Abstract
Most of the terrestrial net primary production enters the decomposer system as dead organic matter, and the subsequent recycling of C and nutrients are key processes for the functioning of ecosystems and the delivery of ecosystem goods and services. Although climatic and substrate quality controls are reasonably well understood, the functional role of biodiversity for biogeochemical cycles remains elusive. Here we ask how altering litter species diversity affects species-specific decomposition rates and whether large litter-feeding soil animals control the litter diversity–function relationship in a temperate forest ecosystem. We found that decomposition of a given litter species changed greatly in the presence of litters from other cooccurring species despite unaltered climatic conditions and litter chemistry. Most importantly, soil fauna determined the magnitude and direction of litter diversity effects. Our data show that litter species richness and soil fauna interactively determine rates of decomposition in a temperate forest, suggesting a combination of bottom-up and top-down controls of litter diversity effects on ecosystem C and nutrient cycling. These results provide evidence that, in ecosystems supporting a well developed soil macrofauna community, animal activity plays a fundamental role for altered decomposition in response to changing litter diversity, which in turn has important implications for biogeochemical cycles and the long-term functioning of ecosystems with ongoing biodiversity loss.
Keywords: biodiversity, soil fauna, temperate forest ecosystem
Plants interact with ecosystem C and nutrient turnover in various ways (1, 2), but the importance of plant species diversity for these processes is not well understood. Living plant diversity can influence decomposition and nutrient dynamics through changes in microclimatic conditions (1, 3), complementary nutrient use (4, 5), and rhizosphere processes. In addition, the amount and quality of plant litter input have a strong impact on C and nutrient cycling (6–8). This litter effect depends on the relative contribution of different species and their characteristic litter traits to the overall community litter pool. There is clear evidence suggesting that observed rates of community litter mass loss and nutrient mineralization may deviate substantially from those expected from single-litter-species decomposition of component species because of nonadditive interactions among litter species (3, 9–13).
We lack a mechanistic understanding of litter species interactions, and the variable results, ranging from clearly negative to strongly positive litter mixing effects, on decomposition are difficult to explain (11–13). Recent theory, and the rare attempts to perform experimental tests of specific mechanisms, have concentrated on bottom-up controls of distinct litter chemistries through nutrient translocation among litter species, or inhibiting compounds, and the results have been conflicting (10–16). Besides potential bottom-up control on litter species interactions, recent laboratory experiments with litter-feeding soil fauna might suggest top-down consumer control through altered food selection and consumption rate in response to changing litter species composition (17, 18). The larger soil invertebrates such as earthworms, millipedes, or isopods, which process large amounts of dead plant material and determine its fate to a great extent in many ecosystems (17, 19–22), are notoriously excluded in decomposition studies, blurring the understanding of the functional significance of litter diversity for decomposition. Moreover, the separation of individual litter species behavior within mixtures appears to be a prerequisite to identify the mechanisms by which litter diversity influences decomposition, but this separation has been done rarely so far and only for two-species mixtures (12).
In this study, we experimentally tested the hypothesis that the activity of litter-feeding invertebrates determines litter diversity effects on ecosystem C and nutrient fluxes in a temperate forest ecosystem. For this test, we used field microcosms to manipulate both tree leaf litter diversity across a broad gradient (from monocultures to six-species mixtures) and the presence of soil fauna, and we recorded the effects of these two factors on the decomposition of individual species within mixtures.
Methods
Site and Experimental Design. Our study site was an old-growth mixed temperate forest (≈120 years old) located 15 km SW of Basel, Switzerland (lat 47°28′N, long 7°30′E), at an elevation of 550 m above sea level. The climate is a typical humid temperate zone climate with mean air temperatures of 2°C and 19°C in January and July, respectively, and an average annual precipitation of 990 mm. The soil is of the rendzina type on calcareous bedrock (topsoil, 0.1–0.15 m; pH ≈ 5.8).
