Abstract
BACKGROUND
Myocardial mass is a key determinant of cardiac muscle function and hypertrophy. Myocardial depolarization leading to cardiac muscle contraction is reflected by the amplitude and duration of the QRS complex on the electrocardiogram (ECG). Abnormal QRS amplitude or duration reflect changes in myocardial mass and conduction, and are associated with increased risk of heart failure and death.
OBJECTIVES
This meta-analysis sought to gain insights into the genetic determinants of myocardial mass.
METHODS
We carried out a genome-wide association meta-analysis of 4 QRS traits in up to 73,518 individuals of European ancestry, followed by extensive biological and functional assessment.
RESULTS
We identified 52 genomic loci, of which 32 are novel, that are reliably associated with 1 or more QRS phenotypes at p < 1 × 10−8. These loci are enriched in regions of open chromatin, histone modifications, and transcription factor binding, suggesting that they represent regions of the genome that are actively transcribed in the human heart. Pathway analyses provided evidence that these loci play a role in cardiac hypertrophy. We further highlighted 67 candidate genes at the identified loci that are preferentially expressed in cardiac tissue and associated with cardiac abnormalities in Drosophila melanogaster and Mus musculus. We validated the regulatory function of a novel variant in the SCN5A/SCN10A locus in vitro and in vivo.
CONCLUSIONS
Taken together, our findings provide new insights into genes and biological pathways controlling myocardial mass and may help identify novel therapeutic targets.
Keywords: electrocardiogram, genetic association study, heart failure, left ventricular hypertrophy, QRS
The heart’s role is to provide adequate circulation of blood to meet the body’s requirements of oxygen and nutrients. The QRS complex on the electrocardiogram (ECG) represents the most widely used measurement of cardiac depolarization, which causes the ventricular muscle to contract, resulting in pulsatile blood flow. The amplitude and duration of the QRS complex reflects conduction through the left ventricle and is well correlated with left ventricular mass as measured by echocardiography (1,2). ECG measurements of the QRS complex are important in clinical and pre-clinical cardiovascular diseases, such as cardiac hypertrophy, heart failure, and various cardiomyopathies; in addition, they can predict cardiovascular mortality (3–6).
Identification of specific genes influencing the QRS complex may thus enhance our understanding of the human heart and ultimately lead to the prevention of cardiovascular disease and death. To further our understanding of the genetic factors influencing the QRS complex, we carried out a large-scale genome-wide association study (GWAS) and replication study of 4 related and clinically used QRS traits: the Sokolow-Lyon, Cornell, and 12-lead-voltage duration products (12-leadsum), and QRS duration. We identified 52 loci that were subsequently interrogated using bioinformatics and experimental approaches to gain more insights into the biological mechanisms regulating cardiac mass and QRS parameters.
METHODS
Additional details about the methods of our study can be found in the Online Appendix.
Our study design is summarized in Online Figure 1. Briefly, we combined summary statistics from 24 studies for up to 2,766,983 autosomal single nucleotide polymorphisms (SNPs) using an inverse-variance fixed-effects meta-analysis for each QRS trait. We performed replication testing for loci showing suggestive association (1 × 10−8 < p < 5 × 10−7) (Online Tables 1 and 2). The threshold for genome-wide significance was set at p < 1 × 10−8.
We performed an intersection between SNPs and regions of deoxyribonuclease hypersensitivity sites (DHS), covalently modified histones, and genomic features (ChromHMM) of cardiac tissues mapped by the National Institutes of Health Roadmap Epigenomics Program, as well as various cardiac transcription factor binding sites (GATA4, MEF2, SRF, TBX5, TBX3, GATA4, and Nkx2-5) measured by Chip-seq.
Single cell suspensions of human ventricular tissue were obtained by dissociation with Ultra Turrax T5 FU (IKA-Works, Breisgau, Germany), followed by dounce homogenization. 4C templates were mixed and sequenced simultaneously in 1 HiSeq 2000 lane (Illumina, Inc., San Diego, California). Enhancer candidate regions with major and minor alleles for rs6781009 were obtained by polymerase chain reaction from human control deoxyribonucleic acid (DNA) and cloned into the Hsp68-LacZ reporter vector. DNA was injected into the pronucleus of a fertilized Friend virus B-type strain egg, and approximately 200 injections/construct were performed. Embryos were harvested and stained with X-gal to detect LacZ activity.
H10 cells, grown in 12-well plates in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal calf serum and glutamine, were transfected using polyethylenimine 25 kDa (PEI) at a 1:3 ratio (DNA:PEI). Transfections were carried out at least 3 times and measured in triplicate. Luciferase measurements were performed using a Modulus Multimode Reader luminometer (Promega Corporation, Madison, Wisconsin).
IDENTIFICATION OF CANDIDATE GENES
We considered genes to be causal candidates on the basis of: 1) the nearest gene and any other gene located within 10 kb of the sentinel SNP; 2) genes containing coding variants in linkage disequilibrium (LD) with the ST-T wave SNPs at r2 > 0.8; 3) GRAIL (Gene Relationships Across Implicated Loci) analyses using the 2006 dataset to avoid confounding by subsequent GWAS discovery; and 4) genes with an expression quantitative trait locus (eQTL) analysis in cis using 4 independent sets of cardiac left ventricle and blood tissues. Ingenuity Pathway Analysis Knowledge Base March 2015 (Ingenuity Systems, Redwood City, California) was used to explore molecular pathways between proteins encoded by the 67 candidate genes from the 52 genome-wide significant loci.
