Abstract
In relapsing remitting multiple sclerosis (RRMS), type I interferon (IFN) is considered immuno-modulatory and recombinant forms of IFN-β are the most prescribed treatment for this disease. However, within the RRMS population, 30–50% of MS patients are nonresponsive to this treatment, and it consistently worsens neuromyelitis optica (NMO) a disease once considered to be a form of RRMS. In contrast to RRMS, type I IFNs have been shown to have properties that drive the inflammatory pathologies in many other autoimmune diseases. These diseases include Sjögren’s syndrome, system lupus erythematosus (SLE), neuromyelitis optica (NMO) rheumatoid arthritis (RA) and psoriasis. Historically, autoimmune diseases were thought to be driven by a TH1 response to auto-antigens. However, since the discovery of the TH17 in experimental autoimmune encephalomyelitis (EAE), it is now generally thought that TH17 plays an important role in MS and all other autoimmune diseases. In this article, we will discuss recent clinical and basic research advances in the field of autoimmunity and argue that IFN-β and other type I IFNs are immuno-modulatory in diseases driven predominantly by TH1 but in contrast are inflammatory in diseases that have a predominant Th17 response.
Keywords: Interferon, TH17, TH1, Autoimmunity
Introduction
Interferon (IFN) β is the most widely prescribed treatment for MS. IFN-β therapy is generally well tolerated and overall reduces the relapse rate by 30% in patients with relapsing remitting multiple sclerosis {RRMS). [1]. There are patients who remain relapse free for several years while on this treatment. The common side effect of IFN-β is moderate to severe flu-like symptoms and IFN-β can cause liver damage. However, a major limitation with IFN-β is that 30–50% of MS patients do not respond to treatment. Therefore, it is highly desirable to identify responders and non-responders prior to the initiation of treatment and thus eliminate the unnecessary treatment of individuals that would have no benefit from this expensive drug.
Type I Interferons (type I IFNs), which include the various IFN-β and IFN-α molecules, were first identified by their role as anti-viral factors produced endogenously during a viral infection[2]; consequently an early hypothesis was that IFN-β treatment provided benefit to MS patients by resolving a viral infection that may have caused the disease. However, it is now established that IFN-β has many immune-suppressive functions [3]. These include blockade of the trafficking of lymphocytes to the central nervous system (CNS), reduction of expression of MHC class II molecules, attenuation of T cell proliferation and alteration of the cytokine milieu from pro-inflammatory to anti-inflammatory.
Two recent publications have illuminated the immune suppressive activity of endogenously expressed type I IFNs[4,5]. During EAE, IFN-β and other type I IFNs are locally elevated at the site of inflammation. The local expression of IFN-β in the CNS targets myeloid cells to inhibit disease. Specifically, IFN-β attenuates several activities of myeloid cells including expression of chemokines, production of pro-inflammatory cytokines and their capacity for antigen presentation. Even with these major insights on the function of endogenous IFN-β, the precise and complete understanding of the mechanisms of action of IFN-β, when it is administered as a treatment for MS or EAE, are still unclear.
In direct contrast to its role in RRMS, type I IFNs have properties that drive the inflammatory pathologies underlying certain other autoimmune diseases, which include Sjögren’s syndrome, system lupus erythematosus (SLE), neuromyelitis optica (NMO) rheumatoid arthritis (RA) and psoriasis[6–8]. And in these diseases it has been proposed that blockade of type I IFN signaling would be an effective treatment strategy for these diseases.
This collection of observations demonstrates that IFN-β is a double-edged sword in autoimmune diseases. In some situations, IFN-β inhibits disease progression whereas in others it promotes the pathology. Understanding the molecular and cellular functional differences of type I IFN in these different diseases will be critical in understanding how this cytokine works as a therapy in RRMS and also will give insights on how to discern which patients might respond to this treatment.
Type I IFN exacerbates TH17 induced autoimmunity
Both TH1 and TH17 cells have been implicated in the initiation and progression of disease in RRMS and its experimental model EAE[9]. IFN-β inhibits TH1 associated pathologies by blocking the inflammatory properties of IFN-γ and IL-12, the prototypical TH1 cytokines[10,11]. Furthermore, IFN-β also inhibits the in vitro differentiation of TH17 cells in humans and in mice[4,12–15]. These conclusions suggest that the IFN-β therapy would be effective in both TH1-driven and TH17-driven autoimmunity.
