Abstract
It has been proposed that aging results from the lifelong accumulation of intracellular damage via reactions with reactive oxygen species (ROS). Metallothioneins are conserved cysteine-rich proteins that function as efficient ROS scavengers and may affect longevity. To better understand mechanisms controlling metallothionein expression, the regulatory factors and pathways that controlled cadmium-inducible transcription of the C. elegans metallothionein gene, mtl-1, were identified. The transcription factor ATF-7 was identified in both ethylmethanesulfonate mutagenesis and candidate gene screens. PMK-1 and members of the insulin signaling pathway, PDK-1 and AKT-1/2, were also identified as mtl-1 regulators. Genetic and previous results support a model for the regulation of cadmium-inducible mtl-1 transcription based on the derepression of the constitutively active transcription factor ELT-2. In addition, knockdown of the mammalian homologs of PDK1 and ATF7 in HEK293 cells resulted in changes in metallothionein expression, suggesting that this pathway was evolutionarily conserved. The insulin signaling pathway is known to influence the aging process; however, various factors responsible for affecting the aging phenotype are unknown. Identification of portions of the insulin signaling pathway as regulators of metallothionein expression supports the hypothesis that longevity is affected by the expression of this efficient ROS scavenger.
Introduction
There are several theories to explain how organisms age [1]. One theory, hypothesized by Harman in 1956, proposes that aging is a consequence of the accumulation of intracellular damage from reactions between macromolecules (DNA, proteins, lipids) and reactive oxygen species (ROS) [2]. ROS are unstable derivatives of molecular oxygen that can be generated both endogenously and exogenously. Although ROS has been implicated in the aging process, the debate remains as to whether its role in aging is through direct damage or the activation of intracellular signaling processes. In mouse models and the nematode C. elegans, changes in the expression of enzymes specifically involved in the oxidative stress response result in varying effects on lifespan [3,4]. Cells respond to ROS through the activation of several signal transduction pathways, including the MAPK pathways, and transcription factors associated with oxidative stress, such as AP-1, NK-κB and Nrf-2 [5]. Moreover, in mammalian cells and C. elegans ROS acts as a second messenger and transfers signals through protein-tyrosine phosphatases, redox-sensitive phosphatases, and the insulin signaling pathway [6]. These observations suggest that ROS, acting as a messenger, could disrupt these pathways ultimately contributing to the aging process.
To combat the cytotoxic effects of ROS, there is an increase in the expression of proteins involved in the repair of ROS-mediated damage and ROS scavenging, including metallothionein (MTs). Metallothioneins are conserved, low molecular weight, cysteine-rich proteins involved in the regulation of copper and zinc homeostasis and protection against non-physiological transition metals, including cadmium. In addition, MTs remove ROS through interactions between hydroxyl radicals and conserved cysteine residues or through the release of zinc and its subsequent uptake into the membrane to suppress lipid peroxidation [7,8]. The role of MT in ameliorating ROS-mediated stress is supported by the observations that MT levels increase in response to chemicals that produce oxidative stress, endogenous ROS producing agents, and ROS byproducts [9–11]. In addition, in vivo MT levels change after treatment with oxidative stressors and MT-null mice are hypersensitive to ROS [12,13]. Interestingly, the overexpression of MT in mice causes increased longevity and variations in MT genotypes are associated with lifespan in humans [14,15].
Structurally and functionally the two C. elegans MTs, MTL-1 and MTL-2, are similar to MTs from other organisms: they contain cysteine motifs; bind cadmium and zinc; and their transcription is inducible by environmental stressors [16]. C. elegans MTs also increase expression in response to ROS generators, such as hydrogen peroxide and paraquat [17–19]. In contrast to higher eukaryotes, where the induction of MT transcription by metals and ROS is regulated through the interaction between metal responsive elements (MREs) and the metal-activated transcription factor MTF1 [20,21], the promoter region of the C. elegans MT gene mtl-1 does not contain MRE consensus sequences and mtl-2 contains one non-functioning MRE [16]. In addition, a C. elegans homolog of MTF1 has not been identified.
Analysis of the C. elegans MT genes defined minimal promoters necessary for stress-inducible mtl-1 and mtl-2 transcription: 366 and 324 bp upstream of the transcription start sites, respectively [22]. The minimal promoters contain at least one functional ELT-2 binding consensus element: GATA elements. ELT-2 is a constitutive transcriptional activator that regulates intestinal cell specific expression that is also required for MT expression in response to cadmium [22,23].
The lack of MREs and MTF1 in C. elegans and the presence of essential GATA elements in the promoters of mtl-1 and mtl-2 suggests a different mode of stress-inducible transcriptional regulation in C. elegans compared to MTs in other organisms [16]. A different mode of regulation is suggested in the earthworm (Lubricus rubellus), which also lacks MTF1, although the promoter region of the earthworm MT-2 gene does contain MREs [24]. To define the alternate mechanisms for metal-inducible MT transcription, transgenic nematodes containing GFP under the control of the minimal mtl-1 promoter were utilized in both candidate gene and mutagenesis gene screens to identify cadmium-responsive regulators of the C. elegans MT gene mtl-1. In both screens, the transcription factor ATF-7 was identified as an important regulator of mtl-1 expression. In addition, PMK-1, which regulates ATF-7 as part of the p38 MAPK pathway, and members of the insulin signaling pathway, PDK-1 and AKT-1/2, were identified as part of this emerging pathway. The insulin signaling pathway has been shown to be involved in both aging and the oxidative stress response in C. elegans [25]. This new metal-responsive regulatory pathway suggests a mechanistic link for MT, not only in regulating metal homeostasis, but also as an effector in the aging process, possibly through the removal of ROS.
Materials and methods
Strains
The following strains were used: N2 Bristol wild type; CB4856 Hawaiian wild type; JF97 mtl-1(tm1770); JF68 mtIs12 (pmtl-1::GFP, rol-6(su1006)); JF99 mtIs12;atf-7(mt12); RB759 akt-1(ok525); TJ1052 age-1(hx546); GR1310 akt-1(mg144); VC204 akt-2(ok393); VC1518 atf-7(gk715); EW15 bar-1(ga80); RB767 C48E7.11(ok532); DR1564 daf-2(m41); DR1572 daf-2(e1368); DR26 daf-16(m26); CF1038 daf-16(mu86); daf-16(mgDf50); FG7 grk-2(gk268); RB2177 hlh-11(ok2994); VC252 hmt-1(gk155); VC358 hsf-1(ok600); KB3 kgb-1(um3); VC1402 mef-2(gk633); FK171 mek-1(ks54); VC1120 nhr-17(gk509); RB1578 nhr-69(ok1926); RB1013 pax-2(ok935); JT709 pdk-1(sa709); KU25 pmk-1(km25); KU4 sek-1(km4); VC345 sgk-1(ok538); VC199 sir2.1(ok434); SP488 smk-1(mn156); PR678 tax-4(p678); DA1750 adEx1750[pmk-1::GFP + rol-6(su1006)]. Unless otherwise indicated, all strains were maintained and experiments conducted at 20°C using NGM agar plates containing E. coli OP50 as a food source [26].
Age synchronization of C. elegans was accomplished as previously described [27]. Briefly, gravid adult nematodes were incubated in alkaline hypochlorite solution (250 μM NaOH, 1% Clorox) to isolate embryos. Embryos were collected by centrifugation and then washed with K medium (32 mM KCl and 51 mM NaCl) [28]. To generate L4 C. elegans, embryos were placed on NGM plates with food and allowed to grow for 48 h at 20°C.
Mutagenesis screen
An ethyl methanesulfonate (EMS) mutagenesis screen was performed to identify regulators of mtl-1 transcription as previously described [26]. Briefly, age synchronized L4 JF68 hermaphrodites were exposed to 50 mM EMS for 4 h. Exposed nematodes (P0) were placed on NGM plates (1 nematode/plate) 24 h post exposure and incubated for 7 d to obtain F2 L4 progeny. F2 nematodes were collected in K+ medium (K medium plus 5 μg/ml cholesterol, 3 mM CaCl2 and 3 mM MgSO4) [28] and then dispensed, five nematodes per well, into 96-well plates using a COPAS Biosort (Union Biometrica, Inc., Holliston, MA) [29]. Each well also contained K+ medium, OP50, and 10 μM cadmium (final concentration). Nematodes were incubated at 20°C for 24 h, after which the levels of GFP were determined by visual observations using a Leica MZ16 FA dissecting fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL). Nematodes with increased levels of GFP, compared to non-mutagenized JF68, were isolated. GFP levels in the isolated strains were confirmed by exposing L4 progeny to 10 μM cadmium on NGM plates for 24 h.
Genomic characterization of EMS mutations
The location and specific nucleotide change present in each EMS mutant with altered mtl-1 transcription was determined using genome-wide single nucleotide polymorphism (SNP) mapping and whole-genome sequencing. SNP mapping was performed to identify genomic locations of the EMS-induced mutations using genomic PCR as previously described [30]. Genomic DNA was isolated from C. elegans by incubating individual nematodes in lysis buffer (50 mM KCl, 10 mM TrisHCl pH 8.3, 2.5 mM MgCl2, 0.45% (v/v) NP-40, 0.45% (v/v) Tween 20 and 0.05 μg/μl proteinase K) for 1 h at 65°C, followed by a 20 min incubation at 95°C. PCR was performed using primers designed to amplify regions surrounding restriction fragment length polymorphism SNPs, the PCR products were digested with Dra1 (New England Biolabs, Inc., Ipswich, MA), and then analyzed by agarose gel electrophoresis [31].