We queried a D. melanogaster dataset containing a genome-wide phenotypic screen of cardiac-specific ribonucleic acid interference (RNAi) silencing of evolutionarily conserved genes under conditions of stress. We also queried the international database resource for the laboratory mouse (MGI [Mouse Genome Informatics]) and manually curated mammalian phenotype (MP) identifiers related to cardiac phenotypes. To illustrate that prioritized genes may play a critical role in heart development, we tested CG4743/SLC25A26, Fhos/FHOD3, Cka/STRN, NACα/NACA, EcR/NR1H, and Hand/HAND1 by performing heart-specific RNAi knockdown with the cardiac Hand4.2-Gal4 driver line.
We collected 43,278 raw Human Genome U133 Plus 2.0 Arrays (Affymetrix, Santa Clara, California) from the Gene Expression Omnibus (GEO) containing human gene expression data. A robust multichip average was used for normalization, and we subsequently conducted stringent quality control and processing of the data, which resulted in a tissue-expression matrix. After quality control, 37,427 samples remained, and we assigned 54,675 different probe sets to 19,997 different Ensembl genes used for human tissue expression profiling. To explore gene-expression of our candidate genes during cardiac differentiation, we performed ribonucleic acid sequencing using E14 Tg (Nkx2-5-EmGFP) mouse embryonic stem cells that were cultured in feeder-free conditions and subsequently differentiated.
STATISTICAL ANALYSIS
Our choice of the statistical threshold (p < 1 × 10−8) for the GWAS was grounded on the guidelines derived from studies of the ENCODE (Encyclopedia of DNA Elements) regions which suggests that p < 5 × 10−8 is the appropriate threshold for genome-wide significance in Europeans, but was also designed to provide us with additional adjustment for the multiple phenotypes tested. This threshold is conservative, considering that our 4 QRS phenotypes are also inter-related: correlation coefficients between the phenotype pairs range from r = 0.22 to 0.80. Additional details on our statistical analysis can be found in the Online Appendix.
RESULTS
Characteristics of studies, participants, genotyping arrays, and imputation are summarized in Online Tables 1 and 2. Together, our studies comprised 60,255 individuals of European ancestry ascertained in North America and Europe, with maximum sample sizes as follows: Sokolow-Lyon (n = 54,993), Cornell (n = 58,862), 12-leadsum (n = 48,632), and QRS duration (n = 60,255). Across the genome, 52 independent loci, 32 of which are novel, reached genome-wide significance for association with 1 or more QRS phenotypes (Figure 1, Online Figure 2, Online Table 3, Online Appendix). At each locus, we defined a single “sentinel” SNP with the lowest p value against any of the 4 phenotypes; regional association plots for the 52 loci are shown in Online Figure 3. Among the 52 loci, 32 were associated with only 1 QRS phenotype, and 20 with at least 2 phenotypes (Online Figure 4). The total number of locus-phenotype associations at p < 10−8 was 79 (72 SNPs), of which 59 are novel (Online Table 3). Full lists of the sentinel SNPs and the SNPs associated with any phenotype at p < 10−6 are provided in Online Tables 4 and 5. All previously known QRS duration loci showed evidence for association (p < 10−6) (Online Table 6). Among the 32 novel loci, 8 demonstrated genome-wide significant association with Sokolow-Lyon, 9 with Cornell, 20 with 12-leadsum, and 9 with QRS duration (Online Table 5). Collectively, the total variance explained by the 52 sentinel SNPs for the QRS traits was between 2.7% (Sokolow-Lyon) and 5.0% (QRS duration) (Online Table 7). At some loci, we found evidence for multiple independent associations with QRS phenotypes at p < 10−8 in conditional analyses (7) (Online Table 8, Online Appendix). Among the 52 loci identified, 8 have been associated previously with PR (reflecting atrial and atrioventricular node function), 5 with QT duration (ventricular repolarization), and 2 with heart rate (sinus node function) (Online Table 6), indicating genetic overlap among the 4 cardiac measures studied. We further demonstrated that there was directional consistency of the association of common variants identified in this study with QRS phenotypes in other ethnic groups (Online Figure 1, Online Table 9, Online Appendix).
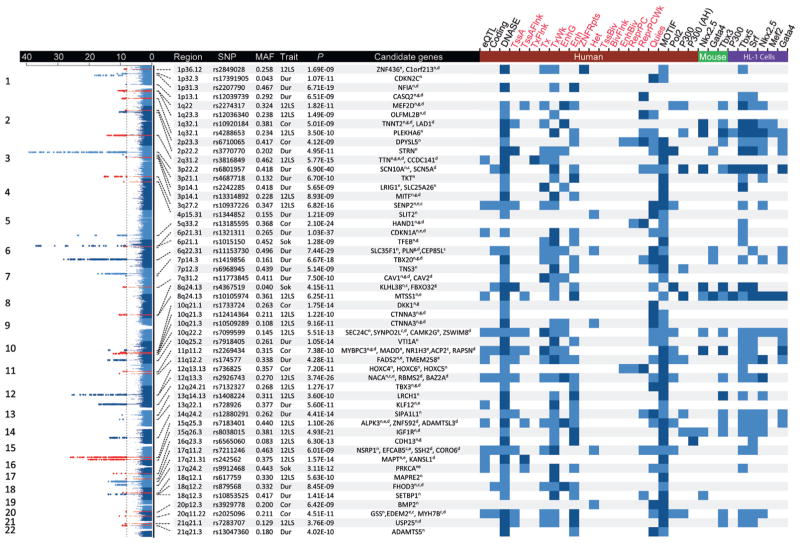
FIGURE 1. Genome-Wide Associations and Candidate Genes.
