demonstrated a mean fasting glucose increase of 17% from day 1 to day 5 of insulin infusion set use.26 On balance, at present the evidence favors clinical convention, which suggests insulin delivery lines currently available should be changed every 3 days.
Extending the Lifetime of Subcutaneous Insulin Infusion Equipment
It has been suggested that steel cannulas may have a greater biocompatibilty given findings by Hojbjerre et al indicating that adipose tissue blood flow and bolus mean infusion pressure increased significantly with a wear time of 48 hours with Teflon cannulas only.27 Nevertheless, studies have not demonstrated a consistent increase in the survival of steel over Teflon cannulas. Patel et al reported comparable failure rates over a 7-day period with both materials in a randomized, crossover trial of 20 pump users.28 Conversely, retrospective survey results suggest longer cannula lifespan with Teflon.29 In an attempt to extend the life of insulin delivery lines the use of alternative materials and innovative designs to minimize equipment failures and reduce tissue trauma are being explored. For example, a novel dual-port catheter reduced flow interruption events, including silent occlusions, over 2.5- to 4.5-hour infusion periods.30 Further advances in the durability of insulin infusion sets and translation into clinical use are required before a combined insulin delivery and glucose sensing platform is practical.
Insulin Formulation Factors
The insulin solution infused can impact the delivery site lifetime. Insulin can cause local skin reactions via immune-mediated inflammatory reactions to the insulin protein and other solution additives, such as cresols.31-33 Changes to the chemical and physical structure of insulin are postulated to contribute to the risk of insulin set occlusions. Isoelectric precipitation is a pharmacokinetic change occurring in an acidic environment and the pH threshold for precipitation differs depending on the isoelectric point of the insulin formulation.34 Poulsen et al demonstrated the greatest resistance to precipitation with insulin aspart, intermediate resistance with buffered human regular insulin, and the least resistance with insulin lispro.34 The clinical relevance of these differences remains unclear.
Insulin fibrillation (insoluble linear aggregates of partially unfolded insulin molecules), another potential cause of insulin delivery line occlusions, can be triggered by changes in the environment such as heat and mechanical agitation with exposure to hydrophobic surfaces.35,36 Physical stabilization of insulin solutions has been enhanced by the addition of certain substances, such as zinc, which strengthens the hexameric structure of insulin, reducing fibril formation.35 Insulin glulisine, which utilizes a surfactant rather than zinc, has been demonstrated to be particularly prone to fibril formation in vitro.37 In addition, there was a trend toward an increase in line occlusions with insulin glulisine when compared with insulin aspart and lispro in a randomized, crossover study of pump users.38 Manipulation of the structure of insulin can also reduce fibrillation. For example, addition of a peptide tether to single chain insulin analogues made them refractory to fibrillation for three months at 37°C without affecting their biological activity.39 Development of insulin formulations with greater stability and less cutaneous side effects may help to extend the lifespan of insulin delivery lines.
The effects of new rapid-acting insulin formations, while theoretically better suited to a closed-loop system, have yet to be widely studied relating to their impact on infusion set lifespan.40-43 A 6-week double-blind, randomized controlled trial utilizing 219 infusion sets in participants trialing a modification of insulin aspart with an enhanced time to onset of action reported seven possible occlusions (unexplained hyperglycemia or leakage) compared with no occlusions using regular insulin aspart.44 No occlusions were macroscopically or microscopically confirmed. Ongoing research is required.
Bihormonal Artificial Pancreas
A bihormonal artificial pancreas, delivering both insulin and glucagon, may improve glycemic control and reduce hypoglycemia. This has recently been demonstrated in short-term studies conducted in inpatient, supervised outpatient and home settings.45-48 There are a number of challenges to be overcome before a bihormonal closed-loop system can be incorporated into a unified platform. Currently, two delivery lines (for insulin and glucagon) and a sensor require a significant proportion of the abdominal wall. Most studies have utilized three separate devices (the control unit, insulin pump and glucagon pump), although recently a single integrated wearable device has been developed.48
At present there is no commercially-available physically and chemically stable glucagon preparation, and frequent reservoir changes are currently necessary to prevent degradation and amyloid fibril formation.49 Prototype formulations that increase stability to allow longer pump compatibility are under development, for example utilizing ferulic acid, surfactant or other surfactant-like excipients.50,51 In the preliminary results of a phase 2 trial, a novel glucagon peptide analog with favorable stability in liquid solution, has shown comparable pharmacological and safety profiles to a commercially-available lyophilized form of native glucagon.52 Little information is available regarding the impact of glucagon on sensor function when infused in close proximity, or the compatibility of insulin and glucagon when administered via the same or closely positioned lines.