Whole-genome sequencing was used to identify specific mutations affecting mtl-1 transcription in the EMS mutagenized strains [32,33]. Initially each mutant strain was outcrossed six times to N2 wild type nematodes to produce strains with homogenous genetic backgrounds. Genomic DNA was isolated using Gentra Puregene Core Kit A (Qiagen, Inc., Valencia, CA) with modifications. Briefly, approximately 300 μl of packed nematodes were collected from six 100 mm NGM plates. Half of the packed nematodes were suspended in lysis solution containing Proteinase K (0.1 mg/ml) and incubated overnight at 55°C. Puregene RNase A was then added and the incubation continued for 1 h at 37°C. DNA was then extracted with phenol:chloroform:isoamyl alcohol and the aqueous phase extracted again with choloroform:isoamyl alcohol. Genomic DNA was precipitated following the addition of ammonium acetate and ice-cold 100% ethanol. Finally, air dried DNA pellets were dissolved in TE buffer.
Whole-genome sequencing was performed at the NIH Intramural Sequencing Center. Sequence data was generated from 10 μg of DNA using 103 nucleotide, paired-end reads, which provided a >40x genomic coverage for each mutant strain. Genome data was analyzed with MAQGene using the default parameters [33,34]. Preliminary analysis identified ~3,800 mutations per strain, relative to the reference genome. To determine which of these mutations was associated with the phenotype of interest, the following scheme was employed: First, mutations that were common to multiple strains were eliminated from consideration, as these variants were likely present in the parent nematode strain or in the wild type strain used to outcross the mutants. Removing the common mutations resulted in ~1,200 unique mutations per strain. Next, mutations not located within the region of the chromosome identified by SNP mapping were eliminated. This reduced the number of mutations to 100–500 per strain. Finally, mutations located within coding, non-coding, or regulatory regions of known or predicted genes were selected; which resulted in 2–5 potential mutant genes per strain. To identify the mutant gene, the ability of RNA interference (RNAi) to phenocopy the GFP response observed in the original mutant strain in both the presence and absence of cadmium was determined. Potential mutant genes were individually tested in the JF68 strain using RNAi as previously described [35,36].
Candidate screen
The ability of a known gene to affect mtl-1 transcription was determined by measuring steady-state mtl-1 mRNA or GFP levels in C. elegans strains with either mutations in the candidate gene (see Strains above) or by RNAi. For candidate genes tested using mutant strains, male nematodes containing the mutation of interest were crossed with JF68 L4 hermaphrodites. Rolling F2 progeny were isolated and lines in which 100% of the F3 progeny expresses the Rol phenotype were examined for the mutation either by genomic PCR or phenotypic analysis. JF68 L4 hermaphrodites that contained a mutation in the candidate gene were then exposed to 10 μM cadmium on NGM plates for 24 h. The level of GFP in treated and untreated animals was assessed by visual observation by fluorescence microscopy.
RNAi exposures were conducted by feeding L4 JF68 hermaphrodites bacteria expressing double stranded RNA (dsRNA) corresponding to the candidate gene of interest on RNAi plates (NGM plates containing 100 mg/ml ampicillin, 12 mg/ml tetracycline and 1 mM IPTG) for 72 h at 20°C [35,36]. L4 progeny were then transferred to RNAi plates with or without 10 μM cadmium and incubated for an additional 24 h. The effect of knocking down the expression of the candidate gene was assessed as described above. A minimum of three biological replicates were performed for each mutant strain or RNAi exposure.
RNA isolation and quantitative RT-PCR
To assess the contribution of candidate genes in regulating mtl-1 transcription, quantitative reverse transcription-real time-PCR (qRT-PCR) was used to measure the cadmium-responsive change in the steady-state mtl-1 mRNA levels in either mutant strains or RNAi-treated nematodes. Total RNA was isolated from age synchronized L4 hermaphrodites (~100) exposed to 100 μM cadmium for 1 h or 5 h at 20°C on NGM plates. Animals were then collected and incubated in K medium for 10 min to remove bacterial food from the intestinal lumen. C. elegans were collected by centrifugation (2000 rpm for 2 min) and rinsed once with K medium. The washed pellet was suspended in TRIZOL (Life Technologies Co. Grand Island, NY) and immediately frozen in liquid nitrogen. The solution was thawed at 37°C for 10 minutes and then transferred to tubes containing zirconia/silica beads. Nematode disruption was accomplished using a Mini- BeadBeater (Biospec Products, Inc., Bartlesville, OK) with a 30 s agitation at maximum speed. RNA was extracted with phenol:cholorofom, the aqueous phase was collected and then an equal volume of 70% ethanol added. Total RNA was isolated using Qiagen RNeasy kits (Qiagen Inc., Valencia, CA). The concentration of the purified RNA was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA).
For qRT-PCR, cDNA was generated from 55 ng of total RNA with SuperScriptTM III First-Strand Synthesis System for RT-PCR (Life Technologies, Grand Island, NY). qRT-PCR was performed using QuantiTect SYBR Green RT-PCR kits (Qiagen) following the manufacturer’s instructions in an ABI Prism 7900HT system (Applied Biosystems, Foster City, CA). The primers used for mtl-1 were: forward 5’-TGGATGTAAGGGAGACTGCAA-3’ and reverse 5’-CATTTTAATGAGCCGCAGCA-3’. Each biological replicate was measured in triplicates and a minimum of three biological replicates were conducted for each strain and condition.
To determine the role of the candidate gene in the regulation of cadmium-inducible mtl-1 transcription, mtl-1 mRNA levels were normalized to mlc-2 (myosin light chain). The primers used for mlc-2 were: forward 5’-TTGACAGGAACTGACCCAGAGG-3’ and reverse 5’-ATAGCCTTGACCTCATCCTCG-3’. The log2 fold change in the steady-state mtl-1 mRNA following cadmium exposure, compared to untreated wild type C. elegans, was then determined using the comparative CT method (2- ΔΔCT method) [37]. All values are presented as the mean log2 fold change ± standard error of means (S.E.M.). Statistical significance was assessed using a two-tailed unpaired t-test. To determine if there was a gene x cadmium effect, an interaction factor was calculated by two-way ANOVA analysis using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). The interaction factor was calculated from the difference in the slopes of the lines when graphing mtl-1 mRNA levels under the four exposure conditions [38]: cadmium treated versus untreated in wild type and mutant strains. Statistical significance was also determined for each interaction comparison.
Brood size analysis and embryonic lethality
Brood size analysis and embryonic lethality were measured in wild type N2, JF68, VC1518, and JF99 strains on NGM plates at 20°C, as previously described [39]. For each strain mean ± S.E.M. were determined from a total of n = 19–26 over six biological replicates. Statistical significance when comparing all strains to N2 wild type C. elegans was determined by one-way ANOVA followed by Dunnett’s multiple comparison test at a significance of p < 0.05. For comparisons of JF68 to JF99 and VC1518 to JF99, an unpaired two-tailed t-test was conducted to determine statistically significance differences.
Growth analysis
The Complex Object Parametric Analyzer and Sorter (COPAS) Biosort was used to determine the effects of cadmium and paraquat on growth as previously described [40]. Mutant strains were synchronized using the alkaline hypochlorite preparation as previously described [27]. Using the COPAS Biosort, 50 L1 larvae were placed in each well of a 96-well microtiter plate. Each well contained K-plus medium, OP50 E. coli, and either cadmium or paraquat at the indicated concentrations. Nematodes were then incubated at 20°C for 48 h. Visual inspection of the animals was made, and the COPAS Biosort was then used to determine the time of flight, which is a measure of the length of the nematode, and extinction, which is a measure of the optical density of the nematode. Both these measurements correspond to the developmental stage of the nematode. Under control conditions, wild type L1 larvae developed to the L4 stage in 48 h. Three independent experiments (n ~ 200 L1/experiment) were conducted at all concentrations. Statistical analysis of growth was accomplished as previously described [40].
PMK-1 nuclear translocation
The transgenic strain DA1750, which expresses a PMK-1-GFP fusion protein, was used to determine the ability of cadmium to induce nuclear accumulation of PMK-1 and the roles of akt-1 and akt-2 in this process. L4 hermaphrodites were exposed to 25, 100 and 200 μM cadmium on NGM plates for 5 h at 20°C. The location of PMK-1-GFP was assessed by fluorescence microscopy. Animals with discernible nuclear localization in the intestine were considered positive for translocation. Average percent ± S.E.M. of positive animals over four biological replicates (n = 153–164) is presented.
To define the contribution of akt-1/-2 in cadmium-induced nuclear translocation, L4 hermaphrodites were placed on NGM plates containing either vector RNAi or both akt-1 and akt-2 RNAi, and then incubated at 20°C for 48 h. L4 progeny were placed on corresponding RNAi plates containing 0, 25, 50, 100 or 200 μM cadmium for 5 h and the nuclear accumulation of PMK-1-GFP assessed. Average percent ± S.E.M. of positive animals over four biological replicates (n = 20–40 animals per replicate) is presented. Significant differences between cadmium-treated to untreated DA1750 nematodes were determined using an unpaired two-tailed t-test.
