Abstract
Herpes zoster is a common disease caused due to varicella zoster virus (VZV) infection with increasing incidence by age. If the patient has a severe, extended, or treatment-recalcitrant course of herpes zoster, this must be a red flag to search for underlying pathologies. Here, we report about a 64-year-old male patient with diabetes, who came to our emergency department because of general malaise, fever, chills, and a pronounced nuchal and facial swelling on the left side. Based on herpetiform-grouped vesicles and yellowish crusts, an impetiginized facial herpes zoster was diagnosed, and combined antiviral and antibiotic treatment was initiated. He was HIV negative. Despite intensified treatment, his situation worsened. We observed blasts in peripheral blood, but bone marrow biopsy was initially denied. Some days later after deterioration of his disease, he accepted further diagnostics. A myelodysplastic syndrome with blast excess (refractory anemia and blast excess II, RAEB II) could be confirmed. The following translocations were detected: t(2;12)(p13; q13) and t(6;9)(p22;q34). REAB II has an unfortunate prognosis. Cytoreductive treatment was initiated by the hematooncologist. Unfortunately, the patient deceased due to septic shock.
Keywords: herpes zoster, varicella zoster virus, myelodysplastic syndrome, sepsis, emergency
Introduction
Herpes zoster is caused by varicella zoster virus (VZV). After a short prodromal stage, a dermatomal eruption of herpetiform-grouped vesicles develops due to reactivation of VZV within ganglia and their anterograde transport into axons innervating the skin. Common symptoms are pain, dysesthesia, and paresthesia.1
Severe zoster subtypes are characterized by ulcerations, necrosis, or dissemination, with or without vasculitis, paresis, central nervous system involvement, or involvement of other internal organs.1 Facial edema is another possible symptom2 (Table 1). Severe zoster infections are a red flag for serious underlying disorders such as infections, immunodeficiency, and malignancies.
Table 1.
Differential diagnosis of facial swelling
| Disorders | Remarks |
|---|---|
| Acute hemorrhagic edema | Children, after infections, vaccination or drug-induced |
| Allergic contact dermatitis | Due to aeroallergen or hair dyes |
| Angioedema | Acquired type (mast cell-related or bradykinin-induced); hereditary type with absolute or functional deficiency of C1 esterase inhibitor |
| Brachiocephalic venous compression, vena cava superior syndrome | Due to multinodal gout, after venous puncture, and due to tumors |
| Cushing syndrome, Mb. Cushing | Steroid-induced (syndrome) ACTH overproduction, buffalo hump |
| Drug induced | Eg by folic acid antagonist pemetrexed or dipeptidyl peptidase 4 inhibitor vildagliptin, periorbital |
| Erysipelas | Fever, erythema, hyperthermia |
| Lepra | Facies leontina |
| Lymphedema | Secondary after surgery and/or irradiation of head-and-neck cancer |
| Mb. Morbihan | Solid facial erythema with rosacea (Glabella, cheeks, lids) |
| Strangulation | Strangulation marks |
| Subcutaneous emphysema | After trauma, skin crackling |
| Lymphomas | Fever, hepato-splenomegaly, fatigue |
| Posttraumatic/postsurgery | Medical history |
| Zygomycosis | Rhino-cerebral type, hyposmia, nasal secretions |
Abbreviation: ACTH, adrenocorticotropic hormone.
Case report
A 64-year-old male was admitted to our department as an emergency. He suffered from general malaise, fever, chills, and a pronounced nuchal and facial swelling on the left side. Since birth, he suffered from left-sided microtia with complete absence of meatus acusticus. His medical history was positive for diabetes mellitus type 2 treated by diet and an arterial hypertension controlled by 160 mg valsartan 160 mg/d.
On examination, we observed a left-sided head-and-neck edema with herpetiform-grouped vesicles around the left ear and on the nose and yellowish crusts and formation of bullae in the perioral region (Figures 1 and 2). Laboratory findings were remarkable for leukocytosis with a white blood cell count of 25.7 (normal range: 3.8–11.0), thrombocytopenia of 64 Gpt/L (120–340), lowered hematocrit of 0.245 L/L (0.40–0.54), hemoglobin of 5.1 mmol/L (8.6–12.1), erythrocyte count of 2.5 Tpt/L (4.6–6.2), neutrophilia of 13.8 Gpt/L (1.8–7.6), lymphopenia of 11% (20–45), increased number of large unstained cells with 2 Gpt/L (0–0.40), and C-reactive protein (CRP) of 333 mg/L (<5). HIV tests were negative.
Figure 1.