Redundancy in Glucose Sensing
Accurate and reliable CGM sensors are vital to the efficacy and safety of an automated closed-loop system. Factors limiting performance include calibration error, sensor delay, and drift.53 A potential approach to improving sensor performance is redundancy, that is, incorporating inputs from ≥2 sensing elements to provide a single integrated output, to more accurately and reliably reflect glucose levels.
The benefits of redundancy have previously been demonstrated using multiple, separate sensors concurrently. In animal studies, improved sensor accuracy was demonstrated by incorporating the median input of multiple closely-positioned glucose sensors into a single combined output.54 Castle et al utilized the mean and median inputs from four amperometric sensors worn simultaneously to improve sensor performance, in particular demonstrating a reduction in the proportion of reported values ≥50% deviation from reference blood glucose.55 Further improvement in sensor accuracy can been achieved by interrogating the sensors to determine their performance and selection of sensors performing superiorly, rather than simple signal averaging.53,55
Given the burden of wearing multiple sensing devices, a novel redundant sensor was investigated by our research group.56 The prototype sensor incorporated two electrochemical electrodes in a fold-over configuration inserted via single subcutaneous needle. The sensor was linked to a processing algorithm that intelligently combined the data from each sensing electrode. In adults with type 1 diabetes studied over 7 days, redundancy enhanced electrochemical glucose sensing performance, particularly increasing sensor accuracy, and display time while reducing variation in performance between sensors. Therefore, many of the benefits attributed to redundancy in glucose sensing no longer require multiple sensor insertions.
Despite redundancy, sensors utilizing identical methodology may all be subject to the same interferences. Orthogonal redundancy combines different sensing methodologies and provides a potential solution. Our research group investigated a prototype orthogonal redundant sensor that combined an optical fluorescence sensor with two electrochemical sensing elements via a single insertion platform.6 This demonstrated a trend to improved sensor accuracy and reliability, and while promising, further technical development of the optical sensing component is needed to fulfill the potential of this approach.
Non-glycemic Biological Parameters
A closed-loop system may benefit from the ability to detect insulin delivery line failures and situations with unpredictable insulin requirements (such as exercise, meals and acute illness). A multi-input sensor that detects parameters such as physical acceleration, lactate, and ketones may enhance the ability of an artificial pancreas to respond to such challenges.
Accelerometers provide information regarding exercise onset, duration and intensity. Stenerson et al studied pump wearers using a PLGS algorithm and wearing a 3-axis accelerometer via a chest strap. During everyday activities the accelerometer-augmented PLGS system reduced hypoglycemia by >10% compared with PLGS alone (74% versus 62% reduction, respectively), though failed to prevent exercise-associated hypoglycemia during a structured soccer session.57,58 While mixed results have been reported using heart rate information to predict hypoglycemia, Jacobs et al have modeled in silico combined accelerometer and heart rate data collected during a 45-minute aerobic treadmill session to propose an exercise algorithm for insulin pump use.59-62 Turksoy et al have shown feasibility of a multivariable arm-band incorporating an accelerometer and other parameters of energy expenditure.7,63 Additional parameters may provide added value. For example, Zanon et al investigated a novel non-invasive glucose monitoring system worn on the arm that also collected accelerometer, temperature and humidity data.64
There are potential clinical advantages to sensing molecules in addition to glucose in a closed-loop system. A ketone sensor may assist in the detection of relative insulin deficiency, such as observed following infusion line failure, during intercurrent illness or diabetic ketoacidosis. A lactate sensor could detect rising levels during high-intensity exercise, aiding adjustment of insulin delivery.65 Ward et al have shown feasibility of oxidase-based amperometric wire micro-sensors detecting changes in glucose and lactate during subcutaneous implantation in rats.66 Development of novel sensor technology detecting these inputs is required.