In our publication in Nature Medicine[16], we addressed this matter. We administered recombinant mouse IFN-β to mice with EAE induced by transferring either TH1 or TH17 myelin specific cells. We found that IFN-β treatment effectively blocked disease symptoms in mice with EAE induced with TH1 cells which was associated with the upregulation of the anti-inflammatory cytokines IL-27 and IL-10. However, we unexpectedly found that IFN-β treatment exacerbated disease symptoms in mice with TH17 induced EAE. This was a surprising observation since a popular theory on the mechanism of IFN-β treatment is that it attenuates disease by inhibiting the TH17 cells. Strikingly, in TH17 EAE we found that IFN-β did indeed inhibit IL-17, yet, IFN-β treatment actually worsened disease.
The large clinical trials for IFN-β therapy in RRMS indicated that there are patients that do not respond to treatment. It has been postulated that the lack of treatment response is due to inadequate bioavailability of IFN-β in these patients, such as the development of neutralizing antibodies[17]. However there is a growing body of evidence that demonstrate that both endogenously expressed and therapeutically administered IFN-β could have an active role in inducing inflammation in non-responders.
A recent study demonstrated that the RRMS patients as a whole have a diverse molecular response to IFN-β[18]. In this study’s patient population, some “non-responder” patients actually had increased gene expression induced by IFN-β treatment which was qualitatively different to that of the responders. Therefore, it can be concluded that IFN-β therapy could indeed exacerbate the disease in some patients.
In a small cohort of RRMS, 12 responders and 14 non-responders, we identified a subset of non-responders that had elevated TH17 and type I IFN signatures in blood[16]. Specifically we found a subset of non-responders had high serum concentrations of the TH17 cytokine, IL-17F, before the initiation therapy. IL-17F is produced by TH17 cells in both human and mouse[19,20], and suggests that the disease in this group of non-responders has a pathology predominantly initiated by TH17 cells. Furthermore, we found these TH17 non-responders also had high serum levels of endogenous IFN-β compared to responders. In fact, we found there to be a significant correlation in the concentrations of IL-17F and IFN-β in serum, suggesting that these two cytokines are biologically linked. These data are congruent with our observation in mice, where IFN-β treatment is not therapeutic in mice with TH17 EAE[16]. Moreover, they are also concordant with the findings from Comabella, et al[21]. This study described a non-responder population of RRMS patients to have elevated serum levels of type I IFN and increased expression of type I IFN-regulated genes prior to initiation of IFN-β therapy compared to responders. The authors conclude that non responding MS patients may have a disease that is similar to Lupus, which has a type I IFN signature[6], and that type I IFN actually contributes to the neuro-inflammation in these non-responders.
Recently, two groups of investigators have shown that IFN-β directly inhibits IL-17A expression in PBMCs from MS patients[14,15], and which suggests that inhibition of TH17 differentiation is a mechanism for IFN-β therapy. We also have seen that IFN-β inhibits IL-17 production in T-cells[16]. Therefore it can be speculated that non-responders that have a highly aggressive TH17-induced disease would up-regulate IFN-β in an attempt to offset the inflammation. This would render IFN-β treatment ineffective, since endogenous IFN-β expression is already elevated.
However, we contend that IFN-β has pro-inflammatory effects during TH17 induced disease. Since IFN-β has such pleotropic effects, it could have effects besides inhibiting IL-17 production that are pro-inflammatory to autoimmune diseases. Our studies with mice provide strong evidence that type I IFN drives inflammation in a TH17 version of adoptively transferred EAE. But, this phenomenon where IFN-β promotes TH17 inflammation also occurs in other human autoimmune diseases.