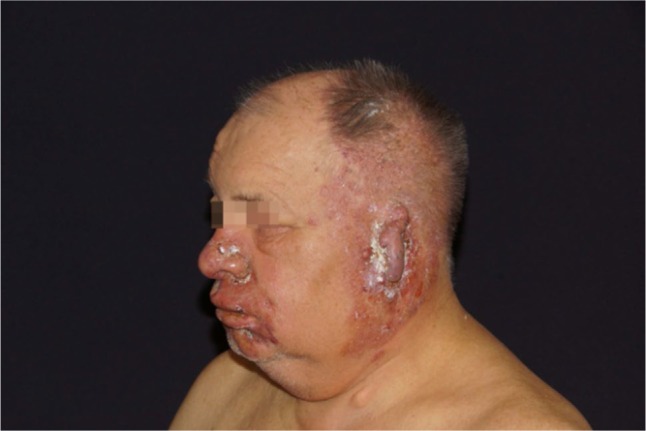
Initial presentation of the patient with temporal herpes zoster.
Figure 2.
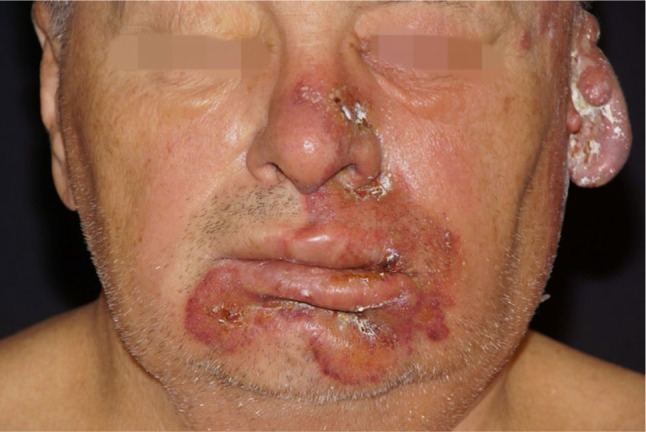
Perioral bullous impetigo, herpetiform vesicles on the nose.
Before submission, antibiotic treatment with sultamicillin was started and continued after submission at a dosage of 3 × 3 g/d intravenously (iv). The facial swelling, however, worsened. Therefore, the patient was referred to our Ear Nose Throat-department for diagnostics. Diagnostic ultrasound and thoracic computerized tomography (CT) were performed to exclude a bacterial or mycotic abscess formation.
With the diagnosis of impetiginized herpes zoster, antibiotic treatment was changed to 3 × 800 mg clindamycin/d plus aciclovir 3 × 500 mg/d. Pain management was realized using metamizole sodium 3 × 30 gtt/d. During the following days, only a minor clinical improvement was seen. In the peripheral blood, 11% blasts were noted. A bone marrow biopsy was suggested, but the patient refused.
The further course was characterized by malodorous oozing, formation of bullae, and increasing edema. On the left chin and left submandibular region, firm infiltrates were noted, and skin biopsies were taken to exclude specific infiltrates of a malignancy. Histopathology, however, was nonspecific, demonstrating only a putrid inflammation. Vesicles showed dissemination suggesting zoster generalisatus (Figures 3 and 4). Accordingly, we increased the dosage of aciclovir to 10 mg/kg (3 × 1000 mg/d) iv. The pain sensations become worse, and pregabalin was started in increasing doses. Certoparin sodium 3000 I.E. anti-Xa/d was injected subcutaneously for thrombosis prophylaxis.
Figure 3.
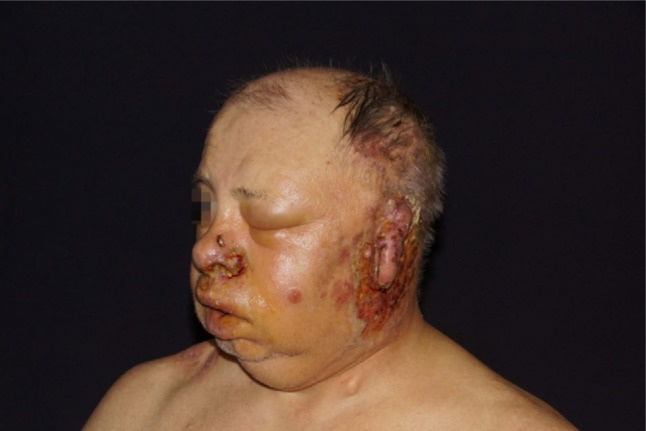
Increased facial edema and necrotic herpes zoster lesions.
Figure 4.

Generalization of herpes zoster vesicles.
Nevertheless, the situation did not improve. CRP increased to >400 mg/L, leukocytosis of 28.4 Gpt/L developed, and procalcitonin increased to 3.65 ng/mL (normal range: 0–0.5).
Under the suspicion of septicemia, we transferred the patient to the intensive care unit. Central venous access was realized by a subclavian catheter. The patient was treated by 4.5 g piperacillin, 500 mg levofloxacin, 1000 mg acyclovir every 8 hours, and initially 100 mg prednisolone. During the treatment course, he developed doughy stool; therefore, the antibiosis was changed to vancomycin 4 × 500 mg per os. Stool samples for Clostridium difficile remained negative. Clinical improvement was accompanied by reduction of vesiculation and edema.