Interactions between litter diversity and soil fauna were examined by using field microcosms (0.15 m in diameter and 0.25 m in height) constructed from plastic cylinders that were inserted 0.2 m deep into the intact forest soil. The top, bottom, and two 0.15- × 0.15-m large openings on the sides were covered with 0.5-mm nylon mesh. This particular mesh size was chosen to avoid the escape of the added macrofauna from, and the entrance of naturally occurring macrofauna into, any of the microcosms, while at the same time allowing a free establishment of mesofauna and microbial communities. Each of 156 microcosms was filled with 0.003 m3 of forest soil (weighed out to an accuracy of ±1 g) and 8 g (±0.01 g) of tree leaf litter, which is equivalent to 1.5 times the average total annual litter fall (302 g/m2) at the experimental site. For the soil used in microcosms, ≈800 kg of fresh topsoil (to a depth of ≈0.15 m) was taken 30 m from the experimental site in October 2001. The soil was freed of large stones and roots by hand, sieved, and homogenized by being passed several times through a 0.005-m sieve, and then it was defaunated (i.e., all macrofauna were removed, but other organisms were kept; ref. 23). For the litter added to the microcosms, we used fresh fallen leaf litter collected at the experimental site every 5–7 days in October and November 2000 by using 30 litter traps (1 m2 each). This litter was air-dried, separated into species, pooled across litter traps, and then divided into three batches to be used in three replicate blocks of microcosm positioning in the forest. Litter from the six most abundant deciduous tree species at the site (Table 1) was added as single species, three different mixtures of two species, three different mixtures of four species, and a mixture of all six species, resulting in a total of 13 different litter treatments with individual litter species contributing equal amounts to the overall litter pool of mixtures. Species combinations were determined randomly, with the exception of Fagus sylvatica, which was present in each mixture. We consider this exception important, because Fagus dominates the study site as it does most mid-European temperate forests, and thus, other litter species rarely occur in the absence of Fagus. The two-species mixtures were Fagus/Quercus, Fagus/Acer, and Fagus/Tilia, and the four-species mixtures were Fagus/Quercus/Carpinus/Tilia, Fagus/Acer/Carpinus/Tilia, and Fagus/Carpinus/Prunus/Tilia. Millipedes (Glomeris marginata and Glomeris conspersa) and the anecic earthworm (Aporrectodea longa), representing the two most important functional groups of litter-feeding macrofauna in temperate forest ecosystems, were collected by hand; earthworms also were collected with the “Octet” electrosampling method at the experimental site in Fall 2001. Glomeris spp. are the most abundant arthropods in the litter layer, and Aporrectodea longa is the most common earthworm species at the experimental site.
Table 1. Mass loss of monocultures (no macrofauna) and initial quality of studied species.
| Species | Mass loss | N | Lignin | Lignin/N | C/N |
|---|---|---|---|---|---|
| Fagus sylvatica | 16 (1) | 0.73 (0.01) | 10.2 (0.9) | 14.0 (0.9) | 60 (1) |
| Quercus petraea | 21 (2) | 0.83 (0.02) | 10.0 (0.2) | 12.0 (0.6) | 52 (1) |
| Acer campestre | 35 (6) | 0.91 (0.01) | 10.9 (0.5) | 12.0 (0.4) | 47 (1) |
| Carpinus betulus | 39 (6) | 1.16 (0.02) | 8.0 (0.5) | 6.8 (0.2) | 37 (1) |
| Prunus avium | 56 (1) | 0.97 (0.02) | 8.2 (0.3) | 8.4 (0.3) | 45 (1) |
| Tilia platyphyllos | 72 (2) | 1.45 (0.02) | 8.9 (0.6) | 6.2 (0.5) | 30 (1) |
Litter C and N were measured with a CHN analyzer (Model 900, LECO Instruments, St. Joseph, MI), and lignin was determined with the thioglycolic acid method (24, 25). Litter chemistry data are expressed as the percentage of total leaf dry mass, and mass loss is expressed as the percentage of total initial mass (n = 3; SE are given in parentheses).
Four microcosms of each litter treatment were placed randomly in each of three blocks (4 microcosms × 13 litter mixtures × 3 blocks) in November 2001. Litter material was added to the microcosms on Dec. 7, 2001, and allowed to decay for a total of 92 days before animals were added on March 8, 2002. We delayed the addition of animals because they generally prefer partially decayed litter over fresh fallen litter and because they are largely inactive during the cold winter months. Two individuals of Glomeris spp. (0.175 g average fresh body weight per individual) or one individual of Aporrectodea (3 g average fresh body weight) were added either alone or in combination to one microcosm per litter treatment within each block, and the fourth microcosm remained without macrofauna. Individuals were chosen from a large pool of animals to achieve a similar animal biomass among microcosms of a given animal treatment. Our animal densities are roughly 1.5 times higher than the natural densities estimated based on our sampling, but they are representative for total macrofauna density and biomass at our site.