MT expression in HEK293 cells
To determine if activating transcription factor 7 (ATF7) and 3-phosphoinositide dependent protein kinase-1 (PDK1) participate in the regulation of mammalian MT expression, steady-state MT1A mRNA levels were measured following siRNA knockdown of ATF7 or PDK1 in HEK 293 cells. Human embryonic kidney 293T (ATCC # CRL-11268) cells were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, and 10% fetal bovine serum. Cells were transfected with siRNA for non-homologous control, metal-regulatory transcription factor 1 (MTF1), ATF7, or PDK1 using Lipofectamine RNAiMax according to manufacturer’s instructions (Life Technologies, Inc.). Twenty-four hours post transfection; cells were treated with 1 μM cadmium for 4 h. Total RNA was then isolated from both cadmium-treated and non-treated cells using RNeasy Mini Kits following manufacturer's instructions (Qiagen, Inc.). MT1A mRNA levels were determined by qRT-PCR as described above and normalized to β-actin. Primers used were: MT1A forward 5’-TCCTTATTCCCGGTGTCGCTA-3’ and reverse 5’-AGGTTTGTGGAATGCGCGTTC-3’; and β-actin forward 5’-GGAAATCGTGCGTGACATTAA G-3’ and reverse 5’-TCAGGCAGCTCGTAGCTCTTCT-3’. Fold change in MT1A mRNA levels for all treatments was compared to that observed in non-homologous siRNA untreated cells. The efficiency of siRNA knock down for MTF1, ATF7 and PDK1 was 60%, 85% and 95%, respectively. Three biological replicates were performed for each condition and significant changes determined as described above.
Results
Identification of novel regulators of mtl-1 transcription
Two approaches were employed to identify regulators of mtl-1 transcription: EMS mutagenesis and candidate gene screens. The EMS screen was conducted using pmtl-1::GFP, a transgenic C. elegans strain that expressed an integrated copy of GFP regulated by the mtl-1 promoter. A total of 8,000 homozygous F2 progeny from EMS-treated P0 nematodes were exposed to 10 μM cadmium. Those expressing increased levels of GFP compared to non-mutagenized pmtl-1::GFP nematodes were further tested. Ultimately, eleven confirmed independent mutagenized lines were isolated. Six of the eleven lines were mapped to chromosomes by SNP mapping: III (JF100, JF101 and JF99), V (JF102), and X (JF103 and JF104). Based on chromosome locations and whole genome sequence data, two to five potential genes per mutant strain were identified (Table 1). The roles of the potential genes in regulating mtl-1 transcription were tested using RNAi. Four of the five EMS mutant strains had one gene that when knocked down in the wild type strain phenocopied the original mutation (Table 1).
Table 1. Candidate genes identified by SNP mapping and whole genome sequencing.
| Mutant Strain |
Chromosome Location a |
Mutation Description | Transcript | Gene | Description b | GFP Expression c | |
|---|---|---|---|---|---|---|---|
| JF99 | III | 8042708 | GCC->CCC[Ala->Pro] | K12H4.6 | uncharacterized | wild type | |
| 4484031 | -5292 downstream | C07G2.2a | atf-7 |
basic-region leucine zipper (bZIP) transcription factor |
increased | ||
| 4484033 | -5290 downstream | ||||||
| JF100 | III | 847235 | TCT->GCT[Ser->Ala] | K02F3.2 | homolog of human aspartate/glutamate carrier), member 13 | wild type |
|
| 847236 | TCT->TGT[Ser->Cys] | ||||||
| 2253914 | ATG->ATT[Met->Ile] | T20H9.4 | fbxa-73 | F-boxA protein | wild type | ||
| 4059720 | GTT->TTT[Val->Phe] | E03A3.4 | his-70 | H3 histone | wild type | ||
| 8068399 | AGT->ATT[Ser->Ile] | K12H4.1 | ceh-26 | protein containing a prospero-related homeodomain | wild type | ||
| 10030880 | CAT->CAA[His->Gln] | M04D8.4 | regulated by cyc-1 and rsr-2; involved in lifespan | increased | |||
| JF101 | III | 7333093 | GGA->AGA[Gly->Arg] | F56C9.10 | orthology of human BCAS3; involved in lifespan | increased | |
| 8583361 | 3’ UTR | C06E1.3 | uncharacterized | wild type | |||
| JF102 | V | 13506615 | GCT->TCT[Ala->Ser] | R11G10.4 | uncharacterized | wild type | |
| 17490432 | AAC->ACC[Asn->Thr] | K03D7.8 | uncharacterized | wild type | |||
| 17490433 | AAC->CAC[Asn->His] | ||||||
| JF103 | X | 4565296 | 5’ UTR | C05E11.5 | amt-4 | member of the ammonium transporter protein family | wild type |
| 4914715 | ATC->AAC[Ile->Asn] | K05B2.3 | ifa-4 | intermediate filament protein | wild type | ||
| 5462742 | AGC->AGG[Ser->Arg] | C25F6.4 | ddr-1 | Discoidin Domain Receptor; protein tyrosine kinase homolog | increased | ||
| 5462743 | GCA->CCA[Ala->Pro] | ||||||
| 5462744 | GCA->GAA[Ala->Glu] | ||||||
a Chromosome number and location of altered base
b From Wormbase (18, 55)
c Level of GFP expression determined by visual observation in JF68 following RNAi treatment and exposure to 10 μM cadmium for 24 h.
Bold indicates genes confirmed to be regulators of mtl-1 transcription.
A candidate gene screen was also conducted to identify genes and pathways that may regulate mtl-1 transcription. Candidate genes from MAPK pathways, transcription factors involved in other stress response pathways, transcriptional regulators, receptors/channels, and PMK-1 interacting factors were tested. A complete list of the tested candidate genes and alleles can be found in S1 Table. Genes that affected mtl-1 transcription included: atf-7, pmk-1, akt-1(gof), pdk-1, mek-2, skn-1, fos-1, zfp-1, par-5, tax-4, ragc-1, and tir-1. Several of these genes were associated with the canonical insulin signaling pathway, pdk-1 and akt-1; and the p38 MAPK pathway, atf-7 and pmk-1.
Confirmation of ATF-7 as a regulator of mtl-1 transcription
In the candidate gene and EMS mutagenesis screens, the basic-region leucine zipper transcription factor, ATF-7 was identified as a potential regulator of mtl-1 transcription (Table 1, S1 Table). The EMS mutant strain pmtl-1::GFP;atf-7(mt12) contained mutations downstream of C07G2.2a, the atf-7 coding region. Knocking down atf-7 expression by RNAi in pmtl-1::GFP nematodes resulted in increased GFP levels, similar to those observed in pmtl-1::GFP;atf-7(mt12) (Table 1). When a strain of C. elegans containing a viable mutant allele of atf-7, gk715, was crossed with pmtl-1::GFP, increased levels of GFP were also observed in the absence of cadmium (Fig 1A). To confirm that pmtl-1::GFP;atf-7(mt12) contained an allele of atf-7, complementation analysis was conducted. Atf-7(gk715) males were crossed with pmtl-1::GFP;atf-7(mt12) hermaphrodites and F1 L4 larvae exposed to 0 or 10 μM cadmium for 24 h, and then GFP levels were assessed. Atf-7(gk715) failed to complement pmtl-1::GFP;atf-7(mt12) for the GFP response to cadmium, suggesting that the mutation in pmtl-1::GFP;atf-7(mt12) also affected atf-7. Further phenotypic comparison between pmtl-1::GFP;atf-7(mt12) and atf-7(gk715), brood size and embryonic lethality, showed no differences (S2 Table).
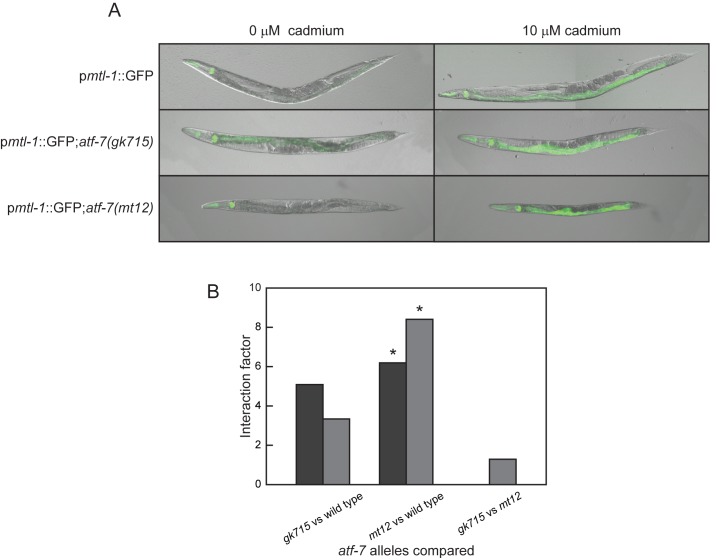
Fig 1. Role of ATF-7 in regulating mtl-1 transcription.