This overlay Manhattan plot shows the results for the genome-wide associations with QRS traits among Europeans. Single nucleotide polymorphisms (SNPs) reaching genome-wide significance (p < 1 × 10−8) are colored red (novel loci) or blue (previously reported loci). Candidate genes have been identified by 1 or multiple strategies: n = nearest; c = coding nonsynonymous variant; g = GRAIL (Gene Relationships Across Implicated Loci) tool; e = expression quantitative trait loci (eQTL); and d = DEPICT (Data-Driven Expression-Prioritized Integration for Complex Traits) tool. The presence of associated eQTL, coding SNPs, deoxyribonuclease (DNAse) hypersensitivity sites, chromatin states, or transcription factor binding sites are indicated for lead SNPs (light blue) or those in high (r2 > 0.8) linkage disequilibrium (dark blue). 12LS = 12-lead sum product; Cor = Cornell voltage product; Dur = QRS duration; MAF = minor allele frequency; Sok = Sokolow-Lyon product.
FUNCTIONAL ANNOTATION OF THE QRS ASSOCIATIONS
To better capture common sequence variants at the 52 loci, we queried the 1000 Genomes Project dataset (8), and identified 41 nonsynonymous SNPs in 17 genes that are in high LD (r2 > 0.8) with 12 of the sentinel SNPs (Online Table 10), representing an initial set of candidate variants that may have a functional effect on the QRS phenotypes through changes in protein structure and function.
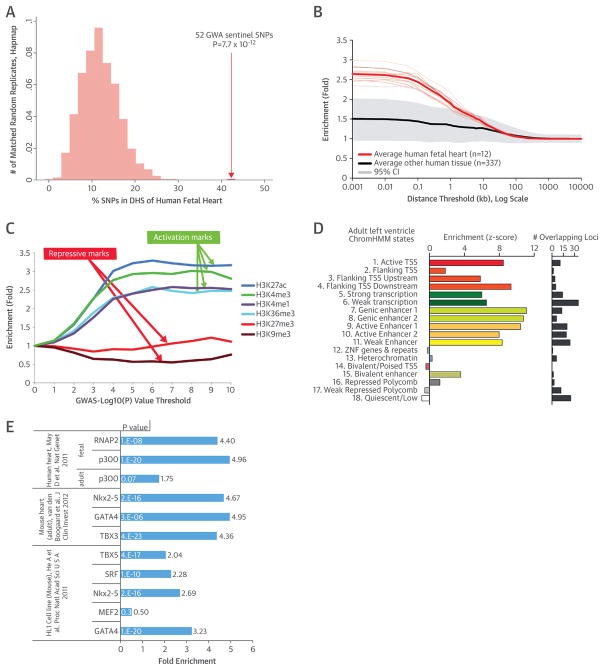
To assess the potential role of gene expression regulation, we tested the 52 loci for enrichment of DHS (9). In an analysis across 349 diverse cell lines, cultured primary cells, and fetal tissues (10) mapped by the ENCODE project (11) and the National Institute of Health Roadmap Epigenomics Program (12), the majority (42 of 52) of sentinel SNPs were located in DHS. In human fetal heart tissue, we found that less than one-half (22 of 52) overlapped DHS, which still represents a ~3.5-fold enrichment compared with the null expectation (p = 7.7 × 10−12) (Figure 2A). Further, the enrichment of genome-wide significant SNPs (p < 10−8) in DHS was strongest within the first 100 base pairs around the sentinel variants (Figure 2B). Additionally, there was a strong enrichment for histone marks and chromatin states (13) associated with active enhancers, promoters, and transcription in the human heart; by contrast, no enrichment was observed for transcriptionally repressive histone marks or states (Figures 2C and 2D, Online Figure 5). Strikingly, we observed increasing enrichment of activating histone marks at the identified QRS loci during the process of differentiating mouse embryonic stem cells into cardiomyocytes (Online Figure 6). Altogether, these findings are consistent with earlier observations of selective enrichment of trait-associated variants within DHS of specific cells of tissue types (10), and they point to a regulatory role of the QRS-associated loci during cardiac development.
FIGURE 2. Functional Annotations.
(A) The 52 sentinel SNPs are significantly enriched in deoxyribonuclease hypersensitivity sites (DHS) of the human fetal heart compared with the matched random distribution of HapMap SNPs. (B) The effect of physical distance between SNPs that meet genome-wide significance (p < 1 × 10−8) on enrichment of fetal heart relative to all other tissues at DHS. The enrichment is strongest at the SNP’s location and decreases after 100 base pairs from the SNP sites. (C) SNPs associated with QRS traits are enriched for the activating histone modifications H3K27ac, H3K4me3, H3K4me1, and H3K36me3 in the human left ventricle, which increased at more stringent genome-wide association study (GWAS) p value thresholds. The repressive mark H3K27me3 is not enriched, whereas H3K9me3 is significantly reduced, suggesting that QRS-trait loci are predominantly expressed in the left ventricle. (D) To capture greater complexity, we performed an integrative analysis in an 18-state “expanded” ChromHMM model representative of different functional regions of the genome. The 52 loci for the 18-state model were enriched using the 6 core histone marks (left); the total number of the 52 loci overlapped by each feature is shown (right). (E) SNPs (p < 1 × 10−8) were also significantly enriched for various factors in the human heart, mouse heart, and the HL-1 cell line. CI = confidence interval; TSS = transcription start site; other abbreviations as in Figure 1.