Data Collection and Analyses. Assessing decomposition of entire litter mixtures is likely to obscure diversity effects on individual species within mixtures; therefore, we analyzed the fate of each individual litter species in response to changing litter diversity separately. Remaining litter was retrieved from all 156 microcosms (4 macrofauna treatments × 13 litter mixtures × 3 replicates) after a total of 204 days of exposure in the field on June 28, 2002. The duration of the experiment was determined by mass loss rates of rapidly decomposing species (Prunus and Tilia) to ensure sufficient food availability in all animal treatments and appropriate remaining litter mass for data analyses. After removing surface litter and millipedes, soil from all microcosms was searched for earthworms and then passed through a 0.004-m sieve to collect litter within the soil. All litter was rinsed with deionized water to remove soil particles, separated into species, and air-dried. On the basis of color, texture, structure of epidermis, and patterns of leaf veins, litter species identification was possible even for pieces as small as 8 mm2. Unidentified litter material accounted for 2–5% of the total litter mass remaining.
Single-factor ANOVA was used to test for differences in species-specific initial litter quality and mass loss in monocultures among the six study species (Table 1). In addition, the relationship between initial litter quality and mass loss from monocultures was assessed by using simple linear regression analyses across species. Mass loss data of individual litter species from the different diversity and animal treatments were analyzed with mixed-model three-factor analyses of covariance to test for effects of initial litter quality (covariable), block (random factor), litter diversity (random factor), macrofauna (fixed factor), and the interactions between litter diversity and macrofauna. Initial litter quality (i.e., N concentration, lignin concentration, C/N ratio, and lignin/N ratio) of litter mixtures was calculated as the average from the relative contribution of each species present. According to the mixed-model structure, mean squares of the factor macrofauna have been tested against the mean squares of the interaction term (diversity × macrofauna) instead of the residual (26). In addition to analyses of covariance, we performed post hoc pairwise comparisons (Tukey adjusted) to identify differences among levels of diversity and macrofauna identity (26). To assess whether litter mixtures differed in their overall mass loss compared with what would be predicted from mass loss of monocultures, we tested the observed total mass loss of entire litter mixtures against the predicted total mass loss calculated as the sum of mass losses of the respective monocultures by using single-factor ANOVA. All mass loss data, N concentration, and lignin concentration were arc sin square root transformed, and C/N ratio and lignin/N ratio were ln transformed before statistical analysis to improve the homogeneity of variance and normality. systat, version 5.2.1 (Systat Inc., Evanston, IL), was used for all statistical analyses.
Results and Discussion
The tree species used in our experiment showed a wide range in litter mass loss when decomposing as monocultures (F5,12 = 31.3, P = 0.0001, Table 1). Likewise, initial litter N concentration (F5,12 = 96.8, P = 0.0001), lignin concentration (F5,12 = 4.6, P = 0.014), C/N ratio (F5,12 = 142.3, P = 0.0001), and lignin/N ratio (F5,12 = 34.9, P = 0.0001) were significantly different among species (Table 1). Across the study species, decomposition correlated positively with litter N concentration (r2 = 0.75, F1,4 = 12.3, P = 0.025) and negatively with C/N ratio (r2 = 0.75, F1,4 = 11.8, P = 0.026), and lignin/N ratio (r2 = 0.72, F1,4 = 10.3, P = 0.032). The good correlation between litter quality and decomposition is well established for many plant species in various ecosystems (27–30). The robustness of this relationship suggests an important predictive value of initial litter chemistry for rates of C and nutrient cycling, and litter quality traits such as N concentration or the ratios of C/N and lignin/N are widely used as input variables in biogeochemical models (27, 31–33).