After patient consent, CT and bone marrow biopsy were performed. Bone marrow analysis disclosed atypical myelopoiesis, reduced atypical erythropoiesis, and micromegakaryocytic megakaryopoiesis with 10% of bone marrow blasts confirming a myelodysplastic syndrome (MDS) refractory anemia and blast excess II (RAEB II). Chromosomal analysis and cytodiagnostics revealed a karyotype 46/XY and translocations t(2;12)(p13; q13) and t(6;9)(p22;q34). Due to anemia, erythrocyte concentrates were required.
Two weeks after admission, the patient suffered from pain in his right arm. The skin surrounding the subclavian catheter became erythematous. Therefore, the catheter was removed, but microbial cultures of the tip remained negative. Venous duplex sonography disclosed vena subclavia thrombosis and soft tissue hematoma.
In the following days, oozing of the scalp and the ear helix was observed. Microbial swaps identified Pseudomonas aeruginosa.
We intensified diuresis because of a progressive edema. Hemato-oncologist initiated cytoreductive treatment with azacitidine. Anemia and thrombocytopenia required erythrocyte and thrombocyte concentrates. Herpes zoster persisted. During intravenous infusion of varicella immunoglobulin, the patient developed an anaphylactic shock, which could be managed by intensive care. Six days later, the patient died due to a septic shock.
Written informed consent has been obtained from the patient before his tragic outcome to have the case details and any accompanying images published.
Discussion
In most cases, the diagnosis of herpes zoster can be confirmed clinically by the presence of herpetiform-grouped vesicles and unilateral localization within a dermatome, in combination with pain and paresthesia.3 In the present case, a severe zoster with delayed and minimal response to treatment was the red flag for a serious underlying disorder in a patient previously healthy and capable. Antiviral drug therapy was modified accordingly during the course.4
Further diagnostics were initially delayed due to patient refusal for bone marrow biopsy. But afterward, the diagnosis of MDS was confirmed.
MDS is accompanied by an increased risk of zoster infection. A recent study using data of Surveillance, Epidemiology and End Result (SEER)-Medicare data base calculated an odds ratio of 1.31.5
MDS with RAEB is characterized by an increased mortality. RAEB I is defined as a peripheral blast excess <5% and bone marrow blasts <10%, without Auer rods. RAEB II shows peripheral blasts >5% and bone marrow blasts >10%, and Auer rods may occur.6 Mean survival of patients with MDS REAB-II is ~16 months.7,8 Translocation t(6;9) (p22;q34) worsens the prognosis with a mean survival of 12 months.9
Severe atypical zoster is a red flag. Patients need a workup for serious underlying diseases. Unfortunately, improvement of individual prognosis is not always possible.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wollina U, Machetanz J. Herpes zoster und postzosterische Neuralgie. Hautarzt. 2016;67(8):653–665. doi: 10.1007/s00105-016-3834-y. [DOI] [PubMed] [Google Scholar]
- 2.Hornstein OP. Differentialdiagnose der Gesichtsschwellungen. HNO. 1979;27(4):129–137. [PubMed] [Google Scholar]
- 3.Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) Guideline on the Management of Herpes Zoster – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 1: diagnosis. J Eur Acad Dermatol Venereol. 2017;31(1):9–19. doi: 10.1111/jdv.13995. [DOI] [PubMed] [Google Scholar]
- 4.Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) Guideline on the Management of Herpes Zoster – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–29. doi: 10.1111/jdv.13957. [DOI] [PubMed] [Google Scholar]
- 5.Titmarsh GJ, McMullin MF, McShane CM, Clarke M, Engels EA, Anderson LA. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol. 2014;38(1):56–61. doi: 10.1016/j.canep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett JM. Changes in the updated 2016: WHO classification of the myelodysplastic syndromes and related myeloid neoplasms. Clin Lymphoma Myeloma Leuk. 2016;16(11):607–609. doi: 10.1016/j.clml.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 7.DiNardo CD, Garcia-Manero G, Pierce S, et al. Interactions and relevance of blast percentage and treatment strategy among younger and older patients with acute myeloid leukemia (AML) and myelodysplastic syndrome. Am J Hematol. 2016;91(2):227–232. doi: 10.1002/ajh.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breccia M, Latagliata R, Cannella L, et al. Analysis of prognostic factors in patients with refractory anemia with excess of blasts (RAEB) reclassified according to WHO proposal. Leuk Res. 2009;33(3):391–394. doi: 10.1016/j.leukres.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M, Ashok Kumar J, Sitaram U, et al. The t(6;9)(p22;q34) in myeloid neoplasms: a retrospective study of 16 cases. Cancer Genet Cytogenet. 2010;203(2):297–302. doi: 10.1016/j.cancergencyto.2010.08.012. [DOI] [PubMed] [Google Scholar]


