Abstract
Background
Skin autofluorescence, a biomarker for advanced glycation end products (AGEs) accumulation, has been shown to predict diabetes-related cardiovascular complications and is associated with several environmental and lifestyle factors. In the present study, we examined the association between various smoking behaviors and skin autofluorescence, as well as the association between several cotinine biomarkers and skin autofluorescence, using both epidemiological and metabolomics data.
Methods
In a cross-sectional study, we evaluated participants from the LifeLines Cohort Study and the Qatar Metabolomics Study on Diabetes (QMDiab). In the LifeLines Cohort Study smoking behavior and secondhand smoking were assessed in 8,905 individuals including 309 individuals (3.5%) with type 2 diabetes. In QMDiab, cotinine biomarkers were measured in saliva, plasma and urine in 364 individuals of whom 188 (51%) had type 2 diabetes. Skin autofluorescence was measured non-invasively in all participants using the AGE Reader.
Results
Skin autofluorescence levels increased with a higher number of hours being exposed to secondhand smoking. Skin autofluorescence levels of former smokers approached levels of never smokers after around 15 years of smoking cessation. Urinary cotinine N-oxide, a biomarker of nicotine exposure, was found to be positively associated with skin autofluorescence in the QMDiab study (p = 0.03).
Conclusions
In the present study, we have demonstrated that secondhand smoking is associated with higher skin autofluorescence levels whereas smoking cessation has a beneficial effect on skin autofluorescence. Finally, urinary cotinine N-oxide might be used as an alternative way for questionnaires to examine the effect of (environmental) tobacco smoking on skin autofluorescence.
Introduction
Advanced glycation end products (AGEs) are the final products of non-enzymatic glycation and oxidative reactions [1] and comprise a group of irreversibly modified proteins, lipids and nucleic acids [2]. AGEs form stable structures with—and accumulate in—tissues as a result of aging [3,4]. The formation and accumulation of AGEs is increased in conditions such as diabetes and renal insufficiency [5,6]. AGE accumulation can be measured non-invasively in the skin with a device known as the AGE Reader (Diagnoptics Technologies, Groningen, The Netherlands) [7]. Previous studies have demonstrated that higher skin autofluorescence (SAF) is associated with, and a good predictor of the development of cardiovascular morbidity and mortality in patients with diabetes and (end-stage) renal failure [8–10]. In addition, recent studies have shown that SAF levels are increased in patients with chronic obstructive pulmonary disease and peripheral artery disease, independent of diabetes status [11,12].
Several studies have shown elevated SAF levels in smokers compared to non-smokers [9,13]. Moreover, we have recently found both current smoking and the number of pack-years to be strongly associated with higher SAF levels in a large-scale general population [14]. Active and passive tobacco smoking increase the risk of cardiovascular disease and type 2 diabetes, while higher SAF levels are associated with both conditions. As tobacco smoke has been reported to be an exogenous source of AGEs, its accumulation might be considered as an underlying mechanism leading to cardiovascular disease and type 2 diabetes [15].
In studies examining the risk of tobacco smoking on disease outcome, smoking behavior is assessed by general questionnaires. Questionnaires regarding tobacco use are prone to underestimation and reporting biases [16]. Furthermore, the majority of these questionnaires do not include questions regarding secondhand smoking. An alternative way to examine exposure to tobacco smoke is through assessment of biomarkers for tobacco smoke, measured in either urine, saliva or blood [17].
Cotinine, the main metabolite of nicotine, is considered to be a valid biomarker for environmental tobacco smoke exposure due to its high sensitivity, specificity and long half-life time [18,19]. Since secondhand smoking might be underestimated using questionnaires, it would be interesting whether SAF is a more accurate and reliable measure to detect—long-term—exposure to secondhand smoking.
The aim of the present study was to examine the effect of both active and secondhand smoking as well as smoking cessation on SAF. In addition, using metabolomics data we also assessed the association between several cotinine biomarkers and SAF.
Materials and methods
Study design
For this cross-sectional study, we used data from two different populations, the Dutch LifeLines Cohort Study and Qatar Metabolomics Study on Diabetes (QMDiab). The LifeLines Cohort Study is a multidisciplinary prospective population-based cohort study with a unique three-generation design that examines the health and health-related behaviors of 8,905 participants in the northern part of The Netherlands [20]. For the current study, we included all participants from Western European origin between 18 and 80 years old with both SAF measurements and information regarding smoking behavior, who participated in our previous studies regarding (genetic) determinants of SAF [14,21]. Exclusion criteria were missing data for smoking status (n = 104) and severely impaired renal function (serum creatinine >140 micromol/L (n = 14) leaving 8,905 individuals, of whom 309 (3.5%) with type 2 diabetes, available for analysis. Before participating in the study, all participants provided written informed consent. The study protocol was approved by the medical ethical review committee of the University Medical Center Groningen, The Netherlands.
The Qatar Metabolomics Study on Diabetes (QMDiab) [22] is a large collaborative effort between the Dermatology Department of Hamad Medical Center (HMC) in Doha, Qatar and Weill Cornell Medical College—Qatar (WCMC-Q).
Subjects were enrolled between February 2012 and June 2012. Before participating in the study, all participants provided written informed consent. Ethical approval was obtained from the Institutional Review Board from both HMC and WCMC-Q. In total, 374 subjects above the age of 18 participated in the study. Smoking data were missing from ten participants, leaving 364 participants of whom 185 (51%) with type 2 diabetes for analyses.