Despite the use of litter material of the same species-specific quality, intraspecific variation in mass loss in response to changing litter diversity and macrofauna presence was substantial in all litter species (Fig. 1). In most species, this treatment-related variation exceeded the litter-chemistry-related variation among species, with a 21-, 16-, 15-, and 14-fold difference in percent litter mass remaining between microcosms with the highest and the lowest litter mass loss in Prunus, Carpinus, Acer, and Tilia, respectively. Spatial variability (block effect) and differences in litter quality (covariable) explained only little of the overall variance in the litter mass remaining, and both the block factor and the covariable were not significant in either species (Table 2). However, mass loss of the three more slowly decomposing species (Fagus, Quercus, and Acer) increased significantly with increasing litter diversity (Table 2). In fact, when we compared the actual measured litter mass remaining with the litter mass that would have been predicted based on data from monocultures of these three species, 71% of all data showed an actual litter mass lower than the predicted mass (Fig. 1). In contrast, in the three species with faster decomposition rates (Carpinus, Prunus, and Tilia), we found no overall significant diversity effect (Table 2). Accordingly, only 60% of all data showed a faster-than-predicted mass loss, which is still somewhat higher than the ≈50% expected from a random distribution. These results indicate that decomposition of relatively recalcitrant litter species is accelerated when other species are present, but accompanying litter species are of limited importance for changing the mass loss of more rapidly decomposing species. Stimulating effects of higher nutrient availability from high-quality litter species on the decomposition of low-quality litter species has been repeatedly proposed as a mechanism for synergistic litter mixture effects (11, 19, 34) but could only rarely be confirmed (15, 16). In other studies, differences in chemistry of pairs of litter species were not related to differences between observed and predicted decomposition (14), or positive effects of low-quality litter species on decomposition of associated litters of higher quality were observed (35).
Fig. 1.
Observed remaining litter mass of individual species as a function of the remaining litter mass predicted from monocultures of the respective species and animal treatments. (Left) Data from the three more slowly decomposing species are shown. (Right) Data from the more rapidly decomposing species. The lines indicate the 1:1 line along which observed and predicted values are equal. Seventy-one percent of all data points lie below this line in Left, and 60% lie below it in Right (i.e., show a faster-than-predicted decomposition).
Table 2. Analyses of covariance to test for differences in litter mass loss of the six study species.
| Litter species | Source of variance | df | Mean square | F value | P value |
|---|---|---|---|---|---|
| Fagus sylvatica | Covariable (C/N ratio) | 1 | 12.56 | 1.30 | 0.26 |
| Block | 2 | 4.07 | 0.42 | 0.66 | |
| Litter diversity (D) | 3 | 60.54 | 6.27 | 0.0008 | |
| Macrofauna (M) | 3 | 80.55 | 2.16 | 0.16 | |
| D × M | 9 | 37.32 | 3.86 | 0.0005 | |
| Error | 71 | 9.65 | |||
| Quercus robur | Covariable (lignin/N ratio) | 1 | 11.28 | 0.87 | 0.36 |
| Block | 2 | 9.50 | 0.73 | 0.49 | |
| Litter diversity (D) | 3 | 46.81 | 3.60 | 0.026 | |
| Macrofauna (M) | 3 | 264.95 | 4.67 | 0.031 | |
| D × M | 9 | 56.73 | 4.36 | 0.0013 | |
| Error | 28 | 13.00 | |||
| Acer campestre | Covariable (lignin/N ratio) | 1 | 15.27 | 0.41 | 0.53 |
| Block | 2 | 59.41 | 1.58 | 0.22 | |
| Litter diversity (D) | 3 | 171.79 | 4.58 | 0.0099 | |
| Macrofauna (M) | 3 | 342.89 | 2.62 | 0.12 | |
| D × M | 9 | 130.87 | 3.49 | 0.0053 | |
| Error | 28 | 37.53 | |||
| Carpinus betulus | Covariable (C/N ratio) | 1 | 67.00 | 0.61 | 0.45 |
| Block | 2 | 313.81 | 2.88 | 0.067 | |
| Litter diversity (D) | 2 | 263.53 | 2.42 | 0.10 | |
| Macrofauna (M) | 3 | 606.60 | 9.44 | 0.011 | |
| D × M | 6 | 64.24 | 0.59 | 0.74 | |
| Error | 45 | 109.06 | |||
| Prunus avium | Covariable (N concentration) | 1 | 54.56 | 1.40 | 0.25 |
| Block | 2 | 19.82 | 0.97 | 0.40 | |
| Litter diversity (D) | 2 | 43.90 | 1.13 | 0.34 | |
| Macrofauna (M) | 3 | 971.47 | 5.93 | 0.032 | |
| D × M | 6 | 163.75 | 4.20 | 0.0062 | |
| Error | 21 | 38.96 | |||
| Tilia platyphyllos | Covariable (lignin/N ratio) | 1 | 36.80 | 0.78 | 0.38 |
| Block | 2 | 16.72 | 0.35 | 0.70 | |
| Litter diversity (D) | 3 | 45.64 | 0.96 | 0.42 | |
| Macrofauna (M) | 3 | 240.12 | 7.39 | 0.0084 | |
| D × M | 9 | 32.47 | 0.69 | 0.72 | |
| Error | 50 | 47.36 |
Analysis of covariance was used to test for effects of litter quality (covariable), blocking (random block factor), litter diversity (random factor), macrofauna presence (fixed factor), and the interaction of litter diversity and macrofauna presence on species-specific litter mass loss. The litter quality parameter explaining the largest portion of variance was used as covariable in the model. According to the mixed model used, the effect of macrofauna was tested against the mean square of D × M as the error term.