One good example of an autoimmune disease where type I IFN and TH17 drive pathology is psoriasis. Psoriatic lesions in skin have the hallmarks of chronic inflammation that promotes uncontrolled keratinocyte proliferation, with subsidiary manifestations like psoriatic arthritis. Type I IFN is an important mediator of the early events in inflammation in psoriasis. Like SLE, a type I IFN signature is evident in the psoriatic plaques[7]. Furthermore, in pre-psoriatic plaque tissue, plasmacytoid dendritic cells are found in abundance where they secrete large quantities of IFNβ and IFN-α[22]. Furthermore, genetic deletion or antibody blockade of type I IFN signaling is therapeutic in animal models of psoriasis. Such experiments demonstrate that type I IFNs play a direct pathogenic role in this disease[7,23]. However the most convincing data demonstrating that type I IFN can drive psoriasis, emanate from the observation of the development of psoriatic lesions in RRMS patients and in Hepatitis C patients who received type I IFN as a treatment for their initial disease[24–31].
In addition to type I IFN, the TH17 pathway also has a major role in driving psoriasis. Through genome-wide association studies, the IL-23/TH17 pathway has been directly implicated in the pathogenesis of psoriasis[32–34]. Moreover, Ustekinumab, a monoclonal antibody (mAb) therapy that blocks IL-23 signaling was successful in clinical trials of psoriasis and is now a FDA-approved marketed drug for this disease[32,35]. Furthermore, neutralizing IL-17A with mAb is also showing promise as a treatment for psoriasis from results in advanced clinical trials[36].
Neuromyelitis optica (NMO) is another autoimmune disease where type I IFN and TH17 drive pathology. NMO is a neuro-inflammatory disease which until recently was considered a variant of RRMS. Unlike MS, the NMO lesions are primarily found in the spinal cord and optic nerve [37]. Furthermore, granulocytes are predominant in NMO lesions and are absent in RRMS[37,38]. Remarkably NMO patients have elevated CSF levels of IL-17 compared to RRMS[39]. Therefore, the high levels of IL-17 in the CNS of NMO patients likely induce the local expression of chemokines that recruit granulocytes to the CNS[40,41]. There have been trials of IFN-β therapy for NMO but it had no therapeutic benefit. In fact several case reports have shown that IFN-β can induce severe relapses and exacerbations in this disease[42–46].
One could argue that the brain may have differential responses to type I IFN and to TH17 cytokines, in comparison to other anatomical target organs, such as the skin and spinal cord. This may indeed be true. However, trials with IFN-β and other type I IFNs have shown some efficacy in ulcerative colitis (UC)[47]. And strikingly, investigators at NIH found that those patients with UC, who were non-responders to IFN-β therapy, had significantly higher levels of IL-17 producing T cells in lamina propria compared to responders[48]. These results are very much concordant with what is seen with non-responders to IFN-β therapy in RRMS. The agreement serves to demonstrate that the anti-inflammatory effects of IFN-β are not strictly confined to autoimmunity of the brain.
The mechanism by which IFN-β promotes symptoms in Th17-mediated autoimmunity is currently unknown. However analysis of the current literature allows us to form a hypothesis, illustrated in Figure 1. Type 1 IFN has a proinflammatory effect on granulocytes. IFN-α stimulates neutrophils to release neutrophil extracellular traps (NETS). These NETS have destructive proteases and cytokines that would promote inflammation and destruction to tissues[49]. B cells might also be affected by type I IFN to enhance pathology. The B-cell-stimulating cytokine BAFF is elevated in NMO and also in MS patients after IFN-β therapy[50,51]. BAFF promotes B-cell survival and IL-17 from T-cells helps orchestrate germinal center formation and autoantibody production[52,53]. So in TH17 driven diseases, elevation in BAFF by IFNβ could lead to increased development of auto-reactive B-cells and production of auto-antibodies which are thought to be major pathogenic mechanisms in NMO[54].
Figure 1.