(A) Effect of 10 μM cadmium exposure for 24 h on wild type (pmtl-1::GFP) and two C. elegans stains containing mutant alleles of atf-7 (pmtl-1::GFP;atf-7(gk715) and pmtl-1::GFP;atf-7(mt15)) on GFP expression. (B) atf-7 gene x cadmium interaction factors were determined by comparing changes in mtl-1 mRNA levels after exposure to 100 μM cadmium for 1 (dark gray) h or 5 (light gray) h. * p < 0.05.
The atf-7 allele, gk715, is a deletion that includes a portion of the 5’ UTR, first exon, and part of the first intron. In contrast, all other atf-7 alleles, which include the second exon, are lethal. This suggests that gk715 may be a partial loss-of-function allele. Similar to the strain carrying gk715, pmtl-1::GFP;atf-7(mt12) nematodes were also viable. In addition, changes in mtl-1 mRNA levels after cadmium exposure were similar to wild type nematodes (Fig 2) suggesting that the atf-7 allele in pmtl-1::GFP;atf-7(mt12) was also a partial loss-of-function mutation. The levels of atf-7 mRNA in untreated atf-7(gk715) and pmtl-1::GFP;atf-7(mt12) nematodes were greater compared to wild type: 0.803±0.061 (p = 0.0013) and 1.16± 0.434 (p = 0.0758), respectively (Log2 fold change ± S.E.M.). In addition, atf-7 mRNA levels in atf-7(gk715) and pmtl-1::GFP;atf-7(mt12) were not significantly different (p = 0.405).
Fig 2. Effect of cadmium and mutations on mtl-1 mRNA levels.
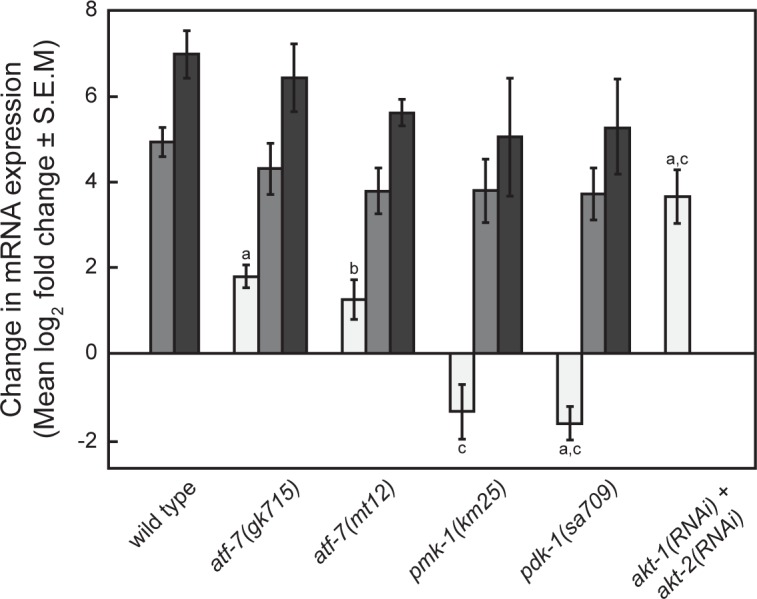
Difference in constitutive mtl-1 mRNA levels (white bars) or exposed to cadmium for 5 h (light gray) or 24 h (dark gray) in mutant strains compared to wild type N2 nematodes. a, p < 0.01 b, p < 0.05 and compared to wild type N2 and c, p < 0.01 compared to atf-7(gk715).
Based on the location of the EMS-induced mutation, the ability of atf-7 RNAi to phenocopy pmtl-1::GFP;atf-7(mt12), the failure of atf-7(gk715) to complement pmtl-1::GFP;atf-7(mt12), and similar phenotypes between these two strains, as well as similar atf-7 mRNA levels, we concluded that pmtl-1::GFP;atf-7(mt12) contained a new allele of atf-7, designated mt12.
Effect of ATF-7 on mtl-1 transcription
In untreated and cadmium-treated animals, increased GFP levels were observed for both pmtl-1::GFP;atf-7(mt12) and pmtl-1::GFP;atf-7(gk715) (Fig 1A). To confirm that these changes corresponded to an increase in the steady-state mtl-1 mRNA, qRT-PCR was performed on nematodes exposed to 0 or 100 μM cadmium for 1 h and 5 h. In the absence of metal, there were no significant differences in mtl-1 mRNA levels between pmtl-1::GFP;atf-7(mt12) and atf-7(gk715); however both had significantly greater levels than that observed in wild type N2 nematodes (Fig 2; p < 0.05). Following exposure to cadmium for 1 h or 5 h, steady state mtl-1 mRNA levels were not significantly different between pmtl-1::GFP;atf-7(mt12), atf-7(gk715), and wild type N2 C. elegans for both exposure conditions (Fig 2).
To determine if atf-7 influenced the ability of cadmium to affect mtl-1 mRNA expression, gene x cadmium interaction factors were calculated [38]. This analysis confirmed that cadmium and atf-7 (both mt12 and gk715) affected mtl-1 mRNA levels (Fig 1B). Interactions were significantly different for atf-7(mt12) at both 1 h and 5 h exposures compared to the wild type allele (p = 0.014 and 0.016, respectively). In addition, the interaction between the two atf-7 alleles showed that both alleles affected mtl-1 mRNA levels in a similar manner in the presence of cadmium (Fig 1B).
Identification of genes in the mtl-1 transcription regulatory pathway
Several of the genes identified in the candidate gene and EMS screens were components of the p38 MAPK and insulin signaling pathways. A detailed investigation of members of these pathways was initiated to further define their interactions and roles in regulating mtl-1 transcription.
PMK-1
PMK-1 is a member of the p38 MAPK signaling pathway that regulates ATF-7 activity[41]. To determine if PMK-1 was also involved in regulating mtl-1 expression, pmk-1 expression was either knocked down by RNAi in pmtl-1::GFP nematodes or through the use of the pmk-1(km25) deletion mutant. In the absence of cadmium, mtl-1 mRNA levels were lower in pmk-1(km25) nematodes compared to wild type N2 animals (p = 0.061) (Fig 2). In response to cadmium, mtl-1 mRNA levels in pmk-1(km25) nematodes increased to a level similar to that observed in wild type N2 and atf-7(gk715) C. elegans. In addition, there was no cadmium x gene (pmk-1) interaction (Fig 3A). The level of GFP decreased in pmk-1 RNAi-treated pmtl-1::GFP nematodes, compared to untreated animals, following cadmium exposure, which also suggested a role of PMK-1 in mtl-1 transcriptional regulation (S1 Table).
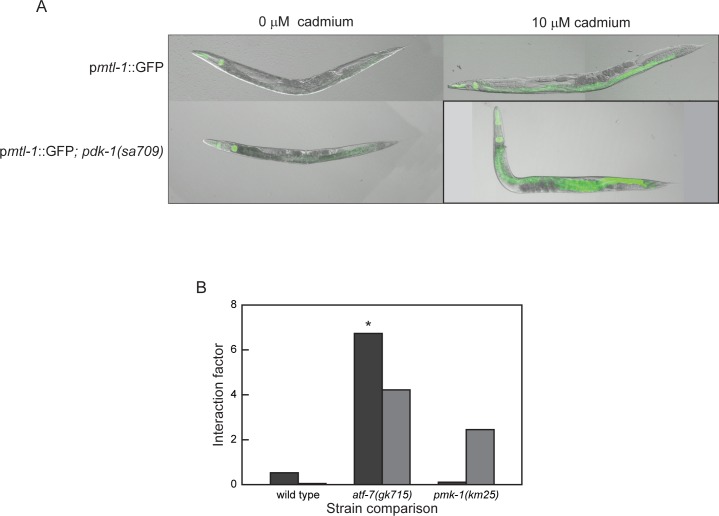
Fig 3. PMK-1 affects mtl-1 transcription and translocates to the nucleus in response to cadmium.