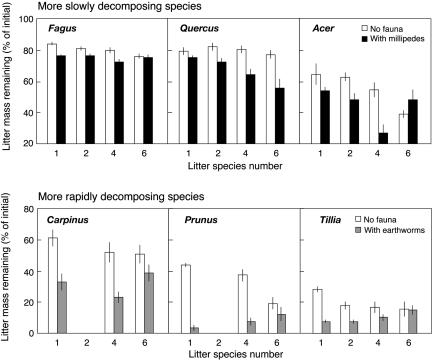
In our study, macrofauna presence changed litter mass loss substantially, and most importantly, the relationship between litter mass loss and litter species number was strongly influenced by animals (Fig. 2 and Table 2). Generally, millipedes had a significant effect on the mass loss of the more slowly decomposing litter species, whereas earthworms were more important in determining mass loss of more rapidly decomposing litter species (Fig. 2). In the presence of millipedes, the recalcitrant Quercus litter disappeared remarkably faster with increasing litter diversity, showing a 26% greater mass loss in the six-species mixture compared with monocultures. However, mass loss of Quercus litter did not change in response to increasing litter diversity without macrofauna or when earthworms alone were present. Likewise, mass loss of Acer litter increased in the presence of millipedes and litter species numbers up to the level of four species, but it slowed again in the 6-species mixture (Fig. 2). No significant macrofauna contribution to the overall positive correlation between mass loss and litter species richness was found in Fagus. The three more easily decomposing species, Carpinus, Prunus, and Tilia, were most affected by earthworms, whereas millipedes contributed only little to the overall stimulation of litter mass loss by macrofauna. Quite unexpectedly, litter of Prunus and Tilia (as a nonsignificant trend in the latter) disappeared more slowly with increasing litter diversity when earthworms were present (Fig. 2). Increasing litter species richness, regardless of the presence or absence of macrofauna, did not influence mass loss of Carpinus litter. Confirming the initial hypothesis, these data show that the activity of litter-feeding soil animals play a fundamental role in shaping litter diversity effects on decomposition, and provide evidence for significant top-down control on the litter diversity–function relationship. Moreover, millipedes and earthworms, two common functional groups of litter-feeding invertebrates in various terrestrial ecosystems, differ considerably in their impacts. Hence, the relationship between species diversity in the litter pool and decomposition is highly dependent on the types of soil animals present, a fact which needs to be considered to understand the functional significance of litter diversity for ecosystem processes. The top-down control of litter-feeding soil animals on litter diversity effects documented here could be driven by changing feeding rates as a function of litter diversity, by indirect effects on microbial or mesofauna communities, or a combination of both. Relative consumption of different litter species has been found to depend on the number of available litter species for millipedes (17) and isopods (18) with a generally increasing relative consumption of higher-quality, and apparently preferred, litter species in more diverse litter mixtures. These results from short-term laboratory feeding trials do not agree with our findings of rather increasing mass loss of low-quality litter and decreasing mass loss of high-quality litter with increasing litter diversity in the presence of macrofauna. This inconsistency might be due to shifting nutritional requirements of animals in the long term, changing palatability of litter species during decomposition, or the particular litter species included in the test. Alternatively, indirect macrofauna effects on microbial decomposition through litter fragmentation, litter mixing, and feces production as it is frequently reported (19–22, 36, 37) could distinctly influence different litter species within the same mixture, leading to the response patterns observed here. To determine conclusively how millipedes and earthworms change the mass loss of particular litter species in relationship to litter diversity, further investigation distinguishing between the direct and indirect effects of macrofauna will be required.
Fig. 2.