Conclusion
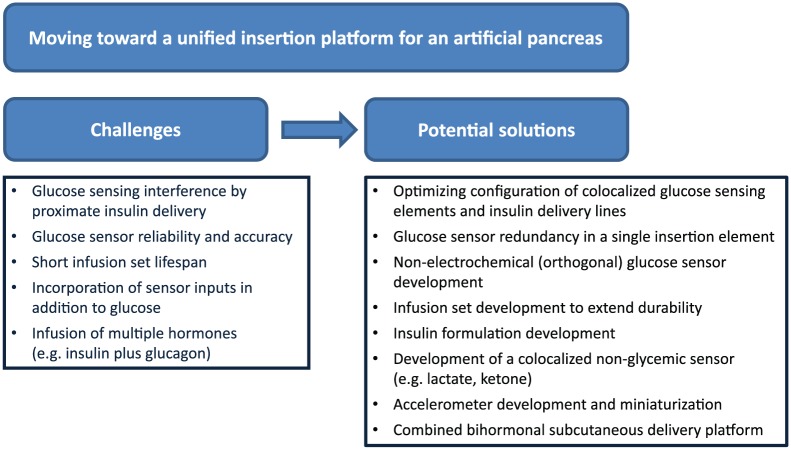
There are a number of challenges faced moving toward a unified closed loop platform (Figure 1). Optimization of glycemia has so far been prioritized throughout the development of current-generation insulin pumps and CGM technology. While the information collected and the hormones delivered are becoming more sophisticated, we need to ensure the physical burden of technology is acceptable to the users.
Figure 1.
Challenges and potential solutions in moving toward a unified insertion platform for an artificial pancreas.
Acknowledgments
We gratefully acknowledge the assistance of Dr Andrea Varsavsky (Medtronic, Northridge, CA).
Footnotes
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; PLGS, predictive low-glucose suspend.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The nonprofit employers of the authors AG, SAM, CS, AJJ, and DNO have received grant support from Medtronic for investigator-initiated studies. AJJ and DNO have previously received honoraria from Medtronic. JU and GV are employees of Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SAM is supported by a University of Melbourne postgraduate scholarship. AJJ is supported by a Sydney Medical School Fellowship from the University of Sydney.
References
- 1. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. [DOI] [PubMed] [Google Scholar]
- 3. Maahs DM, Calhoun P, Buckingham BA, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37(7):1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Innovation Milestones. January 2015. Updated April 2015. http://www.medtronicdiabetes.com/about-medtronic-innovation/milestone-timeline. Accessed July 2016.
- 5. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3(1):17-26. [DOI] [PubMed] [Google Scholar]
- 6. McAuley SA, Dang TT, Horsburgh JC, et al. Feasibility of an orthogonal redundant sensor incorporating optical plus redundant electrochemical glucose sensing. J Diabetes Sci Technol. 2016;10(3):679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turksoy K, Quinn LT, Littlejohn E, Cinar A. An integrated multivariable artificial pancreas control system. J Diabetes Sci Technol. 2014;8(3):498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norgaard K, Scaramuzza A, Bratina N, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15(4):273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Bock M, Cooper M, Retterath A, et al. Continuous glucose monitoring adherence: lessons from a clinical trial to predict outpatient behavior. J Diabetes Sci Technol. 2016;10(3):627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindh M, Lindgren K, Carlström A, Masson P. Electrochemical interferences with the YSI glucose analyzer. Clin Chem. 1982;28(4 pt 1):726. [PubMed] [Google Scholar]
- 11. Kaufmann-Raab I, Jonen HG, Jähnchen E, Kahl GF, Groth U. Interference by acetaminophen in the glucose oxidase-peroxidase method for blood glucose determination. Clin Chem. 1976;22(10):1729-1731. [PubMed] [Google Scholar]
- 12. O’Neal DN, Adhya S, Jenkins A, Ward G, Welsh JB, Voskanyan G. Feasibility of adjacent insulin infusion and continuous glucose monitoring via the Medtronic Combo-Set. J Diabetes Sci Technol. 2013;7(2):381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norgaard K, Shin J, Welsh JB, Gjessing H. Performance and acceptability of a combined device for insulin infusion and glucose sensing in the home setting. J Diabetes Sci Technol. 2015;9(2):215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maran A, Crepaldi C, Tiengo A, et al. Continuous subcutaneous glucose monitoring in diabetic patients: a multicenter analysis. Diabetes Care. 2002;25(2):347-352. [DOI] [PubMed] [Google Scholar]
- 15. Lindpointner S, Korsatko S, Kohler G, et al. Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes. Diabetes Care. 2010;33(3):595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindpointner S, Korsatko S, Kohler G, et al. Glucose levels at the site of subcutaneous insulin administration and their relationship to plasma levels. Diabetes Care. 2010;33(4):833-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Regittnig W, Lindpointner S, Korsatko S, Tutkur D, Bodenlenz M, Pieber TR. Periodic extraction of interstitial fluid from the site of subcutaneous insulin infusion for the measurement of glucose: a novel single-port technique for the treatment of type 1 diabetes patients. Diabetes Technol Ther. 2013;15(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermanides J, Wentholt IM, Hart AA, Hoekstra JB, DeVries JH. No apparent local effect of insulin on microdialysis continuous glucose- monitoring measurements. Diabetes Care. 2008;31(6):1120-1122. [DOI] [PubMed] [Google Scholar]