Data collection
Information regarding ethnicity and smoking behavior was obtained from questionnaires. In QMDiab, ethnicity was determined based on the birthplace of the participant, both parents and four grandparents as described previously [23]. Data on smoking behavior was collected by a detailed self-administered questionnaire. Subjects were classified according to their smoking status as never smoker, former smoker or current smoker (types of tobacco: cigarette, cigarillo, cigar, pipe tobacco). Never smokers were those who had not smoked during the last month and had never smoked for longer than a year. Former smokers were defined as those who had reported smoking for more than a whole year and who had not smoked during the last month and had stopped smoking. Those who had smoked for longer than a year and had not stopped smoking were classified as current smoker. Estimation of total tobacco use of current smokers and their classification into light, moderate and heavy smokers were estimated based on the following quantities: one cigarette = 1 gram tobacco. Light smoking was defined as 10 gram/day or less, moderate as 11 to 20 gram/day and heavy as more than 20 gram/day. Pack-years of smoking was calculated as the number of packs of cigarettes smoked per day multiplied by the number of years a subject has smoked. Finally, information about the age at start and quitting smoking as well as whether a subject have been exposed to secondhand smoking at home was obtained by the questionnaire.
Diagnosis of diabetes mellitus was established at their LifeLines baseline visit either by a single fasting blood plasma glucose level ≥7.0 mmol/L, or when participants reported to have diabetes which was checked with their medication use (i.e. use of oral blood-glucose lowering agents and/or insulin).
Anthropometry
Using standardized protocols, trained technicians measured body weight with the participant wearing light clothing and without shoes with a 0.1 kg precision. Height was measured without shoes to the nearest 0.5 cm. Body Mass Index (BMI) was calculated as weight (kg) divided by height squared (m2).
Biochemical measures
Blood was collected in the fasting state between 8.00 and 10.00 a.m. and transported to the LifeLines laboratory facility at room temperature or at 4°C, depending on the sample requirements. On the day of collection, fasting blood glucose was measured using a hexokinase method. HbA1c (EDTA-anticoagulated) was analyzed using a turbidimetric inhibition immunoassay on a Cobas Integra 800 CTS analyzer (Roche Diagnostics Nederland BV, Almere, The Netherlands). Creatinine clearance was calculated using the Cockcroft-Gault formula [24].
Cotinine was used as a biomarker for environmental tobacco smoke exposure. Cotinine was measured in non-fasting saliva, plasma and urine specimens which were collected and processed using standardized protocols [22]. In brief, saliva was obtained using the Salivette system following the manufacturer’s recommendations (Sarstedt, Germany). After collection, samples were stored on ice for transportation.
Within six hours after sample collection, all samples were centrifuged at 2,500g for 10 minutes, aliquoted, and stored at -80°C.
Metabolic profiling was achieved using ultra-high-performance liquid-phase chromatography (UHPLC) and gas-chromatography separation, coupled with tandem mass spectrometry (GCMS) at Metabolon Inc. using established procedures (Durham, NC, USA) [25]. The units represent ion counts as measured by the mass spectrometer, which represent semi-quantitative values. In total, 1,568 different metabolites were detected. Osmolality in saliva and urine was measured for normalization purposes.
AGE reader
For both study groups, SAF was measured with the AGE Reader (Diagnoptics Technologies, the Netherlands). This method has been described in detail previously [7,13]. SAF measures the accumulation of AGEs in the skin which relationship has been demonstrated by measuring AGEs from skin biopsies [7]. The AGE Reader illuminates a skin surface of approximately 4 cm2, guarded against surrounding light, with an excitation light source whose wavelength is between 300 and 420nm (peak intensity at ~ 370nm).
Emission light and reflected excitation light from the skin are measured with an internal spectrometer in the range 300 to 600nm. Measurements were performed on the volar side of the forearm, 10cm below the elbow, at room temperature. SAF was calculated by dividing the average emitted light intensity per nanometer in the range of 420–600 nm by the average excitated light intensity per nanometer in the range 300–420 nm and multiplying by 100, and is expressed in arbitrary units (AU). Previous studies have shown an error percentage of around 5–6% when repeated SAF measurement were taken over a single day in control subjects and diabetic patients [7]. The AGE Reader measures SAF also in individuals with a pigmented skin, but only when the UV reflection is above 6%. With a UV reflection below 6% SAF is not given when using the current AGE Reader software.
Statistical analysis
Data are shown as mean ± standard deviation (SD) or median and interquartile range (IQR) in case of non-normally distributed data. SAF Z-scores were calculated based on the total population. Univariate Analysis of Variance (ANOVA) with a post-hoc Bonferroni test was used to determine differences between the smoking groups. The effect of secondhand smoking (hours per day) on SAF Z scores (adjusted for age, creatinine clearance and diabetes status) was assessed in never smokers and former smokers. We investigated the effect of smoking cessation (in years after smoking abstinence) on SAF (Z-scores) and adjusted in the analysis for age, BMI, creatinine clearance and diabetes status. Multivariable linear regression analysis was performed to examine the association between cotinine biomarkers measured in different specimens and SAF. SPSS (version 22, IBM, Armonk, NY, USA) was used for statistical analysis. A P-value <0.05 was considered statistically significant.
Results
The clinical characteristics of the LifeLines participants according to their smoking status are shown in Table 1. Mean (±SD) age was 48 ± 12 years in never smokers, 53 ± 11 years in former smokers and 46 ± 10 years for current smokers (p<0.0001). BMI was significantly higher among former smokers compared to never and current smokers (p<0.0001). Current smokers had significantly more pack-years smoked compared to former smokers (16.5 vs 7.7 pack-years, p<0.0001). In former smokers, median time since smoking cessation was 16.7 years (interquartile range 8.0–26.4). Mean SAF levels were significantly higher among current smokers compared to former and never smokers (in non-diabetic subjects, p<0.0001 and in type 2 diabetic individuals, p<0.001). Moreover, in all smoking groups, subjects with type 2 diabetes had significantly higher SAF levels compared to individuals without diabetes (within never smokers, p = 0.001 and within former- and current smokers, p<0.0001).