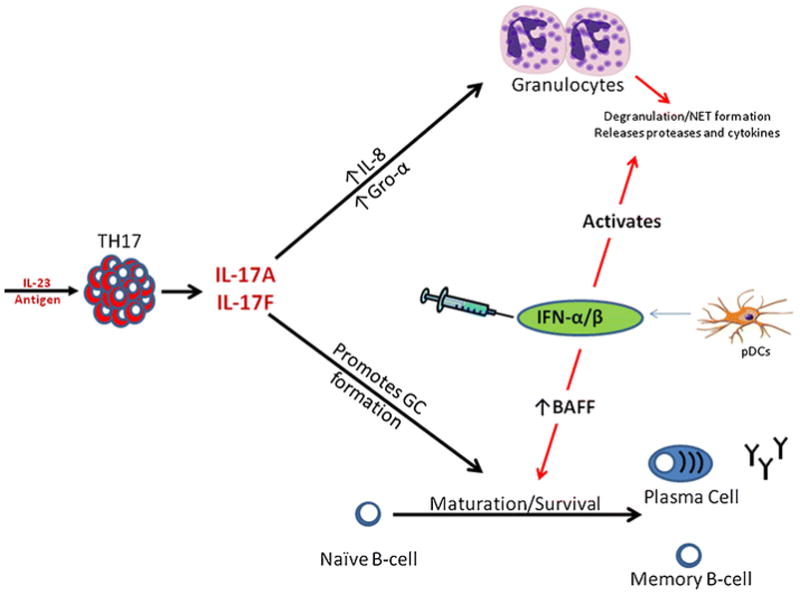
Autoimmune diseases initiated with the IL-23/TH17 response secrete high levels of IL17A and IL-17F. IL-17A and F initiates granulocyte infiltration to the site of inflammation as well as orchestrates germinal center formation and B-cell maturation in lymphoid tissues. Endogenously expressed or therapeutically administered IFN-β could exacerbate TH17 diseases by directly stimulating granulocytes to release tissue destructive proteases and cytokines or by elevating BAFF to enhance the production of auto-reactive antibodies and memory B-cells.
TH1 and IL-7: unlikely partners with IFN-β therapy
Our mouse studies demonstrated TH1 pathways are critical for the anti-inflammatory effect of IFN-β[16]. Inhibition of EAE symptoms by IFN-β treatment required IFN-γ produced by the auto-reactive TH1 cell. However, this biology was not obvious when we assessed serum biomarkers in RRMS patients.
Recently, we reported in Science Translational Medicine that in MS patients serum levels of IL-7 are inversely correlated with IL-17F and high baseline levels of IL-7 is associated with a good response to IFN-β treatment[55]. This discovery was surprising. Recently several groups independently discovered that polymorphisms in the IL-7 receptor gene confer a risk for developing RRMS suggesting that IL-7 signaling plays an important role in this disease[56,57]. The importance of IL-7 signaling in RRMS was further demonstrated when expression of both IL-7R and IL-7 mRNA levels were found to be significantly higher in the CNS of MS patients compared to individuals with non-inflammatory neurological diseases[56]. Since IL-7 signaling is important in lymphocyte development and the homeostasis of naïve and memory T-cells[58–60], it is speculated that IL-7 signaling contributes to the pathogenesis of multiple sclerosis. This hypothesis was supported by EAE models. We and others have successfully treated EAE symptoms in mice using either antibodies or genetic deletions to block IL-7 signaling[55,61–65].
At first glance, the observation that we find high levels of IL-7 to be a biomarker for responsiveness to IFN-β therapy does not appear to support our working hypothesis, namely that TH1 mediated diseases are responsive to IFN-β treatment. Our hypothesis came under even more scrutiny, since recently it was reported that IL-7 promotes the expansion of TH17 cells and that it does not affect TH1 cells[61]. In stark contrast to their experiments, we found that IL-7 actually promotes expansion of IL-12 stimulated TH1 cells and has no effect on TH17 differentiation[55]. Furthermore, we found that IL-7 independently from IL-12 promotes IFN-γ production from human CD4 T-cells. Another, recent study also reported that IL-7 promotes the survival/expansion of TH1 cells. Davis et al demonstrated that ex-vivo expansion of cord blood T cells by IL-7 promotes the survival and expansion of IFN-γ secreting TH1 differentiation[66]. Further biological relationships between TH1, IFN-γ and IL-7 have been demonstrated: First, transcription of the IL-7 gene in non-immune cells is directly induced by IFN-γ [67,68]. Second, IL-7 is a key cytokine for protection against viral pathogens, and this protection is mediated by the TH1 immune pathway[69]. Therefore we speculate that the IFN-γ and IL-7 provide a positive feedback loop to maintain a population of TH1 cells. We are currently identifying how this IL-7/TH1 axis is linked to a favorable response to IFN-β treatment. Our current working hypothesis is that IFN-β synergizes with both IFN-γ and IL-7 to attenuate disease by up-regulating the anti-inflammatory cytokines IL-27 and IL-10 and to decrease chemokine production from macrophages/microglial cells (Figure 2).