- 19. Oliver NS, Toumazou C, Cass AE, Johnston DG. Glucose sensors: a review of current and emerging technology. Diabet Med. 2009;26(3):197-210. [DOI] [PubMed] [Google Scholar]
- 20. Nacht B, Larndorfer C, Sax S, et al. Integrated catheter system for continuous glucose measurement and simultaneous insulin infusion. Biosens Bioelectron. 2015;64:102-110. [DOI] [PubMed] [Google Scholar]
- 21. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9(2):209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christiansen M, Bailey T, Watkins E, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15(10):881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfutzner A, Sachsenheimer D, Grenningloh M, et al. Using insulin infusion sets in CSII for longer than the recommended usage time leads to a high risk for adverse events: results from a prospective randomized crossover study. J Diabetes Sci Technol. 2015;9(6):1292-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmid V, Hohberg C, Borchert M, Forst T, Pfutzner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4(4):976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thethi TK, Rao A, Kawji H, et al. Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications. 2010;24(2):73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sampson Perrin AJ, Guzzetta RC, Miller KM, et al. A web-based study of the relationship of duration of insulin pump infusion set use and fasting blood glucose level in adults with type 1 diabetes. Diabetes Technol Ther. 2015;17(5):307-310. [DOI] [PubMed] [Google Scholar]
- 27. Hojbjerre L, Skov-Jensen C, Kaastrup P, Pedersen PE, Stallknecht B. Effect of steel and Teflon infusion catheters on subcutaneous adipose tissue blood flow and infusion counter pressure in humans. Diabetes Technol Ther. 2009;11(5):301-306. [DOI] [PubMed] [Google Scholar]
- 28. Patel PJ, Benasi K, Ferrari G, et al. Randomized trial of infusion set function: steel versus Teflon. Diabetes Technol Ther. 2014;16(1):15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johansson UB, Adamson U, Lins PE, Wredling R. Patient management of long term continuous subcutaneous insulin infusion. J Adv Nurs. 2005;51(2):112-118. [DOI] [PubMed] [Google Scholar]
- 30. Gibney M, Xue Z, Swinney M, Bialonczyk D, Hirsch L. Reduced silent occlusions with a novel catheter infusion set (BD FlowSmart): results from two open-label comparative studies. Diabetes Technol Ther. 2016;18(3):136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wurzburger MI, Prelevic GM, Despotovic N, et al. Delayed-type allergy against various insulin preparations including human semisynthetic insulin. Ann Allergy. 1987;59(1):44-47. [PubMed] [Google Scholar]
- 32. deShazo RD, Boehm TM, Kumar D, Galloway JA, Dvorak HF. Dermal hypersensitivity reactions to insulin: correlations of three patterns to their histopathology. J Allergy Clin Immunol. 1982;69(2):229-237. [DOI] [PubMed] [Google Scholar]
- 33. Kim D, Baraniuk J. Delayed-type hypersensitivity reaction to the meta-cresol component of insulin. Ann Allergy Asthma Immunol. 2007;99(2):194-195. [DOI] [PubMed] [Google Scholar]
- 34. Poulsen C, Langkjaer L, Worsoe C. Precipitation of insulin products used for continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2005;7(1):142-150. [DOI] [PubMed] [Google Scholar]
- 35. Brange J, Andersen L, Laursen ED, Meyn G, Rasmussen E. Toward understanding insulin fibrillation. J Pharm Sci.1997;86(5):517-525. [DOI] [PubMed] [Google Scholar]
- 36. Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci USA. 1991;88(21):9377-9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senstius J, Poulsen C, Hvass A. Comparison of in vitro stability for insulin aspart and insulin glulisine during simulated use in insulin pumps. Diabetes Technol Ther. 2007;9(6):517-521. [DOI] [PubMed] [Google Scholar]
- 38. van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13(6):607-614. [DOI] [PubMed] [Google Scholar]
- 39. Phillips NB, Whittaker J, Ismail-Beigi F, Weiss MA. Insulin fibrillation and protein design: topological resistance of single-chain analogs to thermal degradation with application to a pump reservoir. J Diabetes Sci Technol. 2012;6(2):277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forst T, Pfutzner A, Flacke F, et al. Postprandial vascular effects of VIAject compared with insulin lispro and regular human insulin in patients with type 2 diabetes. Diabetes Care. 2010;33(1):116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heinemann L, Nosek L, Flacke F, et al. U-100, pH-Neutral formulation of VIAject®: faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes Metab. 2012;14(3):222-227. [DOI] [PubMed] [Google Scholar]