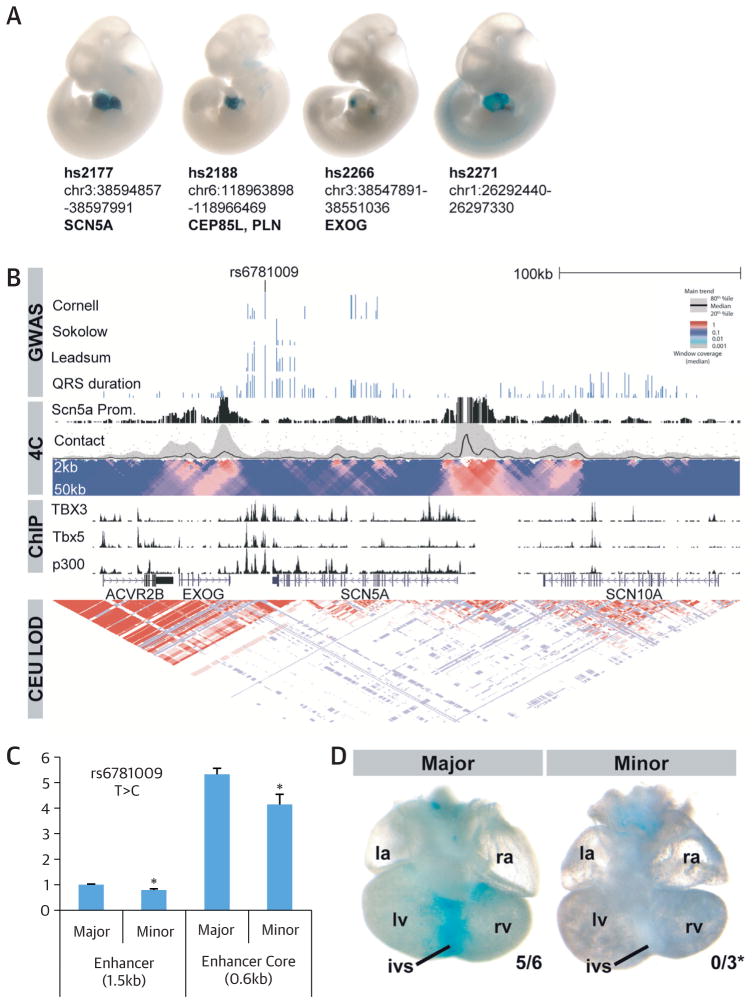
We next surveyed our genome-wide significant SNPs in DHS for perturbation of transcription factor (TF) recognition sequences, because these sites can point directly to binding events (Online Appendix). Of the 22 sentinel SNPs in human fetal heart DHS, 11 are predicted to alter TF recognition sequences (Online Table 11). When considering all genome-wide significant SNPs (p < 10−8) as well as those in high LD (r2 > 0.8), 402 SNPs in the colocalizing DHS perturb transcription recognition sequences, including those of important cardiac and muscle developmental regulators like TBX, GATA-4, and MEF2. When we intersected the GWAS results with ChIP-seq data from mouse and human cardiac tissue (14–16), we found enrichment in enhancers marked by p300, sites bound by RNA polymerase II, and the transcription factors NKX2-5, GATA-4, TBX3, TBX5, and SRF (Figure 2E). A total of 9 of our 52 loci contained not only fetal heart DHS but also ChIP-seq-validated TF binding sites. SNPs overlapping TF binding sites were 5.65-fold enriched within DHS (p = 9.0 × 10−10) but not outside of the DHS (p =0.20). The associations of the 52 sentinel SNPs with all tested functional elements are summarized in Figure 1. We validated several candidate regulatory regions identified earlier as heart enhancers in vivo. Activity of 4 exemplar novel human cardiac enhancers in embryonic transgenic mice stained for LacZ enhancer reporter activity are shown in Figure 3A. Recently, rs6801957 (Figure 1) in the SCN5A/SCN10A locus was reported to influence the activity of a regulatory element affecting SCN5A expression (16,17). Conditional analysis (Online Table 8) revealed that rs6781009 (at 180 kb from the sentinel) is an additional novel independent signal at this locus. Our follow-up in silico and experimental results (Figure 3) indicate the presence of in vivo heart enhancers in genome regions associated with QRS traits.
FIGURE 3. Functional Follow-Up of rs6781009 in the SCN5A Locus.
(A) In vivo activity of 4 exemplar human cardiac enhancers in embryonic transgenic mice stained for LacZ enhancer reporter activity (dark blue) are shown. (For additional examples of previously described enhancers near lead SNPs, see Online Figure 13.) (B) Position of the regulatory element containing rs6781009 on the SCN5A-SCN10A locus. GWAS signals are plotted on a −log(p) scale in dark blue. The regulatory element is bound by TBX3, TBX5, and P300 (lower black traces) in mice, and the contact profile of the SCN5A promoter obtained by 4C-seq human cardiac ventricular tissue revealed an interaction between this regulatory element and the SCN5A promoter (upper black trace and contact profile). Normalized contact intensities (gray dots) and their running median trends (black line) are depicted for the SCN5A promoter viewpoint. Medians are computed for 4-kb windows, and the gray band displays the 20% to 80% percentiles for these windows. Below the profile, statistical enrichment across differently scaled window sizes (from 2 kb [top row] to 50 kb [bottom rom]) is depicted of the observed number of sequenced ligation products over the expected total coverage of captured products, with the latter being estimated on the basis of a probabilistic background model. Local changes in color codes indicate regions that are statistically enriched for captured sequences. The lowest box shows the linkage disequilibrium pattern for the HapMap CEU population. (C) Luciferase assay performed in H10 cells showing a high constitutive activity for the enhancer core element (0.6 kb) containing the major allele for rs6781009, which is reduced for the minor allele in both a large enhancer construct (1.5 kb), as well as in the core enhancer element (0.6 kb). *p < 0.01. (D) Dorsal views of hearts containing the human regulatory element with the major versus minor allele for rs6781009 in a LacZ reporter vector, showing specific expression of the enhancer in the interventricular septum (ivs) for the major allele, which is absent for the minor allele. *p < 0.05. la = left atrium; lv = left ventricle; ra = right atrium; rv = right ventricle; other abbreviations as in Figure 1.