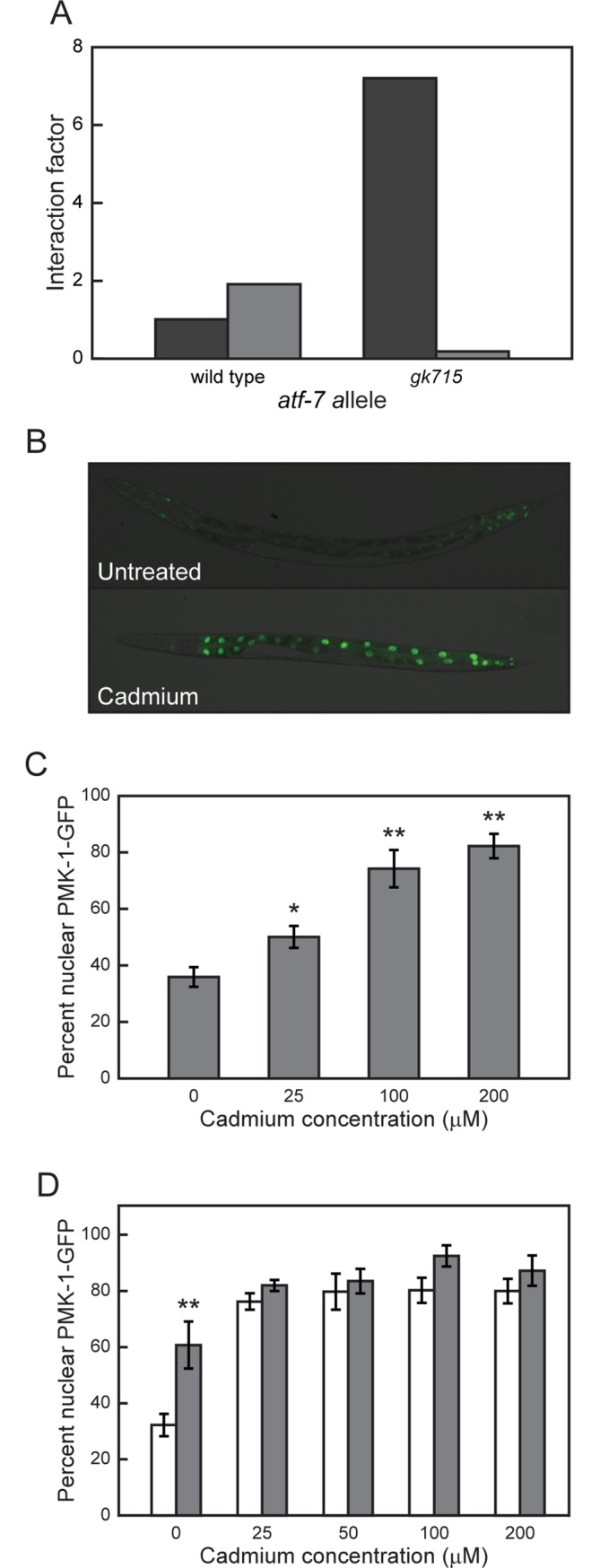
(A) pmk-1 gene x cadmium interaction factor determined by comparing changes of mtl-1 mRNA levels in pmk-1 mutants to either wild type N2 or atf-7(gk715) mutant after exposure to 100 μM cadmium for 1 (dark gray) h or 5 (light gray) h. (B) C. elegans strain pmk-1::GFP, which expresses a PMK-1::GFP fusion protein was exposed to 0, 25, 100 or 200 μM cadmium for 5 h. (C) Percentage of pmk-1::GFP animals in which PMK-1::GFP was localized in intestinal nuclei in response to indicated concentrations of cadmium and (D) after exposure to both cadmium and akt-1(RNAi);akt-2(RNAi) (light gray) or vector RNAi (white). Means ± S.E.M. for four biological replicates are presented. * p < 0.05 and ** p < 0.01.
PMK-1 translocates to the nucleus in response to stress where it then regulates ATF-7[41]. A transgenic strain of C. elegans that expressed a PMK-1::GFP fusion protein was used to assess the ability of cadmium to induce PMK-1 nuclear translocation. PMK-1 accumulated in the nucleus of intestinal cells and throughout the intestine after a 5 h exposure to 25, 100 and 200 μM cadmium (Fig 3B and 3C). In addition, there was a significant, concentration dependent increase in intestinal cell nuclei containing PMK-1::GFP, compared to untreated nematodes (p < 0.05 for all cadmium concentrations) (Fig 3C). These results further support a role for PMK-1 in the regulation of cadmium-inducible mtl-1 transcription.
PDK-1 and AKT-1/-2
PDK-1 (3-phosphoinositide-dependent kinase 1) acts on the AKT-1/AKT-2 complex (serine/threonine kinase Akt/PKB orthologs) in the C. elegans insulin signaling pathway to regulate downstream effectors (22). Loss of PDK-1 activity caused an increase in mtl-1 expression both in the presence and absence of cadmium, as determined by GFP expression in pmtl-1::GFP; pdk-1(sa709) nematodes (Fig 4A and S1 Table). In the presence of cadmium however, there was not a significant difference in mtl-1 mRNA levels between pdk-1(sa709) and wild type N2 nematodes (Fig 2), but mtl-1 mRNA levels were significantly lower than in wild type N2 nematodes in the absence of cadmium (p = 0.0033, Fig 2). Despite the difference in transcript levels, there was not a significant cadmium x gene (pdk- 1) interaction in the pdk-1(sa709) mutant (Fig 4B).
Fig 4. PDK-1 and AKT-1/-2 are involved in mtl-1 transcriptional regulation.
(A) Effect of 10 μM cadmium exposure for 24 h on wild type (pmtl-1::GFP) and pmtl-1::GFP;pdk-1(sa709) GFP expression. (B) pdk-1 gene x cadmium interaction factor determined by comparing changes of mtl-1 mRNA levels in pdk-1 mutants to either wild type N2, atf-7(gk715) or pmk-1(km25) mutants after exposure to 100 μM cadmium gene x cadmuim for 1 (dark gray) h or 5 (light gray) h. * p < 0.05 and ** p < 0.01.
When the expression of either akt-1 or akt-2 was knocked down, by either mutation or RNAi, GFP levels were not different than those observed in pmtl-1::GFP nematodes (S1 Table). GFP levels did increase in pmtl-1::GFP;akt-1(mg144), a gain-of-function allele, in the presence of cadmium. Since AKT-1 and AKT-2 act as a complex, the effect of knocking down the expression of both genes simultaneously using loss-of-function mutant alleles and/or RNAi was examined. In the absence of cadmium, knocking down the expression of both genes caused an increase in GFP expression and mtl-1 mRNA levels (Table 2 and Fig 2, respectively). These results indicated that the AKT-1/-2 complex was also involved in the regulation of mtl-1 transcription.
Table 2. GFP levels in pmtl-1::GFP strains exposed to RNAi and cadmium.
| RNAi | Gene(allele) a | Cadmium Concentration (μM)b | |
|---|---|---|---|
| 0 | 10 | ||
| Vector | Wild type | - | ++ |
| akt-1(mg144) | - | ++ | |
| akt-2(ok393) | ++ | ++ | |
| pdk-1(sa709) | +++ | +++ | |
| atf-7(gk715) | +++ | +++ | |
| akt-1 | Wild type | - | ++ |
| akt-2(ok393) | ++ | +++ | |
| akt-2 | Wild type | ++ | +++ |
| akt-1(mg144) | + | +++ | |
| akt-1+akt-2 | Wild type | ++ | +++ |
| pdk-1(sa709) | +++ | +++ | |
| atf-7(gk715) | +++ | +++ | |
| atf-7 | Wild type | ++ | +++ |
| pdk-1(sa709) | ++ | +++ | |
| pmk-1 | Wild type | - | +++ |
| pdk-1(sa709) | + | ++ | |
| atf-7(gk715) | - | ++ | |
a Mutant allele present in pmtl-1::GFP. Wild type refers to pmtl-1::GFP nematodes.
b L4 nematodes were exposed to cadmium for 24 h and the level of GFP in gut quantitated by visual observation using a Leica MZ16 FA dissecting fluorescence microscope. Three biological replicates were performed for each mutant-RNAi combination.
‘-‘ no visible GFP; ‘+’ light level of GFP; ‘++’ medium level of GFP; ‘+++’ high level of GFP
GFP levels were also assessed in pmtl-1::GFP strains either crossed with mutant alleles or exposed to RNAi for other members of the insulin signaling pathway: the receptor, daf-2; PI3 kinase, age-1; the serine/threonine protein kinase that complexes with akt-1/-2, sgk-1; and the FOXO transcription factor, daf-16. Under all circumstances, including double mutants, mtl-1 expression did not change (S1 Table). This suggested that PDK-1 and the AKT-1/-2 complex acted independently of the insulin signaling pathway to regulate mtl-1 transcription.
Relation among ATF-7, PMK-1, PDK-1, and AKT-1/-2
Pathway analysis was conducted to define the relation among the five genes shown to modulate the transcription of mtl-1: atf-7, pmk-1, pdk-1, and akt-1/-2. GFP levels were assessed in pmtl-1::GFP strains carrying pdk-1(sa709), akt-1(ok525), akt-2(ok393) or atf-7(gk715) loss-of-function alleles exposed to dsRNA for akt-1, akt-2, akt-1+akt-2, atf-7 or pmk-1 in the absence or presence of 10 μM cadmium (Table 2).
When pmtl-1::GFP;pdk-1(sa709) nematodes were simultaneously exposed to akt-1(RNAi) and akt-2(RNAi) the GFP levels increased in both the presence and absence of metal (Table 2). This suggested that the AKT-1/-2 complex was acting downstream of PDK-1 to regulate mtl-1 transcription, and that it was acting as a negative regulator. Further pathway analysis was conducted to determine if ATF-7 acted downstream of AKT-1/-2 to modulate mtl-1 transcription. When pmtl-1::GFP;atf-7(gk715) strains were exposed to akt-1(RNAi) and akt-2(RNAi) simultaneously, the GFP levels were identical to pmtl-1::GFP;atf-7(gk715), which suggested that ATF-7 was downstream from this complex (Table 2).