Table 1. Subjects characteristics of the study populations.
| Characteristics LifeLines cohort | Never smokers | Former smokers | Current smokers |
| N | 3614 | 3321 | 1970 |
| Type 2 diabetes/non-diabetes | 106 (3) / 3508 (97) | 147 (4) / 3174 (96) | 56 (3) / 1914 (97) |
| Age (years) | 48 ± 12 | 53 ± 11*** | 46 ± 10 |
| Gender (male/female) n (%) | 1361 (38) / 2253 (62) | 1423 (43) / 1898 (57) | 913 (46) / 1057 (54) |
| Body mass index (kg/m2) | 26.4 ± 4.4 | 27.0 ± 4.2 *** | 26.1 ± 4.2 |
| Creatinine clearance (ml/min) | 113 ± 31 | 110 ± 31 | 119 ± 32 *** |
| Fasting blood glucose | |||
| type 2 diabetes | 7.7 ± 1.9 | 8.2 ± 2.6 | 7.8 ± 2.4 |
| non-diabetes | 5.0 ± 0.5 | 5.1 ± 0.5 *** | 5.0 ± 0.5 |
| HbA1c (%) | |||
| type 2 diabetes | 6.7 ± 1.1 | 7.0 ± 1.2 | 6.7 ± 1.2 |
| non-diabetes | 5.5 ± 0.3 | 5.6 ± 0.3 *** | 5.6 ± 0.3 |
| HbA1c (mmol/mol) | |||
| type 2 diabetes | 50.0 ± 12.3 | 52.7 ± 12.7 | 50.2 ± 12.9 |
| non-diabetes | 36.9 ± 3.3 | 37.3 ± 3.5 *** | 37.3 ± 3.4 |
| Estimated diabetes duration (years) | 6.5 (2.9–11.2) | 6.6 (3.1–12.3) | 4.3 (3.7–7.3) |
| Duration since stop smoking (years) | n.a. | 16.7 (8.0–26.4) | n.a. |
| Pack-years | n.a. | 7.7 (3.2–15.0) | 16.5 (9.6–25.0) *** |
| SAF (AU) | |||
| type 2 diabetes | 2.27 ± 0.49 | 2.48 ± 0.53 | 2.65 ± 0.61 ** |
| non-diabetes | 1.94 ± 0.40 | 2.09 ± 0.43 | 2.14 ± 0.48 *** |
| Age-adjusted SAF Z-scores | |||
| type 2 diabetes | 0.05 ± 0.16 | 0.65 ± 0.16 | 1.98 ± 0.26 *** |
| non-diabetes | -0.34 ± 0.02 | -0.15 ± 0.03 | 0.57 ± 0.04 *** |
| Characteristics QMDiab cohort | Never Smokers | Former Smokers | Current smokers |
| N | 269 | 61 | 34 |
| Type 2 diabetes/non-diabetes | 133 (49) / 136 (51) | 35 (57) / 26 (43) | 17 (50) / 17 (50) |
| Age (years) | 46 ± 13 | 51 ± 12 | 47 ± 11* |
| Gender (male/female) n (%) | 101 (38) / 168 (62) | 56 (92) / 5 (8) | 29 (85) / 5 (15) * |
| Body mass index (kg/m2) | 29.9 ± 6.3 | 28.4 ± 4.2 | 28.1 ± 4.8 |
| Ethnicity | |||
| Arab (%) | 157 (58) | 27 (44) | 18 (53) |
| South Asian (%) | 75 (28) | 25 (41) | 12 (35) |
| Filipino (%) | 27 (10) | 6 (10) | 3 (9) |
| Other or mix (%) | 10 (4) | 3 (5) | 1 (3) |
| Serum creatinine (umol/L) | 70 ± 17 | 89 ± 23 * | 77 ± 16 |
| HbA1c (%) | |||
| type 2 diabetes | 8.0 ± 1.8 | 8.3 ± 1.6 | 8.2 ± 2.4 |
| non-diabetes | 5.5 ± 0.4 | 5.7 ± 0.4 | 5.7 ± 0.4 |
| HbA1c (mmol/mol) | |||
| type 2 diabetes | 64.3 ± 19.4 | 67.2 ± 17.5 | 66.5 ± 26.2 |
| non-diabetes | 36.6 ± 4.6 | 38.5 ± 0.3 | 38.0 ± 4.7 |
| Estimated diabetes duration (years) | 10.5 ± 10.0 | 11.4 ± 10.6 | 8.7 ± 10.0 |
| Pack-years | n.a. | 10.8 ± 14.3 | 14.0 ± 13.9 |
| Cotinine (saliva, ion counts) | 51.4 x 104 ± 45.2 x 104 (n = 23) | 34.6 x 104 ± 21.2 x 104 (n = 12) | 71.4 x 104 ± 83.8 x 104 (n = 27) |
| Cotinine (plasma, ion counts) | 28.1 x 104 ± 14.4 x 104 (n = 17) | 22.3 x 104 ± 13.4 x 104 (n = 14) | 35.0 x 104 ± 19.8 x 104 (n = 30) |
| Cotinine (urine, ion counts) | 30.6 x 104 ± 67.8 x 104 (n = 37) | 43.6 x 104 ± 42.2 x 104 (n = 20) | 81.3 x 104 ± 87.1 x 104 (n = 32) * |
| Cotinine N oxide (urine, ion counts) | 3.0 x 104 ± 4.8 x 104 (n = 88) | 4.1 x 104 ± 3.8 x 104 (n = 28) | 12.9 x 104 ± 10.1 x 104 (n = 33) *** |
| Hydroxy-cotinine (urine, ion counts) | 191.8 x 104 ± 148.0 x 104 (n = 10) | 158.6 x 104 ± 68.1 x 104 (n = 5) | 277.8 x 104 ± 275.9 x 104 (n = 14) |
| SAF (AU) | |||
| type 2 diabetes | 2.43 ± 0.72 | 2.29 ± 0.58 | 2.49 ± 0.62 |
| non-diabetes | 2.10 ± 0.51 | 2.04 ± 0.58 | 2.23 ± 0.74 |
| Age-adjusted SAF Z-scores | |||
| type 2 diabetes | 0.24 ± 0.25 | 0.66 ±0.38 | 0.50 ± 0.67 |
| non-diabetes | -0.10 ± 0.18 | -0.72 ± 0.41 | -0.04 ± 0.59 |
Data are presented as means ± standard deviation, or median (interquartile range) and number (%).SAF, Skin autofluorescence; AU, Arbitrary Units.