IDENTIFICATION OF CANDIDATE GENES
Across the 52 loci, 974 annotated genes are located within 1 megabase of all sentinel SNPs. Among these genes, we prioritized potential candidates using an established complementary strategy (18,19) by choosing: 1) genes nearest to the sentinel SNP and any other genes within 10 kb (56 genes) (Figure 1); 2) genes containing a nonsynonymous SNP in high LD (r2 > 0.8) with the sentinel SNP (11 genes) (Online Table 10); 3) protein-coding genes with cis-eQTL associated with sentinel SNP (14 genes) (Online Table 12); and 4) GRAIL analysis of the published data (20) (16 genes) (Online Table 13) with “cardiac,” “muscle,” and “heart” as the top 3 keywords describing the observed functional connections. In total, this strategy identified 67 candidate genes at the 52 loci (Figure 1). Pathway analysis confirmed that the list of 67 candidate genes is strongly enriched for genes known to be involved in cardiovascular and muscular system development and function (p = 1 × 10−56) (Online Tables 14 and 15). We have summarized the available functional annotations for all 67 candidates in Online Table 16, including established links from the Online Mendelian Inheritance in Man between candidate genes and familial cardiomyopathies (TNNT2, TTN, PLN, and MYBPC3) and cardiac arrhythmias (CASQ2). We also identified genes that are associated with atrial septal defects (TBX20) and more complex syndromes involving cardiac abnormalities such as the Schinzel-Giedion midface retraction syndrome (SETBP1) (21) and the ulnar-mammary syndrome (TBX3) (22).
INSIGHTS FROM GENE EXPRESSION PROFILING AND MODEL ORGANISMS
We explored gene expression profiles of our candidate genes in data derived from 37,427 U133 Plus 2.0 arrays (Affymetrix) across 40 annotated tissues. We could reliably assign a probe for 63 of our 67 candidate genes. On average, expression levels for these transcripts were higher in cardiac-derived samples compared with other transcripts in the same sample (p = 9.8 × 10−6 for heart tissue; Wilcoxon test) (Online Figure 7) and also when compared to the same transcripts in other tissues (p = 0.005 after Bonferroni correction) (Online Figure 8). To further investigate the potential role of these candidate genes in cardiac development, we assessed temporal gene expression patterns during in vitro differentiation of mouse embryonic stem cells via mesoderm and cardiac precursor cells to cardiomyocytes. A total of 7% of genes are mainly expressed during the embryonic stem cell stage, 22% during the mesoderm stage, 7% in the cardiac precursor stage, and 64% in the cardiomyocyte stage. Compared with other genes, the candidate genes were more highly expressed in cardiomyocytes (p = 5.4 × 10−8; Wilcoxon test) (Online Figure 9). These results suggest that our candidate gene set was enriched for genes that were differentially expressed in cardiac tissue and increasingly expressed during cardiac development.
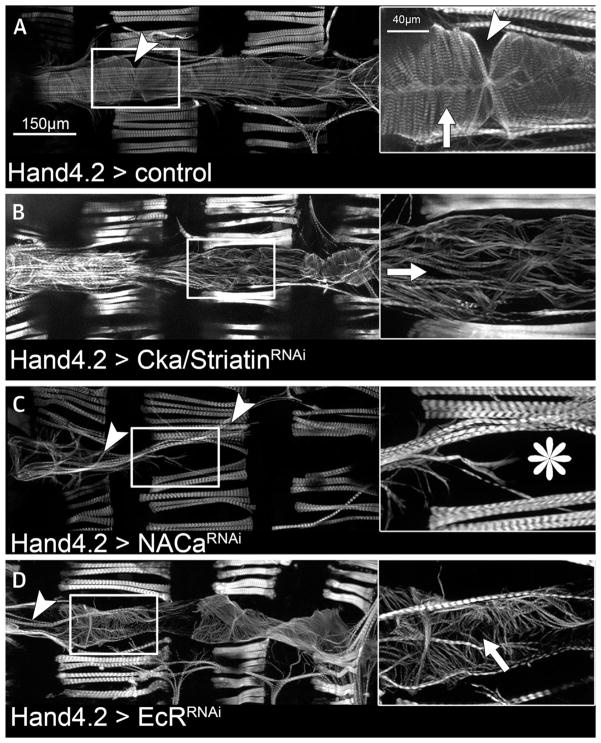
Next, we analyzed data from model organisms to explore the function of the selected candidate genes. From cardiac tissue-specific RNAi knockdown data collected in D. melanogaster, we found that the 67 candidate genes were 2.3-fold enriched for stress-induced cardiac death (9 genes; p = 1.84 × 10−2) (Online Figure 10). To illustrate that prioritized genes may play a critical role in heart development, we tested 4 (CG4743/SLC25A26, Fhos/FHOD3, Cka/STRN, and NACα/NACA) of these 9 genes with unknown cardiac function by performing heart-specific RNAi knockdown with the cardiac Hand4.2-Gal4 driver line. We also retested EcR/NR1H, which has multiple homologous genes in mammals, as well as Hand/HAND1, as this gene was only tested as a full-knockout in early development but not in the adult D. melanogaster heart using cardiac-specific knockdown. Adult hearts of Cka/STRN, NACα/NACA, and EcR/NR1H RNAi showed severe cardiac defects (Figure 4). Knockdown of Hand/HAND1 and Cka/STRN both had a reduced cardiac heart rate. We also expanded on gene-by-gene analysis and identified 6 further genes causing cardiac abnormalities (Online Appendix, Online Table 17). From the Mouse Genome Informatics database, knockout models were annotated for 45 orthologues of the 67 candidate genes, of which 18 (40%) revealed a cardiac phenotype (Online Table 16). This represents a 5.2-fold enrichment compared with randomly matched sets of 67 genes (p = 3.4 × 10−14) (Online Figure 10). Given the evolutionary conservation, the observed heart phenotypes in these model organisms suggest potentially important roles for the significant GWAS loci in electrical and contractile properties of the human heart.