Figure 2.
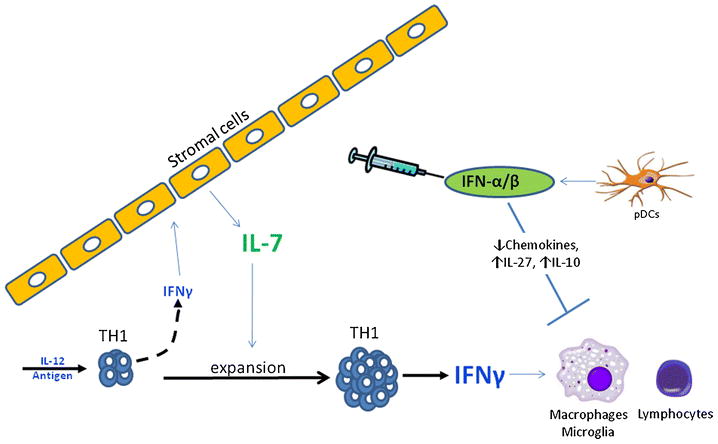
Autoimmune diseases initiated by TH1 cells have high levels of IFN-γ that drive lymphocytic and macrophage infiltration in to sites of inflammation. IFN-γ upregulates IL-7 expression in lymphoid tissue stromal cells during T-cell differentiation and provides signals to expand and maintain the TH1 population. IFN-β treatment synergizes with both IFN-γ and IL-7 to attenuate inflammation by up-regulating the anti-inflammatory cytokines IL-27 and IL-10 and decrease chemokine production from macrophages/microglial cells.
Blocking IL-7 receptor signaling as a potential treatment for RRMS has recently been gaining traction since preclinical EAE studies have shown that this approach attenuates disease symptoms[55,61]. However, it may be that IL-7 signaling has protective effects in this disease. For one, we find that high levels of IL-7 confer responsiveness to IFN-β treatment and therefore blocking IL-7 during IFN-β treatment could neutralize the therapeutic effects[55]. Furthermore, the polymorphism in the IL7R gene that is a risk allele for RRMS modulates the increased expression of a soluble version of this receptor. This soluble receptor might act as a molecular decoy and decrease signaling initiated by IL-7, or the other IL7R ligand TSLP, in RRMS[57]. The biology of IL-7 signaling in MS is complex, and is likely to be quite important in the pathophysiology of MS, since to date MS is the only autoimmune disorder where IL-7 signaling has been demonstrated in genome-wide association studies [70].
Concluding Remarks
As we have discussed, MS has unique features as an autoimmune disorder, features that distinguish it from other autoimmune diseases where blockade of TNF is effective and where blockade of IL-12p40 is beneficial. Both anti-TNF and anti-IL-12p40, in MS[71,72], fail to modulate RRMS, and indeed the anti-TNF approaches carry with them a “Black Box” warning from the FDA. [72] [71]. Furthermore, IFN-β and the other type I IFNs have been shown to contribute to the progression of many autoimmune diseases like RA and psoriasis. In contrast in RRMS, IFN-β is a commonly used therapy that can be an extremely effective drug for some patients. RRMS is a highly complex disease that some believe could be collection of different syndromes. This could help explain the heterogeneity in response to IFN-β therapy in RRMS. At present time there is no rational way to instruct an individual with RRMS to use IFN-β or to try the other first line drugs for RRMS, glatiramer and FTY420. However, clinical biomarker studies will hopefully lead to a diagnostic test that will predict whether one should take IFN-β or not.