* p<0.05
** p <0.001
*** p <0.0001
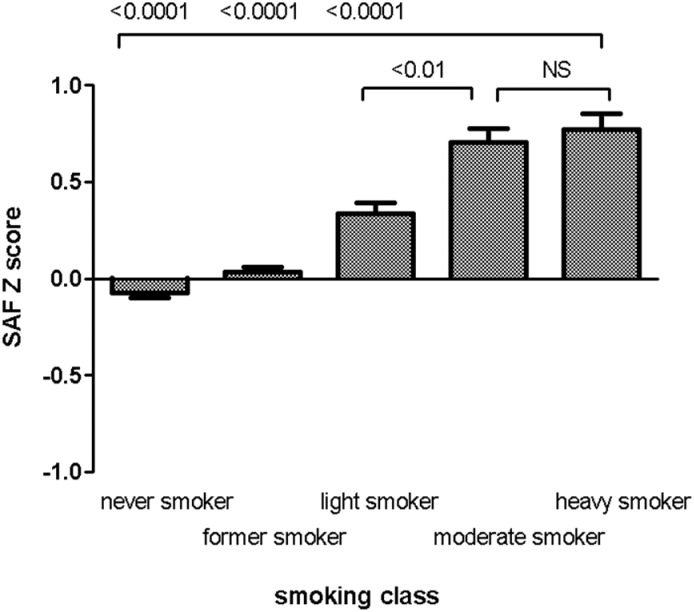
Fig 1 shows SAF levels for different smoking classes according to the amount of tobacco smoked per day. SAF levels were highest among heavy smokers compared to light smokers, former and never smokers (p<0.0001). Significantly higher SAF levels were found in moderate vs. light smokers (p<0.01) but not in heavy vs. moderate smokers (p = 0.08).
Fig 1. Skin autofluorescence stratified for smoking class (LifeLines Cohort Study).
Bars represent mean SAF Z scores (adjusted for age, creatinine clearance and diabetes), whiskers reflect standard error of the mean. Never smoker (n = 3670), Former smoker (n = 3321), Light smoker (0–10 gram tobacco per day, n = 878), Moderate smoker (10–20 gram tobacco per day, n = 537), heavy smoker (>20 gram tobacco per day, n = 475). SAF, skin autofluorescence; AU, arbitrary units; NS, not significant.
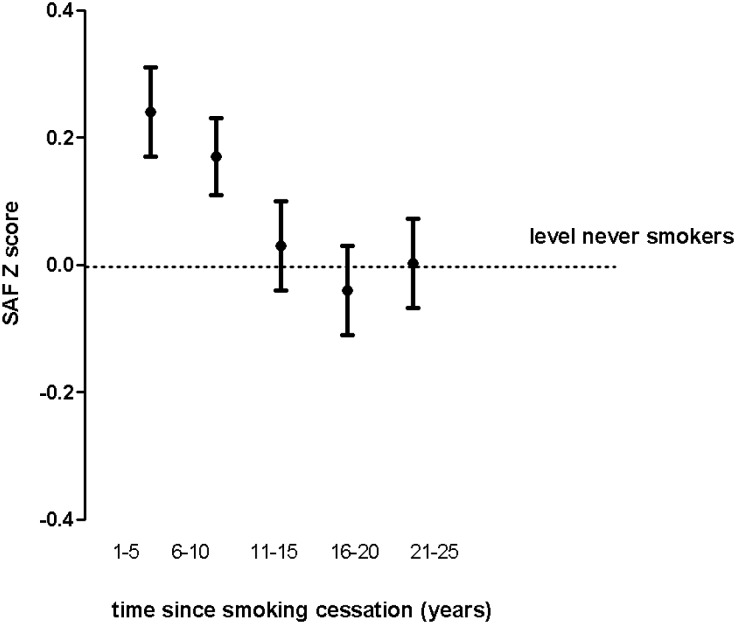
The effect of smoking cessation on SAF is shown in Fig 2. SAF Z-scores of former smokers approached levels of never smokers after 15 years of smoking cessation, also after adjusting for age and BMI.
Fig 2. Effect of smoking cessation on skin autofluorescence in former smokers participating in the LifeLines study.
Dots show mean SAF Z-scores (adjusted for age, BMI, creatinine clearance and diabetes status). Whiskers reflect standard error of the mean. SAF, skin autofluorescence
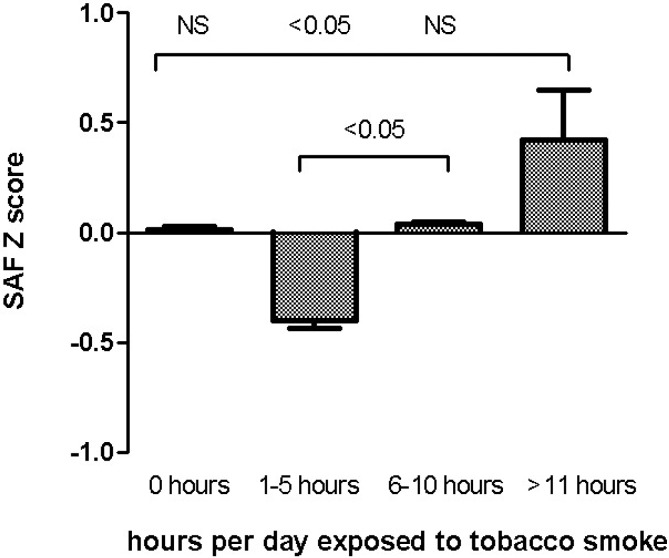
At last, we examined the effect of secondhand smoking on SAF (adjusted for age and diabetes status) in never smokers (as well as former smokers who have stopped smoking for more than 15 years, which is based on the previous analysis). A gradual increase in SAF with the number of hours reportedly being exposed to secondhand was observed (Fig 3).
Fig 3. Effect of secondhand smoking on skin autofluorescence in never- and former smokers participating in the LifeLines study.