To define the relation between PDK-1, ATF-7 and PMK-1, pmtl-1::GFP;pdk-1(sa709) and pmtl-1::GFP;atf-7(gk715) nematodes were treated with pmk-1(RNAi). Following cadmium exposure, pmk-1(RNAi) caused a reduction in GFP levels in both strains (Table 2). If PMK-1 was a regulator of ATF-7 then, the double knockout should have resulted in a phenotype similar to that of atf-7(gk715) alone. This was not observed, however, likely because atf-7(gk715) is a partial loss-of-function allele. In the absence of PMK-1 any ATF-7 bound to the DNA could not be released (38). Both PMK-1 and PDK-1 were negative regulators and their effects on mtl-1 mRNA levels were similar: levels in untreated animals were -1.36±0.63 and -1.63±0.40, respectively (Mean log2 fold change ± S.E.M.; p = 0.7157, Fig 2); and a similar gene x cadmium effect, as determined by the interaction factor (Fig 4B). In addition, an increase in the amount of PMK-1 localized in intestinal cell nuclei significantly increased when both akt-1 and akt-2 were simultaneously knocked down via RNAi in pmk-1::GFP transgenic animals, compared to vector (Fig 3D; p < 0.01). This data along with the observation of an increase in mtl-1 expression after the simultaneous knockdown of akt-1 and akt-2 (Fig 2) suggests that the AKT-1/-2 complex acts as an inhibitor of PMK-1 translocation into the nucleus. These results suggested that PMK-1 may regulate ATF-7, which in turn controls mtl-1 transcription. In addition, this interaction was regulated by PDK-1 and the AKT-1/-2 complex.
Role in ROS response
Growth in response to paraquat exposure was analyzed in atf-7(gk715), atf-7(mt12), pdk-1(sa709), atf-7(gk715);pdk-1(sa709) double mutant, and mtl-1(tm1770) mutant lines to determine the effect of the presence of mtl-1 on the response to ROS. The EC50 was calculated based on growth response curves for all strains after exposure to paraquat. The atf-7(gk715) strain was hypersensitive to paraquat as compared to wild type (0.1341 and 0.2184, respectively; Table 3). Additionally, atf-7(mt12), pdk-1(sa709), and atf-7(gk715);pdk-1(sa709) double mutant also displayed a level of hypersensitivity to paraquat suggesting a role for mtl-1 in the ROS response.
Table 3. Calculated EC50 based on growth response curve after exposure to paraquat.
| Strain | EC50 (mM) |
|---|---|
| Wild type | 0.2184 |
| atf-7(gk715) | 0.1341 |
| atf-7(mt12) | 0.1656 |
| pdk-1(sa709) | 0.1688 |
| atf-7(gk715);pdk-1(sa709) | 0.1682 |
| mtl-1(tm1770) | 0.3228 |
Roles of ATF7 and PDK1 in regulating mammalian MT transcription
To determine if the mammalian homologs of ATF-7 or PDK-1, ATF7 and PDK1, respectively, regulated MT transcription, levels of MT1A mRNA were measured in cadmium treated and non-treated HEK293 cells following gene knock down using siRNA. MT1A mRNA levels significantly decreased after ATF7 or PDK1 knock down in cells not exposed to metal, relative to the non-homologous control (Table 4). Interestingly, the levels of MT1A mRNA were not significantly different than those observed when the expression of the metal-responsive transcription factor MTF1 [14,15] was knocked down (p = 0.292 and p = 0.338 for ATF7 and PDK1, respectively). Following cadmium exposure, MT1A mRNA levels significantly decreased when PDK1 expression was knocked down, relative to cells treated with the non-homologous siRNA (Table 4). For those in which ATF7 expression was knocked down, MT1A levels after Cd exposure were similar to those treated with the non-homologous siRNA. These results suggest that the regulation of MT transcription by at least PDK1 is an evolutionarily conserved process.
Table 4. Effect of siRNA on MT1A mRNA levels.
| siRNA | Cadmium concentration | |||
|---|---|---|---|---|
| 0 μM | 1 μM | |||
| Fold change a | p-value | Fold change | p-value | |
| non-homologous | 0 | N/A | 5.08 ± 0.27 | N/A |
| MTF1 | -1.12 ± 0.29 | 0.06 | 2.97 ± 0.26 | < 0.01 |
| ATF7 | -1.68 ± 0.36 | 0.04 | 4.01 ± 0.37 | 0.05 |
| PDK1 | -1.62 ± 0.36 | 0.05 | 2.63 ± 0.73 | 0.01 |
a All values, is mean log2 fold change +/- S.E.M, are relative to MT1A mRNA levels in cells treated with non-homologous siRNA in the absence of cadmium; N/A, not applicable.
Discussion
Data from molecular, biochemical, and epidemiological studies suggest linkages among exposure to environmental toxicants, the generation of ROS, and aging [2,6]. To ameliorate the damaging effect of ROS, and potentially increase longevity, several ROS detoxifying proteins are produced [3–5]. One class of these proteins are efficient scavengers of ROS, the MTs. C. elegans expresses two MT isoforms, MTL-1 and MTL-2, whose transcription is regulated by metals and other environmental stressors [16].
Based on studies with mammalian systems, several mechanisms, as both positive and negative regulation, have been proposed for the regulation of stress-inducible MT transcription. One proposed mechanism is that zinc binds to zinc-finger domains in MTF1 causing its nuclear translocation, binding to MREs, and then transcriptional activation. In an alternative model, stressors activate various intracellular signal transduction pathways that converge on MTF1 to activate the factor to induce transcription [42]. Negative regulation pathways involving chromatin remodeling and DNA methylation have also been investigated [43]. Although mechanisms for MT transcriptional activation have been extensively examined, several important questions remain regarding its regulation and MT’s role in the stress response. For example: how do non-zinc stressors such as heavy metals or ROS, activate transcription; what are the roles of MTF1 interacting proteins in transcriptional regulation [44]; and what are the mechanistic linkages among MT expression, aging and diet? In contrast to MT genes from higher eukaryotes, the C. elegans MT genes lack conserved MREs and a MTF1 homolog has not been identified. The uniqueness of the C. elegans genes provides an opportunity to investigate alternative mechanisms for the regulation of MT stress-inducible expression.
EMS mutagenesis and a candidate gene screen were used to identify novel pathways involved in MT transcriptional regulation in response to cadmium. Both screens identified the transcription factor ATF-7 to be involved in the regulation; a new allele, mt12, was identified from the EMS screen. ATF-7 is an ortholog of the mammalian ATF2/ATF7/CREB5 family of basic leucine zipper transcription factors, where it acts as a negative regulator of transcription [45]. As part of the innate immunity response in C. elegans ATF-7 is regulated by phosphorylation via PMK-1 [41]. PMK-1 is an ortholog of mammalian p38, which phosphorylates mammalian ATF7 to release it from the promoter in response to stress [45]. In mammalian systems, p38, as well as other MAPK signaling pathways proteins, contribute to the regulation of cadmium-inducible transcription [46,47]. PMK-1 regulates ATF-7 to affect mtl-1 transcription, as evidenced by a decrease in mtl-1 mRNA in pmk-1(km25) mutants, PMK-1 translocation to intestinal nuclei after cadmium exposure, and pathway analysis using RNAi (Figs 2 and 3; Table 2).
Genetic and RNAi analyses confirmed that PDK-1 and AKT-1/-2 act independently of the insulin signaling pathway to regulate cadmium-inducible mtl-1 transcription (S1 Table). In addition, RNAi pathway analysis suggests that PDK-1 and AKT-1/-2 act in the same pathway as PMK-1 and ATF-7 to regulate mtl-1 transcription (Table 2). PDK-1 is a 3-phosphoinositide-dependent kinase that acts downstream of the insulin/IGF-1-like receptor, DAF-2, and the PI3 kinase, AGE-1, within the insulin signaling pathway. PDK-1 is known to phosphorylate the serine/threonine kinase AKT-1/-2 complex [48]. This complex directly binds the FOXO transcription factor DAF-16 to inhibit its activation [49]. The data in this report indicates that mtl-1 transcription is activated through part of the insulin signaling pathway (PDK-1 and AKT-1/-2), bypassing the DAF-2 insulin-like receptor to activate ATF-7 to derepress MT transcription.
Mammalian ATF7 binds to CRE motifs (TGACGTCA) as a homodimer in vitro [50]. The promoter of mtl-1 contains three CRE-like sequences: AGACGTCA at -811; TCAGCGTCA at -794; and AAACGTCA at -337 bp, relative to the transcription start site. The latter is located within the 366 bp minimal mtl-1 promoter that is necessary for cadmium-inducible expression in all C. elegans life stages [22]. Interestingly, deletion of the region between -366 and -320 bp, which includes the CRE-like sequence, limits cadmium-inducible mtl-1 expression to L1 and L2 larva. In addition, deletion of the region between -366 and -253 bp, which includes the CRE-like sequence and a functional ELT-2 binding site (-290), blocks cadmium-inducible mtl-1 transcription [22]. ELT-2 is a constitutively active C. elegans transcription factor that limits transcription to intestinal cells [23]. Previous results suggested that ELT-2 binding was necessary for MT transcription and that additional metal-responsive factors were required to induce transcription. In addition, data suggested that this factor may be a negative regulator [22]. Further evidence of CRE motifs in earthworms (Lubricus rubellus) found them be important in the transcriptional regulation of mtl-2 suggesting an evolutionary role of these motifs and CREB as a transcriptional activator [24].