- 42. Morrow L, Muchmore DB, Hompesch M, Ludington EA, Vaughn DE. Comparative pharmacokinetics and insulin action for three rapid-acting insulin analogs injected subcutaneously with and without hyaluronidase. Diabetes Care. 2013;36(2):273-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heinemann L, Muchmore DB. Ultrafast-acting insulins: state of the art. J Diabetes Sci Technol. 2012;6(4):728-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zijlstra E, Demissie M, Graungaard T, Heise T, Nosek Bode B. Compatibility and safety of faster-acting insulin aspart used in continuous subcutaneous insulin infusion therapy in patients with type 1 diabetes. Paper presented at: Endocrine Society’s 98th Annual Meeting and Expo; April 1-4, 2016; Boston, MA. [Google Scholar]
- 45. Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(8):595-604. [DOI] [PubMed] [Google Scholar]
- 48. Blauw H, van Bon AC, Koops R, DeVries JH. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18(7):671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jackson MA, Caputo N, Castle JR, David LL, Roberts CT, Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep. 2012;12(6):705-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pohl R, Li M, Krasner A, De Souza E. Development of stable liquid glucagon formulations for use in artificial pancreas. J Diabetes Sci Technol. 2015;9(1):8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bakhtiani PA, Caputo N, Castle JR, et al. A novel, stable, aqueous glucagon formulation using ferulic acid as an excipient. J Diabetes Sci Technol. 2015;9(1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dasiglucagon (ZP4207). Single-dose rescue treatment for acute, severe hypoglycaemic events. August 2016. http://www.zealandpharma.com/portfolio/proprietary-pipeline/glucagon-rescue-peng. Accessed October 2016.
- 53. Castle JR, Ward WK. Amperometric glucose sensors: sources of error and potential benefit of redundancy. J Diabetes Sci Technol. 2010;4(1):221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ward WK, Casey HM, Quinn MJ, Federiuk IF, Wood MD. A fully implantable subcutaneous glucose sensor array: enhanced accuracy from multiple sensing units and a median-based algorithm. Diabetes Technol Ther. 2003;5(6):943-952. [DOI] [PubMed] [Google Scholar]
- 55. Castle JR, Pitts A, Hanavan K, et al. The accuracy benefit of multiple amperometric glucose sensors in people with type 1 diabetes. Diabetes Care. 2012;35(4):706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharifi A, Varsavsky A, Ulloa J, et al. Redundancy in glucose sensing: enhanced accuracy and reliability of an electrochemical redundant sensor for continuous glucose monitoring. J Diabetes Sci Technol. 2016;10(3):669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stenerson M, Cameron F, Wilson DM, et al. The impact of accelerometer and heart rate data on hypoglycemia mitigation in type 1 diabetes. J Diabetes Sci Technol. 2014;8(1):64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stenerson M, Cameron F, Payne SR, et al. The impact of accelerometer use in exercise-associated hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cichosz SL, Frystyk J, Hejlesen OK, Tarnow L, Fleischer J. A novel algorithm for prediction and detection of hypoglycemia based on continuous glucose monitoring and heart rate variability in patients with type 1 diabetes. J Diabetes Sci Technol. 2014;8(4):731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cichosz SL, Frystyk J, Tarnow L, Fleischer J. Combining information of autonomic modulation and CGM measurements enables prediction and improves detection of spontaneous hypoglycemic events. J Diabetes Sci Technol. 2015;9(1):132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jacobs PG, Resalat N, El Youssef J, et al. Incorporating an exercise detection, grading, and hormone dosing algorithm into the artificial pancreas using accelerometry and heart rate. J Diabetes Sci Technol. 2015;9(6):1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zanon M, Sparacino G, Facchinetti A, et al. Non-invasive continuous glucose monitoring with multi-sensor systems: a Monte Carlo-based methodology for assessing calibration robustness. Sensors. 2013;13(6):7279-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goodwin ML, Harris JE, Hernandez A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol. 2007;1(4):558-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ward WK, House JL, Birck J, Anderson EM, Jansen LB. A wire-based dual-analyte sensor for glucose and lactate: in vitro and in vivo evaluation. Diabetes Technol Ther. 2004;6(3):389-401. [DOI] [PubMed] [Google Scholar]