FIGURE 4. Heart-Specific RNAi Knockdown in Drosophila.
Cardiac defects upon heart-specific ribonucleic acid interference (RNAi) knockdown are seen in Drosophila. (A) Wild-type dorsal heart tube stained with the F-actin stain phalloidin. The magnified region (right) is highlighted. Arrowheads point to ostia (inflow tracks), and the arrow shows the circumferential orientation of myofibrils. (B) Cka/Striatin RNAi induces myofibrillar disarrangement. Myofibrils are oriented in a disorganized, mainly anterior-posterior orientation with gaps in between (arrow). (C) Knockdown of NACα/NACA causes severe cardiac tissue disintegration. Adult cardiomyocyte tissue may be completely absent (asterisk), whereas some heart-associated longitudinal muscles are still present (arrowheads). At larval stages, the heart is much less affected, suggesting a maturation or remodeling defect. (D) Knockdown of EcR/NR1H blocks cardiac remodeling and causes myofibrillar disarray (arrow). Ventral longitudinal muscles are also abnormal (arrowhead).
Interestingly, the 11p11.2 locus harbors multiple candidate genes (Figure 1), including MYBPC3, ACP2, MADD, and NR1H3. MYBPC3 deficiency is well established to cause hypertrophic and dilated cardiomyopathies in both human and mouse models and, thus, represents a plausible candidate gene (Online Table 16). In addition to MYBPC3, eQTL and histone modification data also suggest a potential role for NR1H3 (Online Figure 11), as decreased expression of NR1H3 was associated with higher QRS voltages. However, NR1H3-deficient mice do not spontaneously develop a cardiac hypertrophic phenotype (MGI: 1352462). To study the potential cardiac effects of NR1H3, we created a transgenic mouse with cardiac-specific overexpression of NR1H3 under the control of the Myh6 promoter and found a diminished susceptibility to perturbations such as transverse aortic constriction and angiotensin II infusion that provoke cardiac hypertrophy (23). This observation is in line with protective effects due to treatment with T0901317, a synthetic NR1H3 agonist, in mice challenged with aortic constriction (24). These data highlight the importance of systematic approaches to identify causal genes beyond well-known candidates.
INSIGHTS FROM DEPICT
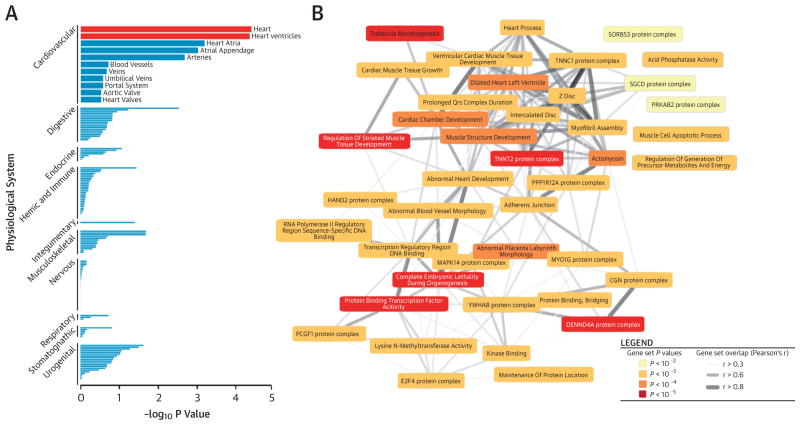
As a complementary approach, we employed the newly developed computational tool DEPICT (Data-Driven Expression-Prioritized Integration for Complex Traits) (25) to analyze functional connections among associated loci (Online Appendix). Enrichment of expression in 209 particular tissues and cell types identified heart and heart ventricles as the most relevant tissue for our association findings (Figure 5A, Online Table 18) and identified 404 significantly (false discovery rate <5%) enriched gene sets (Online Table 19). Comparing the names of these sets with those of the remaining 14,057 gene sets showed an over-representation of the common key words “abnormal,” “muscle,” “heart,” “cardiac,” and “morphology” (Online Table 20). We investigated similarities among gene sets by clustering them on the basis of the correlation between scores for all genes (Online Appendix). Many of the resulting 43 meta-gene sets were correlated and relevant to cardiac biology (Figure 5B). As an example, we showed the correlation structure within the second most significant meta-gene set “dilated heart left ventricle” (Online Figure 12). When prioritizing genes on the basis of functional similarities among genes from different associated regions, DEPICT identified 35 genes (false discovery rate <5%) at 27 of the 52 loci (Figure 1, Online Table 21).
FIGURE 5. Functional Connections of Gene Expression Networks.