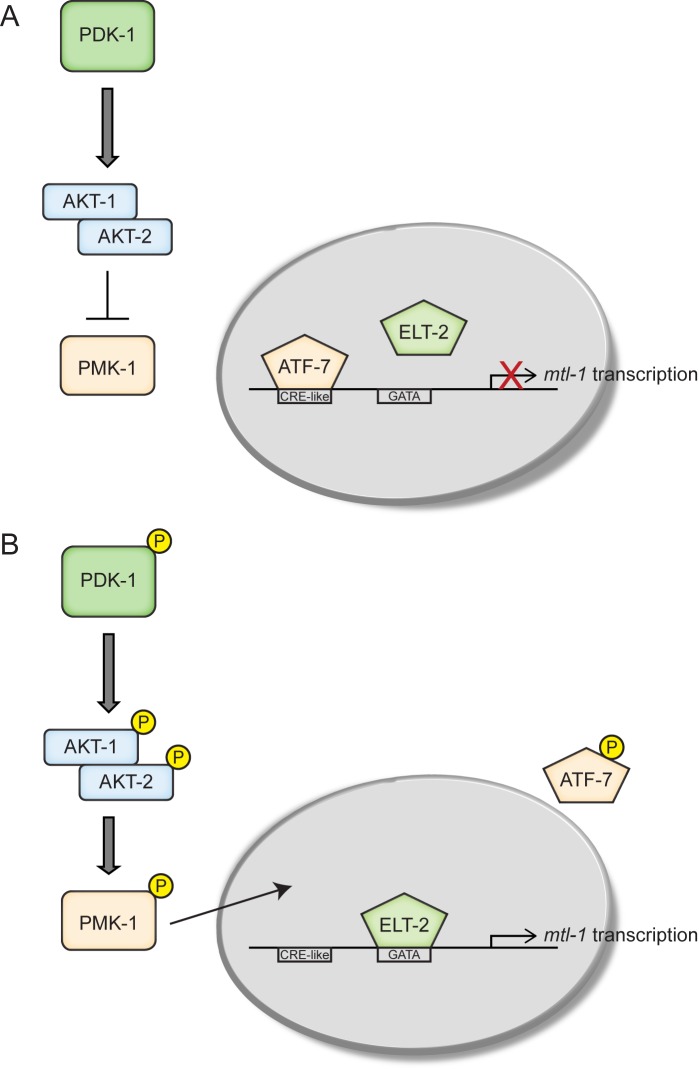
Based on previous studies and current genetic and RNAi data, a mechanism can be proposed for the regulation of cadmium-inducible mtl-1 transcription (Fig 5). In the absence of stress, ATF-7 resides on the CRE-like sequence at -337 in the promoter of mtl-1 to inhibit ELT-2 from binding at -290 (Fig 5A). Based on Drosophila and mouse data it is likely that this inhibition is due to possibly the formation of heterochromatin structures from histone H3K9 trimethylation [51–53]. ATF-7 is known to act as either a homodimer or a heterodimer with ATF-2 [51,52]. Our data suggests that for the regulation of mtl-1, ATF-7 acts as a homodimer given that the knockdown of atf-2 did not affect mtl-1 expression (S1 Table). Upon cadmium exposure, upstream factors are affected that ultimately activate PDK-1 that subsequently phosphorylates the AKT-1/-2 complex. Based on the current data (increase in mtl-1 expression when akt-1 and akt-2 are knockdowned, Fig 2) and that fact that the AKT-1/-2 complex is known to bind DAF-16 to inhibit activation it is likely that the complex may also interact with PMK-1 thus inhibiting its activation. Thus the AKT-1/-2 complex, after being phosphorylated by PDK-1, releases PMK-1 causing it to translocate to the nucleus and phosphorylate ATF-7, which releases it from the mtl-1 promoter. The release of ATF-7 allows ELT-2 to bind thus initiating transcription (Fig 5B).
Fig 5. Proposed pathway for mtl-1 transcriptional activation in C. elegans.
(A) In times of no stress, ATF-7 is bound to the CRE-like sequence located in the promoter region of mtl-1 thus blocking transcriptional activation. Additionally, the AKT-1/-2 complex is inhibiting the ability of PMK-1 to translocate to the nucleus. (B) Upon the introduction of stress PDK-1 is phosphorylated which leads to the phosphorylation of the AKT-1/-2 complex. This activation causes the release and subsequent activation of PMK-1 allowing it to translocate to the nucleus. PMK-1 phosphorylated ATF-7 releasing it from the DNA which allows ELT-2 to bind the GATA sequence initiating transcription of mtl-1.
Based on the data presented in this report, MT transcription is activated through part of the insulin signaling pathway. The insulin signaling pathway is important for various cellular responses including growth factor signaling in humans, and regulating metabolism, development and longevity in C. elegans. PDK-1 and AKT-1/-2 can initiate insulin signaling phenotypes, such as adult longevity and stress resistance independent of AGE-1 [54] and germline apoptosis independent of DAF-16 [55]. In addition, PDK-1 and AKT-1/-2 regulate dauer formation and pathogen resistance independent of the insulin signaling pathway [56,57]. AKTs are activated by multiple inputs to affect the activity of a variety of downstream target proteins that regulate the transcription of multiple genes suggesting they play central roles in the convergence of many pathways [58]. Thus, it is consistent with previous observations that PDK-1 and AKT-1/-2 are acting independent of the insulin signaling pathway to regulate cadmium-inducible mtl-1 transcription.
In HEK293 cells, knock down of PDK1 and ATF7 resulted in significant decreases in steady-state MT1A mRNA levels, in the absence of cadmium and in the presence of cadmium after the knock down of PDK1 (Table 4). In contrast to mammalian cells, the loss of ATF7 in C. elegans causes an increase in mtl-1 mRNA levels. This suggests that at least at the level of PDK1, the signaling pathway identified in C. elegans may also function in higher eukaryotes; however additional factors may be involved in the regulation of cadmium-responsive transcription.
Evidence suggests that MTs not only function to ameliorate the toxic effects of transition metal exposure, but also protect against ROS-mediated damage. MT mRNA levels increase in response to ROS; MTs confer resistance to ROS in cell culture; and MT loss results in hypersensitivity to various oxidizing agents [13,19]. Additionally, in our study, all strains related to MT expression were hypersensitive to paraquat (Table 3). The observation that part of the insulin signaling pathway regulates MT transcription provides a mechanistic model that links this pathway to changes in longevity: by increasing MT expression to reduce the levels of ROS. Overexpression of MT causes a decrease in free radical activity with a concomitant increase in longevity in mice [15,59]. In C. elegans, the insulin signaling pathway component PDK-1 regulates oxidative stress resistance and is involved in regulating lifespan [57]. Together these observations support a hypothesis that the longevity phenotype associated with the insulin signaling pathway are mediated by MT. That is, increasing the expression of MT expression via this pathway reduces the levels of ROS, ultimately increasing lifespan.