References
- 1.Arnason BG. Immunologic therapy of multiple sclerosis. Annu Rev Med. 1999;50:291–302. doi: 10.1146/annurev.med.50.1.291. [DOI] [PubMed] [Google Scholar]
- 2.Pena-Rossi C, et al. Clinical trial: a multicentre, randomized, double-blind, placebo-controlled, dose-finding, phase II study of subcutaneous interferon-beta-1a in moderately active ulcerative colitis. Aliment Pharmacol Ther. 2008 doi: 10.1111/j.1365-2036.2008.03778.x. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste EN, Qin H. Type I interferons as anti-inflammatory mediators. Sci STKE. 2007;2007(416):pe70. doi: 10.1126/stke.4162007pe70. [DOI] [PubMed] [Google Scholar]
- 4.Guo B, et al. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118(5):1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prinz M, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28(5):675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Fits L, et al. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J Invest Dermatol. 2004;122(1):51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- 8.van der Pouw Kraan TC, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66(8):1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stromnes IM, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McRae BL, et al. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J Immunol. 1998;160(9):4298–4304. [PubMed] [Google Scholar]
- 11.Nagai T, et al. Interferon-beta mediates opposing effects on interferon-gamma-dependent Interleukin-12 p70 secretion by human monocyte-derived dendritic cells. Scand J Immunol. 2007;65(2):107–117. doi: 10.1111/j.1365-3083.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Saavedra FM, et al. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol Immunol. 2008;45(15):4008–4019. doi: 10.1016/j.molimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Ramgolam VS, et al. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183(8):5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 15.Durelli L, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 16.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesse D, Sorensen PS. Using measurements of neutralizing antibodies: the challenge of IFN-beta therapy. Eur J Neurol. 2007;14(8):850–859. doi: 10.1111/j.1468-1331.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 18.Rani MR, et al. Heterogeneous, longitudinally stable molecular signatures in response to interferon-beta. Ann N Y Acad Sci. 2009;1182:58–68. doi: 10.1111/j.1749-6632.2009.05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boniface K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185(1):679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 20.Haak S, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comabella M, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132(Pt 12):3353–3365. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- 22.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hida S, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13(5):643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 24.Scavo S, et al. Verrucous psoriasis in a patient with chronic C hepatitis treated with interferon. Clin Drug Investig. 2004;24(7):427–429. doi: 10.2165/00044011-200424070-00006. [DOI] [PubMed] [Google Scholar]
- 25.Horev A, Halevy S. New-onset psoriasis following treatment with pegylated interferon-alpha 2b and ribavirin for chronic hepatitis C. Isr Med Assoc J. 2009;11(12):760–761. [PubMed] [Google Scholar]
- 26.La Mantia L, Capsoni F. Psoriasis during interferon beta treatment for multiple sclerosis. Neurol Sci. 2010;31(3):337–339. doi: 10.1007/s10072-009-0184-x. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Lerma I, et al. New-onset psoriasis in a patient treated with interferon beta-1a. Br J Dermatol. 2009;160(3):716–717. doi: 10.1111/j.1365-2133.2008.09005.x. [DOI] [PubMed] [Google Scholar]
- 28.Webster GF, et al. Cutaneous ulcerations and pustular psoriasis flare caused by recombinant interferon beta injections in patients with multiple sclerosis. J Am Acad Dermatol. 1996;34(2 Pt 2):365–367. doi: 10.1016/s0190-9622(07)80010-7. [DOI] [PubMed] [Google Scholar]
- 29.Seckin D, et al. Concomitant vitiligo and psoriasis in a patient treated with interferon alfa-2a for chronic hepatitis B infection. Pediatr Dermatol. 2004;21(5):577–579. doi: 10.1111/j.0736-8046.2004.21512.x. [DOI] [PubMed] [Google Scholar]
- 30.Imafuku S, et al. Ciclosporin treatment of psoriasis in a patient with chronic hepatitis C. Br J Dermatol. 2007;156(6):1367–1369. doi: 10.1111/j.1365-2133.2007.07873.x. [DOI] [PubMed] [Google Scholar]
- 31.Downs AM, Dunnill MG. Exacerbation of psoriasis by interferon-alpha therapy for hepatitis C. Clin Exp Dermatol. 2000;25(4):351–352. doi: 10.1046/j.1365-2230.2000.00655-4.x. [DOI] [PubMed] [Google Scholar]
- 32.Kimball AB, et al. Efficacy and safety of ABT-874, a monoclonal anti-interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36-week observation/retreatment and 60-week open-label extension phases of a randomized phase II trial. J Am Acad Dermatol. 2008 doi: 10.1016/j.jaad.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair RP, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128(7):1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 36.Hueber W, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 37.Lucchinetti CF, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hengstman GJ, et al. Neuromyelitis optica with clinical and histopathological involvement of the brain. Mult Scler. 2007;13(5):679–682. doi: 10.1177/1352458506070145. [DOI] [PubMed] [Google Scholar]