In the DEPICT (Data-Driven Expression-Prioritized Integration for Complex Traits) analysis, (A) plots show the enrichment of loci associated with QRS traits in specific physiological systems. (B) In a graphic display of DEPICT gene set enrichment analysis, meta-gene sets are represented by nodes colored according to statistical significance, and similarities between them are indicated by edges scaled according to their correlation (only correlations with r > 0.3 are shown).
DISCUSSION
In this study, we performed a meta-analysis of GWAS in 73,518 individuals for 4 quantitative QRS phenotypes and identified 52 independent genetic loci influencing these traits with 79 locus-phenotype associations; the majority of these discoveries are novel. Our loci were colocalized with open chromatin, histone modification, and TF binding sites, specifically in cardiac tissue, and contain in vivo functional enhancers. We also provided direct evidence that rs6781009, located in a cardiac enhancer, interacts with the promoter of SCN5A to modify expression levels. On the basis of multiple criteria, we defined a core set of 67 candidate genes that we believe are likely to influence cardiac mass and function. We have provided several exemplar experiments to further support this hypothesis.
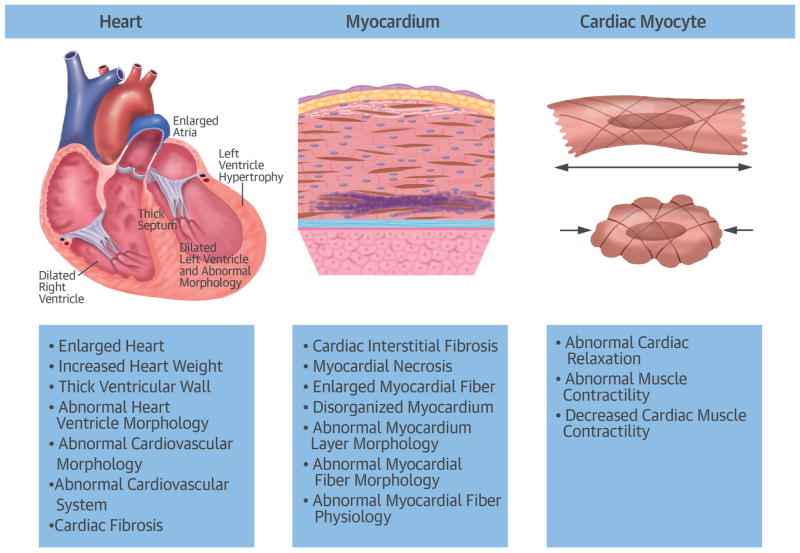
We identified a number of loci containing genes that are directly or indirectly key to the function of cardiomyocytes and cardiac function (Central Illustration). TTN, MYBPC3, TNNT2, SYNPO2L, and MYH7B are essential components of the cardiac sarcomere; PLN, CTNNA3, PRKCA, CASQ2, and STRN are also examples of genes essential for cardiac myocyte function; whereas several key cardiac transcription factors are prominently involved in cardiac muscle and tissue development, such as MEF2D, HAND1, TBX20, TBX3, and NACA. The abundance of candidate genes known to be involved in cardiac muscle function strengthens the hypothesis that the easily obtainable QRS-voltage phenotypes of the ECG are effective in capturing unknown loci that harbor genes likely to play an important role in left ventricular mass, but these are currently not well understood. The colocalization of our genetic loci with regulatory DNA elements (e.g., enhancers, promoters, and transcription factor binding sites) that are active in cardiac tissues further supports the relevance of the genes within these loci. The current work was not designed to provide an explanation for association of each loci and each individual gene.
CENTRAL ILLUSTRATION. Gene-Related Cardiac Conditions.
In this study, a number of gene-containing loci were identified that influence a variety of abnormalities in the dysfunctional heart, myocardium, and cardiac myocytes. Analyzing the QRS genome-wide association study results using the DEPICT (Data-Driven Expression-Prioritized Integration for Complex Traits) tool identified 43 meta-gene sets (Figure 5B); 1 of these is the “dilated heart left ventricle,” of which the effects of the individual gene sets are visualized in the illustration. The correlation substructure and the p values of this gene set are also displayed in Online Figure 12.
Clearly, future translational efforts should be undertaken to resolve the causal genes and exact molecular machinery resulting in the phenotype and should consider mapping the effect of genetic variants on these functional elements at each of the identified loci. Nevertheless, we have provided some exemplar preliminary elements to offer early insights into strategies that can be undertaken to follow-up our findings. For example, we performed a series of experiments to demonstrate in vivo effects of rs6781009 on expression. Dedicated experiments might also elucidate loci containing effects on multiple plausible genes. In 1 of our loci, we identified a very strong candidate gene (MYBPC3) that is well known to be involved in hypertrophic cardiomyopathies. However, using additional layers of information derived from gene expression and histone modifications, we also considered NR1H3 and were able to link overexpression of this gene to cardiac protection of hypertrophy. These examples fuel our expectation that the presented shortlist of SNP associations and the identified candidate genes provided in this work are valuable resources that will help to prioritize and guide future translational studies to further our knowledge on the (patho)physiology of cardiac hypertrophy.
STUDY LIMITATIONS
As in all current GWAS, we have only studied a finite number (~2.8 million) of markers on the genome. Additional fine-mapping studies might be required to narrow the signal of association even further and to identify the potential causal variants with higher accuracy. Also, additional exome-focused arrays or whole-genome sequencing might lead to a stronger signal within a locus or to multiple additional independent signals within a locus. To understand genetic mechanisms and to identify candidate genes, we have studied eQTLs. Although we studied the largest set of human cardiac eQTLs available to date, the absolute number of studied samples is relatively small compared to eQTL data available in easily accessible peripheral blood. Finally, our ECG indexes are generally considered to be markers of cardiac hypertrophy; they also might reflect electrical remodeling of the action potential and not mass per se. Nevertheless, the variables studied here harbor important prognostic information, independently from cardiac mass parameters as assessed by echocardiography (26). This further underscores the relevance of the trait studied and the importance of understanding its genetic determinants.