Genetic analysis identified portions of the insulin signaling pathways as regulators of cadmium-inducible mtl-1 transcription. Further analysis confirmed that the genes in this pathway act upstream of the p38 MAPK, PMK-1, and the bZIP transcriptional repressor, ATF-7. Based on this analysis and previous results a model for regulation of cadmium-inducible transcription based on the derepression of the constitutively active transcription factor ELT-2 was proposed. In addition, knockdown of the mammalian homologs of PDK1 and ATF7 in HEK293 cells results in changes in MT expression, suggesting that this pathway is evolutionarily conserved. The insulin signaling pathway affects the aging process, thus an association between the activation of portions of the insulin signaling pathway to control MT expression provides a mechanistic link between MT, ROS, and aging.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the following people for their assistance with this manuscript: Ginger Miley for the construction of the pmtl-1::GFP strain; Rachel Goldsmith for assistance with fluorescence microscopy; Julie Rice for assistance with the COPAS Biosort; and Marjolein Smith and Sandra McBride (SRA International) for assistance with the statistical analysis. Nematode strains used in this work were provided by the Caenorhabditis Genetics Center.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported [in part] by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01ES102045 and Z01ES102046). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jin K. Modern Biological Theories of Aging. Aging Dis. 2010;1: 72–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11: 298–300. [DOI] [PubMed] [Google Scholar]
- 3.Back P, Braeckman BP, Matthijssens F. ROS in aging Caenorhabditis elegans: Damage or signaling? Oxidative Medicine and Cellular Longevity. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790: 1005–1014. doi: 10.1016/j.bbagen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicology and Applied Pharmacology. 2009. pp. 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radical Biology and Medicine. 2012. pp. 539–555. doi: 10.1016/j.freeradbiomed.2011.10.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas JP, Bachowski GJ, Girotti AW. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986;884: 448–461. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta (BBA)/Protein Struct Mol. 1985;827: 36–44. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14: 325–37. Available: http://www.ncbi.nlm.nih.gov/pubmed/8458590 [DOI] [PubMed] [Google Scholar]
- 10.Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110: 347–354. [DOI] [PubMed] [Google Scholar]
- 11.Braithwaite EK, Mattie MD, Freedman JH. Activation of metallothionein transcription by 4-hydroxynonenal. J Biochem Mol Toxicol. 2010;24: 330–334. doi: 10.1002/jbt.20342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270: 5506–10. Available: http://www.ncbi.nlm.nih.gov/pubmed/7890668 [DOI] [PubMed] [Google Scholar]
- 13.Swindell WR. Metallothionein and the biology of aging. Ageing Research Reviews. 2011. pp. 132–145. doi: 10.1016/j.arr.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipriano C, Malavolta M, Costarelli L, Giacconi R, Muti E, Gasparini N, et al. Polymorphisms in MT1a gene coding region are associated with longevity in Italian Central female population. Biogerontology. 2006;7: 357–365. doi: 10.1007/s10522-006-9050-x [DOI] [PubMed] [Google Scholar]
- 15.Malavolta M, Basso A, Piacenza F, Giacconi R, Costarelli L, Pierpaoli S, et al. Survival study of metallothionein-1 transgenic mice and respective controls (C57BL/6J): influence of a zinc-enriched environment. Rejuvenation Res. 2012;15: 140–3. doi: 10.1089/rej.2011.1261 [DOI] [PubMed] [Google Scholar]
- 16.Freedman JH, Slice LW, Dixon D, Fire A, Rubin CS. The novel metallothionein genes of Caenorhabditis elegans: Structural organization and inducible, cell-specific expression. J Biol Chem. 1993;268: 2554–2564. [PubMed] [Google Scholar]
- 17.Cui Y, McBride SJ, Boyd W a, Alper S, Freedman JH. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 2007;8: R122 doi: 10.1186/gb-2007-8-6-r122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LW S, JH F, CS R. Purification, characterization, and cDNA cloning of a novel metallothionein-like, cadmium-binding protein from Caenorhabditis elegans. J Biol Chem. 1990;265: 256–263. [PubMed] [Google Scholar]
- 19.Zeitoun-Ghandour S, Leszczyszyn OI, Blindauer C a, Geier FM, Bundy JG, Stürzenbaum SR. C. elegans metallothioneins: response to and defence against ROS toxicity. Mol Biosyst. 2011;7: 2397–2406. doi: 10.1039/c1mb05114h [DOI] [PubMed] [Google Scholar]
- 20.Dalton TP, Li Q, Bittel D, Liang L, Andrews GK. Oxidative stress activates metal-responsive transcription factor-1 binding activity. Occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J Biol Chem. 1996;271: 26233–26241. [DOI] [PubMed] [Google Scholar]
- 21.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39: 267–294. doi: 10.1146/annurev.pharmtox.39.1.267 [DOI] [PubMed] [Google Scholar]
- 22.Moilanen LH, Fukushige T, Freedman JH. Regulation of metallothionein gene transcription. Identification of upstream regulatory elements and transcription factors responsible for cell-specific expression of the metallothionein genes from Caenorhabditis elegans. J Biol Chem. 1999;274: 29655–29665. [DOI] [PubMed] [Google Scholar]
- 23.McGhee JD, Fukushige T, Krause MW, Minnema SE, Goszczynski B, Gaudet J, et al. ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev Biol. 2009;327: 551–65. doi: 10.1016/j.ydbio.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hockner M, Dallinger R, Stürzenbaum SR. Metallothionein gene activation in the earthworm (Lumbricus rubellus). Biochem Biophys Res Commun. 2015;460: 537–542. doi: 10.1016/j.bbrc.2015.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaletsky R, Murphy C. The role of insulin/IGF-like signaling in C. elegans longevity and aging. Dis Model Mech. 2010;3: 415–419. doi: 10.1242/dmm.001040 [DOI] [PubMed] [Google Scholar]
- 26.Sulston J, J H. Methods Wood W B (ed), The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 587–606. [Google Scholar]
- 27.Khanna N, Cressman CP, Tatara CP, Williams PL. Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch Environ Contam Toxicol. 1997;32: 110–114. [DOI] [PubMed] [Google Scholar]
- 28.Williams PL, Dusenbery DB. Using the Nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health. 1988;4: 469–478. doi: 10.1177/074823378800400406 [DOI] [PubMed] [Google Scholar]
- 29.Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol Biol. 2006;351: 275–286. doi: 10.1385/1-59745-151-7:275 [DOI] [PubMed] [Google Scholar]
- 30.Davis MW, Hammarlund M, Harrach T, Hullett P, Olsen S, Jorgensen EM. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6: 118 doi: 10.1186/1471-2164-6-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuhrman LE, Shianna K V., Aballay A. High-throughput isolation and mapping of C. elegans mutants susceptible to pathogen infection. PLoS One. 2008;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flibotte S, Edgley ML, Chaudhry I, Taylor J, Neil SE, Rogula A, et al. Whole-genome profiling of mutagenesis in Caenorhabditis elegans. Genetics. 2010;185: 431–441. doi: 10.1534/genetics.110.116616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarin S, Bertrand V, Bigelow H, Boyanov A, Doitsidou M, Poole RJ, et al. Analysis of multiple ethyl methanesulfonate-mutagenized Caenorhabditis elegans strains by whole-genome sequencing. Genetics. 2010;185: 417–430. doi: 10.1534/genetics.110.116319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigelow H, Doitsidou M, Sarin S, Hobert O. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat Methods. 2009;6: 549 doi: 10.1038/nmeth.f.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421: 231–7. doi: 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- 36.Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263: 103–112. [DOI] [PubMed] [Google Scholar]
- 37.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 38.McDonald J. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014. [Google Scholar]
- 39.Hall J, Haas KL, Freedman JH. Role of MTL-1, MTL-2, and CDR-1 in mediating cadmiumsensitivity in Caenorhabditis elegans. Toxicol Sci. 2012;128: 418–426. doi: 10.1093/toxsci/kfs166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyd WA, McBride SJ, Rice JR, Snyder DW, Freedman JH. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol Appl Pharmacol. 2010;245: 153–159. doi: 10.1016/j.taap.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivers RP, Pagano DJ, Kooistra T, Richardson CE, Reddy KC, Whitney JK, et al. Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet. 2010;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Günther V, Lindert U, Schaffner W. The taste of heavy metals: Gene regulation by MTF-1. Biochim Biophys Acta—Mol Cell Res. 2012;1823: 1416–1425. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi S. Positive and negative regulators of the metallothionein gene (Review). Mol Med Rep. 2015;12: 795–799. doi: 10.3892/mmr.2015.3459 [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Kimura T, Huyck RW, Laity JH, Andrews GK. Zinc-induced formation of a coactivator complex containing the zinc-sensing transcription factor MTF-1, p300/CBP, and Sp1. Mol Cell Biol. 2008;28: 4275–84. doi: 10.1128/MCB.00369-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maekawa T, Jin W, Ishii S. The role of ATF-2 family transcription factors in adipocyte differentiation: antiobesity effects of p38 inhibitors. Mol Cell Biol. 2010;30: 613–25. doi: 10.1128/MCB.00685-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 2000;21: 1423–1432. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10874022 [PubMed] [Google Scholar]
- 47.Hung JJ, Cheng TJ, Lai YK, Chang MD. Differential activation of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinases confers cadmium-induced HSP70 expression in 9L rat brain tumor cells. J Biol Chem. 1998;273: 31924–31931. [DOI] [PubMed] [Google Scholar]
- 48.Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13: 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from age-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters CS, Liang X, Li S, Kannan S, Peng Y, Taub R, et al. ATF-7, a Novel bZIP Protein, Interacts with the PRL-1 Protein-tyrosine Phosphatase. J Biol Chem. 2001;276: 13718–13726. doi: 10.1074/jbc.M011562200 [DOI] [PubMed] [Google Scholar]
- 51.Seong K, Li D, Shimizu H, Nakamura R, Ishii S. Inheritance of Stress-Induced, ATF-2-Dependent Epigenetic Change. Cell. Elsevier Inc.; 2011;145: 1049–1061. doi: 10.1016/j.cell.2011.05.029 [DOI] [PubMed] [Google Scholar]
- 52.Maekawa T, Kim S, Nakai D, Makino C, Takagi T, Ogura H, et al. Social isolation stress induces ATF-7 phosphorylation and impairs silencing of the 5-HT 5B receptor gene. EMBO J. Nature Publishing Group; 2010;29: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida K, Maekawa T, Zhu Y, Renard-guillet C, Chatton B, Inoue K, et al. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol. 2015;16: 1034–1043. doi: 10.1038/ni.3257 [DOI] [PubMed] [Google Scholar]
- 54.Gami MS, Wolkow CA. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell. 2006. pp. 31–37. doi: 10.1111/j.1474-9726.2006.00188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quevedo C, Kaplan DR, Derry WB. AKT-1 Regulates DNA-Damage-Induced Germline Apoptosis in C. elegans. Curr Biol. 2007;17: 286–292. doi: 10.1016/j.cub.2006.12.038 [DOI] [PubMed] [Google Scholar]
- 56.Evans EA, Chen WC, Tan MW. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7: 879–893. doi: 10.1111/j.1474-9726.2008.00435.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertweck M, Göbel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6: 577–588. [DOI] [PubMed] [Google Scholar]
- 58.Shmookler Reis RJ, Bharill P, Tazearslan C, Ayyadevara S. Extreme-longevity mutations orchestrate silencing of multiple signaling pathways. Biochimica et Biophysica Acta—General Subjects. 2009. pp. 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malavolta M, Cipriano C, Costarelli L, Giacconi R, Tesei S, Muti E, et al. Metallothionein downregulation in very old age: a phenomenon associated with cellular senescence? Rejuvenation Res. 2008;11: 455–9. doi: 10.1089/rej.2008.0679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.