Bars represent mean SAF Z scores (adjusted for age, creatinine clearance and diabetes status) in never smokers and former smokers who stopped smoking for more than 15 years, whiskers reflect standard error of the mean. 0 hours (n = 4213), 1–5 hours (n = 676), 6–10 hours (n = 78), >11 hours (n = 15). SAF, skin autofluorescence; Arbitrary Units, AU; NS, not significant.
Individuals who had been exposed to secondhand smoking for more than 11 hours per day had significantly higher SAF levels compared to subjects who had been exposed 1–5 hours per day, but not compared to individuals exposed for 6–10 hours or 0 hours per day.
The characteristics of the QMDiab Study participants are shown in Table 1. Mean (±SD) age was 46 ± 13 years in never smokers, 51 ± 12 years among former smokers and 47 ± 11 years in current smokers (p = 0.02). The prevalence of type 2 diabetes was highest among former smokers (57%). There were no significant differences regarding both salivary cotinine and plasma cotinine among the smoking groups. Both urinary cotinine (p<0.05) as well as cotinine N-oxide (p<0.0001) levels were highest for current smokers compared to former and never-smokers.
Five markers for tobacco smoke were evaluated: cotinine in saliva, plasma and urine, and cotinine N-oxide and hydroxy-cotinine in urine. The sensitivity, specificity and the predictive value of the five markers for current smoking are shown in S1 Table. Measured in 148 subjects, cotinine N-oxide had the highest sensitivity (100%) but a relatively low specificity (63.8%) for current smoking.
Cotinine N-oxide was detected in 115 out of 316 individuals (36.4%) who reported to be former or never smoker. However, cotinine in plasma (detected in 61 subjects) had the highest predictive value for current smoking (AUC = 0.94, 95% CI: 0.89, 0.98).
Table 2 shows the results from the multivariable linear regression analyses between smoking variables (smoking status and cotinine biomarkers) and SAF. Current smoking status was significantly associated with higher SAF in the total population. After adjusting for confounders, urinary cotinine N-oxide was found positively and significantly (p = 0.03) associated with SAF in the total population and in individuals without diabetes.
Table 2. Smoking status and cotinine markers related to skin autofluorescence using multivariable analyses stratified by diabetes status (QMDiab Study).
| Coefficient Beta (CI) | P value | Coefficient Beta(CI) | P value | Coefficient Beta (CI) | P value | |
|---|---|---|---|---|---|---|
| All subjects | Non-diabetes | Type 2 diabetes | ||||
| Questionnaire | ||||||
| Current smoker | 0.24 (0.04, 0.45) | 0.02 | 0.22 (-0.03, 0.47) | 0.08 | 0.35 (0.00, 0.69) | 0.05 |
| Former smoker | 0.05 (-0.12, 0.21) | 0.59 | 0.17 (-0.19, 0.22) | 0.87 | 0.08 (-0.19, 0.35) | 0.56 |
| Cotinine markers | ||||||
| Cotinine saliva | 0.01 (-0.05, 0.07) | 0.85 | 0.02 (-0.04, 0.08) | 0.53 | -0.02 (-0.15, 0.11) | 0.75 |
| Cotinine plasma | 0.05 (-0.01, 0.10) | 0.12 | 0.07 (-0.01, 0.15) | 0.07 | 0.04 (-0.05, 0.13) | 0.40 |
| Cotinine urine | 0.05 (-0.00, 0.11) | 0.07 | 0.06 (-0.01, 0.12) | 0.06 | 0.08 (-0.05, 0.22) | 0.23 |
| Cotinine N Oxide (urine) | 0.06 (0.01, 0.12) | 0.03 | 0.08 (-0.01, 0.15) | 0.03 | 0.06 (-0.04, 0.16) | 0.21 |
| Hydroxy Cotinine (urine) | 0.04 (-0.02, 0.10) | 0.17 | 0.04 (-0.03, 0.10) | 0.29 | 0.05 (-0.05, 0.16) | 0.32 |
Values represent regression coefficients (95% confidence interval) and their corresponding p-values. Model was adjusted for age, gender, ethnicity, body mass index, reflectance, creatinine clearance and the presence of type 2 diabetes. For categorical or dichotomous variables, the effect estimates represent the difference in skin AF compared to the reference group. Markers in saliva and urine were normalized by osmolality and subsequently Z-score normalized to make effect sizes comparable. Thus, effect estimates represent change in SAF per standard deviation increase of cotinine marker. Cotinine metabolites were measured in n = 330 (saliva), n = 358 (plasma), and n = 360 (urine).
Discussion
In the present study, we combined data from a large population-based cohort study in the Netherlands with metabolomics data from a smaller cohort study in Qatar to examine the effect of smoking intensity, secondhand smoking and smoking cessation on SAF, as well as the association between cotinine biomarkers and SAF.
In our previous study, we have already reported have higher SAF levels in smokers compared to non-smokers [14]. The present study shows that smoking intensity influences SAF levels as well. SAF levels were highest in heavy smokers compared to light smokers, whereas no significant difference was found between heavy and moderate smokers.
Hoonhorst et. al. found a positive association between the amount of pack-years smoked and higher SAF levels among patients with chronic obstructive pulmonary disease indicating a dose-dependent effect [12]. Ohkuma et. al. examined the association between tobacco smoking and glycaemic control in type 2 diabetes patients and reported a significant increase in HbA1c levels with higher smoking intensity [26].