CONCLUSIONS
In this study, we identified 52 genomic loci, of which 32 are novel and associated with electrically active cardiac mass. We prioritized 67 candidate genes and showed their relevance in cardiac biology using bioinformatic approaches, and we performed in vitro and in vivo experiments, going beyond the classical GWAS approach. To facilitate and accelerate future studies aimed at a better understanding of cardiac hypertrophy, heart failure, and related diseases, we made our results of genome-wide associations publicly available.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Numerous genetic loci have been identified that are associated with electrocardiographic markers of ventricular hypertrophy.
TRANSLATIONAL OUTLOOK
A better understanding of the genetic pathways underlying increased myocardial mass could be used to target therapeutic interventions that improve clinical outcomes for patients with hypertension, heart failure, and various forms of congenital heart disease.
Acknowledgments
A detailed list of acknowledgments is provided in the Online Appendix.
ABBREVIATION AND ACRONYMS
- DHS
deoxyribonuclease hypersensitivity sites
- ECG
electrocardiogram
- eQTL
expression quantitative trait locus
- GWAS
genome-wide association study
- LD
linkage disequilibrium
- RNAi
ribonucleic acid interference
- SNP
single nucleotide polymorphism
- TF
transcription factor
Footnotes
APPENDIX For an expanded Methods section as well as supplemental figures and tables, please see the online version of this article.
Dr. Abecasis has served on the scientific advisory board for Regeneron Genetics Center. Dr. Haugen’s current employer (Altius Institute) receives research funding from GlaxoSmithKline. Dr. Pennacchio is a salaried employee and owns stock in Metabiota. Dr. Stamatoyannopoulos is the director of a nonprofit research institute. Dr. Psaty has served on the data and safety monitoring board for a clinical trial funded by Zoll LifeCor; and has served on the steering committee of the Yale Open Data Access project funded by Johnson & Johnson. Dr. de Bakker is currently an employee of and owns equity in Vertex Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 2.Okin PM, Roman MJ, Devereux RB, Pickering TG, Borer JS, Kligfield P. Time-voltage QRS area of the 12-lead electrocardiogram: detection of left ventricular hypertrophy. Hypertension. 1998;31:937–42. doi: 10.1161/01.hyp.31.4.937. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89–105. doi: 10.7326/0003-4819-71-1-89. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–90. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 5.Usoro AO, Bradford N, Shah AJ, Soliman EZ. Risk of mortality in individuals with low QRS voltage and free of cardiovascular disease. Am J Cardiol. 2014;113:1514–7. doi: 10.1016/j.amjcard.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Kamath SA, de P Meo Neto J, Canham RM, et al. Low voltage on the electrocardiogram is a marker of disease severity and a risk factor for adverse outcomes in patients with heart failure due to systolic dysfunction. Am Heart J. 2006;152:355–61. doi: 10.1016/j.ahj.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Ferreira T, Morris AP, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–75. S1–3. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abecasis GR, Altshuler D, Auton A, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stergachis AB, Neph SJ, Reynolds AP, et al. Epigenetic memory of developmental fate and time encoded in human regulatory DNA landscapes. Cell. 2013;154:888–903. doi: 10.1016/j.cell.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurman RE, Rynes E, Humbert R, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein BE, Stamatoyannopoulos JA, Costello JF, et al. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol. 2010;28:1045–8. doi: 10.1038/nbt1010-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundaje A, Meuleman W, Ernst J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci U S A. 2011;108:5632–7. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May D, Blow MJ, Kaplan T, et al. Large-scale discovery of enhancers from human heart tissue. Nat Genet. 2012;44:89–93. doi: 10.1038/ng.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Boogaard M, Smemo S, Burnicka-Turek O, et al. A common genetic variant within SCN10A modulates cardiac SCN5A expression. J Clin Invest. 2014;124:1844–52. doi: 10.1172/JCI73140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Boogaard M, Wong LY, Tessadori F, et al. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J Clin Invest. 2012;122:2519–30. doi: 10.1172/JCI62613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gieger C, Radhakrishnan A, Cvejic A, et al. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201–8. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Harst P, Zhang W, Mateo Leach I, et al. Seventy-five genetic loci influencing the human red blood cell. Nature. 2012;492:369–75. doi: 10.1038/nature11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raychaudhuri S, Plenge RM, Rossin EJ, et al. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 2009;5:e1000534. doi: 10.1371/journal.pgen.1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoischen A, van Bon BW, Gilissen C, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42:483–5. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 22.Linden H, Williams R, King J, Blair E, Kini U. Ulnar Mammary syndrome and TBX3: expanding the phenotype. Am J Med Genet A. 2009;149A:2809–12. doi: 10.1002/ajmg.a.33096. [DOI] [PubMed] [Google Scholar]
- 23.Cannon MV, Sillje HHW, Sijbesma JW, et al. Cardiac LXRα overexpression protects against pathological hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Mol Med. 2015;7:1229–43. doi: 10.15252/emmm.201404669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuipers I, Li J, Vreeswijk-Baudoin I, et al. Activation of liver X receptors with T0901317 attenuates cardiac hypertrophy in vivo. Eur J Heart Fail. 2010;12:1042–50. doi: 10.1093/eurjhf/hfq109. [DOI] [PubMed] [Google Scholar]
- 25.Pers TH, Karjalainen JM, Chan Y, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–51. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.