- 39.Ishizu T, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128(Pt 5):988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 40.Smith E, et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179(12):8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, et al. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46(1):79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palace J, et al. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010;67(8):1016–1017. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu J, et al. IFNbeta-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology. 2010;75(16):1423–1427. doi: 10.1212/WNL.0b013e3181f8832e. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu Y, et al. Development of extensive brain lesions following interferon beta therapy in relapsing neuromyelitis optica and longitudinally extensive myelitis. J Neurol. 2008;255(2):305–307. doi: 10.1007/s00415-007-0730-5. [DOI] [PubMed] [Google Scholar]
- 45.Uzawa A, et al. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol. 2010;17(5):672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 46.Warabi Y, et al. Interferon beta-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J Neurol Sci. 2007;252(1):57–61. doi: 10.1016/j.jns.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Genovese MC, et al. A randomized, controlled trial of interferon-beta-1a (Avonex(R)) in patients with rheumatoid arthritis: a pilot study [ISRCTN03626626] Arthritis Res Ther. 2004;6(1):R73–R77. doi: 10.1186/ar1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannon PJ, et al. Suppression of inflammation in ulcerative colitis by interferon-{beta}-1a is accompanied by inhibition of IL-13 production. Gut. 2010;60(4):449–455. doi: 10.1136/gut.2010.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinelli S, et al. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279(42):44123–44132. doi: 10.1074/jbc.M405883200. [DOI] [PubMed] [Google Scholar]
- 50.Krumbholz M, et al. Interferon-beta increases BAFF levels in multiple sclerosis: implications for B cell autoimmunity. Brain. 2008;131(Pt 6):1455–1463. doi: 10.1093/brain/awn077. [DOI] [PubMed] [Google Scholar]
- 51.Vaknin-Dembinsky A, et al. Preferential increase of B-cell activating factor in the cerebrospinal fluid of neuromyelitis optica in a white population. Mult Scler. 2010;16(12):1453–1457. doi: 10.1177/1352458510380416. [DOI] [PubMed] [Google Scholar]
- 52.Xie S, et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184(5):2289–2296. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9(2):166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 54.Meinl E, et al. Humoral autoimmunity in multiple sclerosis. J Neurol Sci. 2011;306(1–2):180–182. doi: 10.1016/j.jns.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Lee LF, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci Transl Med. 2011;3(93):93ra68. doi: 10.1126/scitranslmed.3002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundmark F, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39(9):1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 57.Gregory SG, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39(9):1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 58.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seddon B, et al. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 60.Puel A, et al. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 61.Gao X, et al. Adjuvant treatment suppresses IL-17 production by T cell-independent myeloid sources in nonobese diabetic mice. Mol Immunol. 2010;47(14):2397–2404. doi: 10.1016/j.molimm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Walline CC, et al. IL-7Ralpha confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 2011;12(1):1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee LF, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci Transl Med. 3(93):93ra68. doi: 10.1126/scitranslmed.3002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walline CC, et al. IL-7Ralpha confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 12(1):1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 16(2):191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 66.Davis CC, et al. Interleukin-7 permits Th1/Tc1 maturation and promotes ex vivo expansion of cord blood T cells: a critical step toward adoptive immunotherapy after cord blood transplantation. Cancer Res. 2011;70(13):5249–5258. doi: 10.1158/0008-5472.CAN-09-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oshima S, et al. Interferon regulatory factor 1 (IRF-1) and IRF-2 distinctively up-regulate gene expression and production of interleukin-7 in human intestinal epithelial cells. Mol Cell Biol. 2004;24(14):6298–6310. doi: 10.1128/MCB.24.14.6298-6310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ariizumi K, et al. IFN-gamma-dependent IL-7 gene regulation in keratinocytes. J Immunol. 1995;154(11):6031–6039. [PubMed] [Google Scholar]
- 69.Nanjappa SG, et al. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117(19):5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363–391. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellesi M, et al. CNS demyelination during anti-tumor necrosis factor alpha therapy. J Neurol. 2006;253(5):668–669. doi: 10.1007/s00415-005-0055-1. [DOI] [PubMed] [Google Scholar]
- 72.Segal BM, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]