While Cerami et. al. have shown AGEs to be present in aqueous extracts of tobacco, Nicholl et. al. found higher AGEs levels in the lenses and blood vessels of tobacco smokers, demonstrating that tobacco smoke is an exogenous source of AGEs [15,27]. Tobacco smoke is associated with increased systemic oxidative stress, which may in turn contribute to exogenous AGEs formation as well [28]. AGEs have negative effects on insulin sensitivity, which might be due to increased oxidative stress and inflammation [29,30]. In addition, several AGEs can form cross-links within the vascular wall which results in impaired protein function and as a result increased vascular stiffness [31]. Finally, interaction of circulating AGEs with the AGEs receptor (RAGE) stimulates the production of pro-inflammatory cytokines, enhances oxidative stress [32] and causes endothelial dysfunction [33]. Therefore, tobacco smoke as an exogenous source of AGEs accumulation, may play a potential role in the underlying pathophysiological mechanism of type 2 diabetes [13] and a wide range of cardiovascular diseases, including (sub)clinical atherosclerosis, [34] coronary artery [35] and peripheral artery disease [36],
Using metabolomics data we have shown that cotinine N-oxide, a biomarker for environmental tobacco smoke exposure, is significantly associated with higher SAF levels in a group of individuals without diabetes. Therefore, urinary cotinine N-oxide might be used as an alternative way for questionnaires in demonstrating the effect of tobacco smoking on SAF. Remarkably, we found high levels of cotinine in never smokers, particularly in urine and saliva. Cotinine concentrations are four to five times higher in urine than in plasma and saliva which makes urine more eligible for detecting low-level exposure [37]. This could explain our detected sensitivity of 100% for cotinine N-oxide and supports our thoughts regarding the high levels of cotinine N-oxide in never smokers caused by secondhand smoking. Moreover, data from the third National Health and Nutrition Examination Survey (NHANES) suggest that around 90% of never smokers have detectable levels of serum cotinine [38]. It has been reported that the serum cut-off point to discriminate adult active smokers from non-smokers is 3 ng/ml [39]. Several explanations might be given for the fact that we observed high levels of cotinine in non-smokers. Firstly, in Qatar and the Middle-East, many public areas (such as restaurants) in the Qatar and the Middle-East still allow smoking, increasing the exposure to secondhand smoking in non-smokers. Secondly, we cannot exclude the possibility of heavy secondhand smoke exposure for some of the individuals, as well as the fact of misclassification of subjects smoking status according to their self-reports (e.g. smokers who occasionally smoke might be in fact considered as active smokers). In the study from Benowitz et. al, some of the individuals who reported to be a non-smoker had cotinine levels similar to levels of active smokers [39]. According to the cotinine levels reported in our study, it is likely that at least a proportion of non-smokers were in fact active smokers. Thirdly, racial/ethnic differences in the rate of metabolism of nicotine and cotinine have been described [40]. This supports our thoughts about secondhand exposure in (non-caucasian) non-smokers having a slower nicotine metabolism, and as a result, have elevated cotinine levels.
Fourthly, exposure to thirdhand smoke might also be an explanation for the high cotinine levels observed in non-smokers. Thirdhand smoke is generally considered to be residual tobacco smoke left on (indoor) surfaces such as sofas and wallpapers. Finally, several kinds of nutrition, such as vegetables and black tea, contain small amounts of nicotine but these levels are almost negligible.
In addition to active smoking, we were able to demonstrate that secondhand smoking is associated with higher SAF levels as well. Although we did not find all groups to be significantly different, a marked increase in SAF levels with higher exposure to secondhand smoking was observed. From these results, we learn that secondhand smoking can be added to the other determinants associated with SAF as demonstrated in our previous study [14].
Secondhand smoke is a combination of sidestream smoke and mainstream smoke which is exhaled smoke by an active smoker [41]. Sidestream smoke contains a relatively higher concentration of unfiltered toxic gases and small respirable particles than mainstream smoke [42] and is thus potentially more hazardous compared to the smoke which is directly inhaled by active smokers [43]. Accumulating evidence has shown that secondhand smoking increases the risk of type 2 diabetes [44] and coronary artery disease [45,46] by increasing oxidative stress, systemic inflammation and endothelial dysfunction [28,47]. Furthermore, tobacco smoking may contribute to insulin resistance [48] and has been demonstrated to be associated with a higher risk of pancreatitis and pancreatic cancer suggesting that chemical compounds in tobacco smoke may have direct toxic effects on pancreatic β cells [49]. In a similar way as active smoking, it is highly likely that enhanced AGEs formation is involved in the pathophysiological pathway by which secondhand smoking increases the risk of both type 2 diabetes and cardiovascular diseases.
To our knowledge, this is the first study showing that quitting smoking has a reversible effect on SAF levels. We observed that SAF Z-scores of former smokers gradually decreased with increasing years since quitting smoking, even after adjusting for BMI.
After around 15 years of smoking abstinence, SAF Z-scores had returned to levels of never smokers. Ohkuma et. al. have shown a decrease in HbA1c levels in subjects with type 2 diabetes as the years since quitting smoking increased [26]. Since HbA1c is actually an intermediate product of glycation, our findings might be considered in line with their results.
Our data provide some indication that at least part of the effect of tobacco smoking on AGEs accumulation is reversible in individuals who quit smoking, indicating a beneficial effect of smoking cessation on both metabolic and glycaemic control.
It has been reported that smoking cessation reduces the risk of type 2 diabetes [50] and cardiovascular disease [51]. Paradoxically, smoking cessation is commonly associated with subsequent weight gain [52] which in turn might attenuate the beneficial effects of smoking cessation. Several potential mechanisms may contribute to a lower risk of type 2 diabetes and cardiovascular disease associated with smoking cessation, including improved insulin sensitivity and lipoprotein levels as well as reduced inflammation [53–55]. Considering the role of increased AGE accumulation as a consequence of tobacco smoking in its association with type 2 diabetes and cardiovascular disease, it is likely that reduced formation and accumulation of AGEs may result in reduced type 2 diabetic and cardiovascular risk. However, our hypothesis needs to be confirmed by future translational studies.
Our study has some strengths and limitations. Firstly, part of this study is based on a large number of participants, resulting in a good statistical power and the ability to perform analysis for different smoking status and classes. A limitation of the LifeLines Study is the potential misclassification of individuals with regards to their smoking status as we cannot completely rule out misreporting of smoking habits or history. Secondly, we were not able to stratify the analyses for diabetes status since the number of subjects with type 2 diabetes was too small.
A limitation of the QMDiab study is the small number of study participants which may have caused a reduced statistical power in finding significant associations. Since the QMDiab study did not include questions regarding secondhand smoking, we were not able to assess any correlation with cotinine biomarkers.
Conclusions
This study clearly demonstrated that secondhand smoking is associated with higher SAF levels whereas smoking cessation led to a gradual normalization of SAF levels as the years since smoking abstinence increase. Moreover, we have demonstrated that urinary cotinine N-oxide, a biomarker for environmental tobacco smoke, is significantly associated with higher SAF levels and might therefore be used as an alternative way for questionnaires to examine the effect secondhand smoking on SAF. These findings should be taken into consideration in future studies on SAF or using SAF as a screening tool for populations at risk for diabetes and cardiovascular diseases.
Supporting information
Missing saliva collection (n = 44), plasma collection (n = 16), urine collection (n = 14). Missing smoking data (n = 10).
(DOCX)
Acknowledgments
This work was supported by Netherlands Consortium for Healthy Ageing (NCHA), Bio-SHaRE-EU, Biobank Standardisation and Harmonisation for research excellence in the European Union. Bioresource research impact factor BRIF4568.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The manuscript is based on data from the LifeLines cohort study. LifeLines adheres to standards for open data availability. The data catalogue of LifeLines is publicly accessible on www.lifelines.net. All international researchers can apply for data at the LifeLines research office (LLscience@umcg.nl). The LifeLines system allows access for reproducibility of the study results. The QMDiab study was supported by ‘Biomedical Research Program’ funds at Weill Cornell Medical College in Qatar, a program funded by the Qatar Foundation. Support for some of the experiments was provided by the Weill Cornell Medical College in Qatar (WCMC-Q) bioinformatics and virtual metabolomics core which is funded by the Qatar Foundation. Dennis Mook-Kanamori is supported by Dutch Science Organization (ZonMW-VENI Grant 916.14.023). This work was supported by Netherlands Consortium for Healthy Ageing (NCHA), Bio-SHaRE-EU, Biobank Standardisation and Harmonisation for research excellence in the European Union. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000; 28(12):1708–1716 . [DOI] [PubMed] [Google Scholar]
- 2.Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol. 1990; 45(4):B105–11:. [DOI] [PubMed] [Google Scholar]
- 3.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991; 30(5):1205–1210:. [DOI] [PubMed] [Google Scholar]
- 4.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993; 91(6):2463–2469 doi: 10.1172/JCI116481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994; 43(6):836–841:. [DOI] [PubMed] [Google Scholar]
- 6.Meerwaldt R, Zeebregts CJ, Navis G, Hillebrands JL, Lefrandt JD, Smit AJ. Accumulation of advanced glycation end products and chronic complications in ESRD treated by dialysis. Am J Kidney Dis. 2009; 53(1):138–150 doi: 10.1053/j.ajkd.2008.08.031 . [DOI] [PubMed] [Google Scholar]
- 7.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004; 47(7):1324–1330 doi: 10.1007/s00125-004-1451-2 . [DOI] [PubMed] [Google Scholar]
- 8.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007; 30(1):107–112 doi: 10.2337/dc06-1391 . [DOI] [PubMed] [Google Scholar]
- 9.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006; 29(12):2654–2659 doi: 10.2337/dc05-2173 . [DOI] [PubMed] [Google Scholar]
- 10.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005; 16(12):3687–3693 doi: 10.1681/ASN.2005020144 . [DOI] [PubMed] [Google Scholar]
- 11.de Vos LC, Boersema J, Mulder DJ, Smit AJ, Zeebregts CJ, Lefrandt JD. Skin autofluorescence as a measure of advanced glycation end products deposition predicts 5-year amputation in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2015; 35(6):1532–1537 doi: 10.1161/ATVBAHA.115.305407 . [DOI] [PubMed] [Google Scholar]
- 12.Hoonhorst SJ, Lo Tam Loi AT, Hartman JE, Telenga ED, van den Berge M, Koenderman L, et al. Advanced glycation end products in the skin are enhanced in COPD. Metabolism. 2014; 63(9):1149–1156 doi: 10.1016/j.metabol.2014.06.006 . [DOI] [PubMed] [Google Scholar]
- 13.Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010; 12(5):399–403 doi: 10.1089/dia.2009.0113 . [DOI] [PubMed] [Google Scholar]
- 14.van Waateringe RP, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, Paterson AD, et al. Lifestyle and clinical determinants of skin autofluorescence in a population-based cohort study. Eur J Clin Invest. 2016; 46(5):481–490 doi: 10.1111/eci.12627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997; 94(25):13915–13920:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009; 11(1):12–24 doi: 10.1093/ntr/ntn010 . [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999; 107 Suppl 2:349–355:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996; 18(2):188–204:. [DOI] [PubMed] [Google Scholar]
- 19.Seccareccia F, Zuccaro P, Pacifici R, Meli P, Pannozzo F, Freeman KM, et al. Serum cotinine as a marker of environmental tobacco smoke exposure in epidemiological studies: the experience of the MATISS project. Eur J Epidemiol. 2003; 18(6):487–492:. [DOI] [PubMed] [Google Scholar]
- 20.Stolk RP, Rosmalen JG, Postma DS, de Boer RA, Navis G, Slaets JP, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol. 2008; 23(1):67–74 doi: 10.1007/s10654-007-9204-4 . [DOI] [PubMed] [Google Scholar]
- 21.Eny KM, Lutgers HL, Maynard J, Klein BE, Lee KE, Atzmon G, et al. GWAS identifies an NAT2 acetylator status tag single nucleotide polymorphism to be a major locus for skin fluorescence. Diabetologia. 2014; 57(8):1623–1634 doi: 10.1007/s00125-014-3286-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mook-Kanamori DO, Selim MM, Takiddin AH, Al-Homsi H, Al-Mahmoud KA, Al-Obaidli A, et al. 1,5-Anhydroglucitol in saliva is a noninvasive marker of short-term glycemic control. J Clin Endocrinol Metab. 2014; 99(3):E479–83 doi: 10.1210/jc.2013-3596 . [DOI] [PubMed] [Google Scholar]
- 23.Mook-Kanamori MJ, Selim MM, Takiddin AH, Al-Homsi H, Al-Mahmoud KA, Al-Obaidli A, et al. Ethnic and gender differences in advanced glycation end products measured by skin auto-fluorescence. Dermatoendocrinol. 2013; 5(2):325–330 doi: 10.4161/derm.26046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16(1):31–41:. [DOI] [PubMed] [Google Scholar]
- 25.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009; 81(16):6656–6667 doi: 10.1021/ac901536h . [DOI] [PubMed] [Google Scholar]
- 26.Ohkuma T, Iwase M, Fujii H, Kaizu S, Ide H, Jodai T, et al. Dose- and time-dependent association of smoking and its cessation with glycemic control and insulin resistance in male patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. PLoS One. 2015; 10(3):e0122023 doi: 10.1371/journal.pone.0122023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholl ID, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med. 1998; 4(9):594–601:. [PMC free article] [PubMed] [Google Scholar]
- 28.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007; 131(5):1557–1566 doi: 10.1378/chest.06-2179 . [DOI] [PubMed] [Google Scholar]
- 29.Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007; 76(2):236–244 doi: 10.1016/j.diabres.2006.09.016 . [DOI] [PubMed] [Google Scholar]
- 30.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A. 2012; 109(39):15888–15893 doi: 10.1073/pnas.1205847109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006; 114(6):597–605 doi: 10.1161/CIRCULATIONAHA.106.621854 . [DOI] [PubMed] [Google Scholar]
- 32.Schmidt AM, Hasu M, Popov D, Zhang JH, Chen J, Yan SD, et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci U S A. 1994; 91(19):8807–8811:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002; 105(7):816–822:. [DOI] [PubMed] [Google Scholar]
- 34.den Dekker MA, Zwiers M, van den Heuvel ER, de Vos LC, Smit AJ, Zeebregts CJ, et al. Skin autofluorescence, a non-invasive marker for AGE accumulation, is associated with the degree of atherosclerosis. PLoS One. 2013; 8(12):e83084 doi: 10.1371/journal.pone.0083084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulder DJ, van Haelst PL, Gross S, de Leeuw K, Bijzet J, Graaff R, et al. Skin autofluorescence is elevated in patients with stable coronary artery disease and is associated with serum levels of neopterin and the soluble receptor for advanced glycation end products. Atherosclerosis. 2008; 197(1):217–223 doi: 10.1016/j.atherosclerosis.2007.03.027 . [DOI] [PubMed] [Google Scholar]
- 36.de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, et al. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2013; 33(1):131–138 doi: 10.1161/ATVBAHA.112.300016 . [DOI] [PubMed] [Google Scholar]
- 37.Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, et al. Assessing secondhand smoke using biological markers. Tob Control. 2013; 22(3):164–171 doi: 10.1136/tobaccocontrol-2011-050298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996; 275(16):1233–1240:. [PubMed] [Google Scholar]
- 39.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009; 169(2):236–248 doi: 10.1093/aje/kwn301 . [DOI] [PubMed] [Google Scholar]
- 40.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, et al. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health. 1990; 80(9):1053–1056:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. A position paper from the Council on Cardiopulmonary and Critical Care, American Heart Association. Circulation. 1992; 86(2):699–702:. [DOI] [PubMed] [Google Scholar]
- 42.Flouris AD, Vardavas CI, Metsios GS, Tsatsakis AM, Koutedakis Y. Biological evidence for the acute health effects of secondhand smoke exposure. Am J Physiol Lung Cell Mol Physiol. 2010; 298(1):L3–L12 doi: 10.1152/ajplung.00215.2009 . [DOI] [PubMed] [Google Scholar]
- 43.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005; 14(6):396–404 doi: 10.1136/tc.2005.011288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Ji J, Liu YJ, Deng X, He QQ. Passive smoking and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. PLoS One. 2013; 8(7):e69915 doi: 10.1371/journal.pone.0069915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005; 111(20):2684–2698 doi: 10.1161/CIRCULATIONAHA.104.492215 . [DOI] [PubMed] [Google Scholar]
- 46.Iversen B, Jacobsen BK, Lochen ML. Active and passive smoking and the risk of myocardial infarction in 24,968 men and women during 11 year of follow-up: the Tromso Study. Eur J Epidemiol. 2013; 28(8):659–667 doi: 10.1007/s10654-013-9785-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard DJ, Ota RB, Briggs LA, Hampton M, Pritsos CA. Oxidative stress induced by environmental tobacco smoke in the workplace is mitigated by antioxidant supplementation. Cancer Epidemiol Biomarkers Prev. 1998; 7(11):981–988:. [PubMed] [Google Scholar]
- 48.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992; 339(8802):1128–1130:. [DOI] [PubMed] [Google Scholar]
- 49.Chowdhury P, Rayford PL, Chang LW. Pathophysiological effects of nicotine on the pancreas. Proc Soc Exp Biol Med. 1998; 218(3):168–173:. [DOI] [PubMed] [Google Scholar]
- 50.Wannamethee SG, Shaper AG, Perry IJ, British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001; 24(9):1590–1595:. [DOI] [PubMed] [Google Scholar]
- 51.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013; 368(4):341–350 doi: 10.1056/NEJMsa1211128 . [DOI] [PubMed] [Google Scholar]
- 52.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004; 5(2):95–103 doi: 10.1111/j.1467-789X.2004.00131.x . [DOI] [PubMed] [Google Scholar]
- 53.Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011; 161(1):145–151 doi: 10.1016/j.ahj.2010.09.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest. 1997; 27(5):450–456:. [DOI] [PubMed] [Google Scholar]
- 55.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010; 55(18):1988–1995 doi: 10.1016/j.jacc.2010.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Missing saliva collection (n = 44), plasma collection (n = 16), urine collection (n = 14). Missing smoking data (n = 10).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.