Remaining mass of individual litter species after 204 days of exposure in the field (mean ± SE). Litter decomposed as monocultures or within litter mixtures, with or without macrofauna present. For the more slowly decomposing species (Upper), data for microcosms without macrofauna (open bars) or with millipedes (solid bars) are shown [earthworms had no effect (Fagus and Quercus) or a similar effect as millipedes (Acer)]. For the more rapidly decomposing species (Lower), data without macrofauna (open bars) or with earthworm presence (shaded bars) are shown [millipedes had either no effect (Carpinus) or a much smaller effect than earthworms (Prunus and Tilia)]. See Table 2 for statistics.
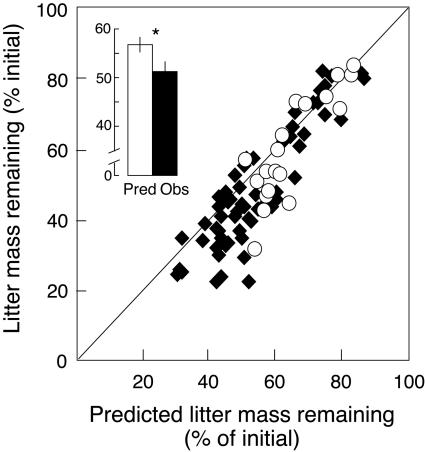
Here we show that altering litter diversity in a temperate forest ecosystem significantly changes mass loss of specific litter species during decomposition. The large differences of effects among species changes their relative contribution to the litter mixture compared with that predicted from monocultures, with a generally faster disappearance of recalcitrant litter types in mixtures. Consequently, the litter layer composition and the temporal dynamics of litter decay are altered, which has important implications for litter turnover. For example, an accelerated decomposition of the slowly decomposing species Fagus and Quercus reduces their residence time in the litter layer, therefore improving the average litter pool quality, which is further accentuated by the earthworm-driven slow-down in decomposition of high-quality litter such as Prunus or Tilia. A shift in relative residence time of different species in the litter pool may directly affect C mineralization and nutrient release rates. Perhaps more importantly, the altered litter layer composition is also changing habitat structure, microclimatic conditions, and resource availability for mesofauna and microbial communities, and thus, may have significant indirect effects on nutrient fluxes and the fate of C. As a likely consequence of these different feedback effects, the litter mixture as a whole might decompose at a different rate than that predicted from monocultures of component species (11, 12). We found that, across all treatment combinations, the observed mass loss of litter mixtures significantly increased by an average of 10% compared with that predicted when calculated as the arithmetic mean of monocultures (Fig. 3), which is strong evidence for an overall positive litter mixture effect on decomposition in our study system.
Fig. 3.
Total remaining litter mass of entire microcosms as a function of total predicted litter mass remaining. Data points represent individual microcosms either without macrofauna (open circles) or with macrofauna (i.e., millipedes, earthworms or both; solid diamonds). The line indicates the 1:1 line along which observed and predicted values are equal. Inset shows the average predicted (open bar) and observed (solid bar) total remaining litter mass across all treatments. Predicted litter mass loss of mixtures was calculated as the average of component species mass loss observed in monocultures and compared with the actual observed community litter mass.
In the light of these data, it is questionable whether initial substrate chemistry and climatic conditions alone sufficiently predict ecosystem C and nutrient cycling in species-rich forest communities, as it is usually assumed on the basis of single litter species decomposition and the common exclusion of larger soil animals (27–30). Litter mixing effects on decomposition can change plant nutrient availability (34, 38) and consequently plant growth and net primary productivity (34, 39). The interplay of litter species diversity and soil animals reported here, thus, are directly relevant for nutrient supply rates and forest productivity. It may further influence the fate of C during the decomposition pathway as a key determinant for C cycling and sequestration.
Acknowledgments
We thank E. Spehn for discussions and advice on experimental design and for comments on previous versions of the manuscript. We also thank T. Handa, C. Körner, S. Scheu, P. Vitousek, D. Wardle, and two anonymous reviewers for helpful suggestions and comments improving the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hobbie, S. E. (1992) Trends Ecol. Evol. 7, 336-339. [DOI] [PubMed] [Google Scholar]
- 2.Eviner, V. T. & Chapin, F. S., III (2003) Annu. Rev. Ecol. Evol. Syst. 34, 455-485. [Google Scholar]
- 3.Hector, A., Beale, A. J., Minns, A., Otway, S. J. & Lawton, J. H. (2000) Oikos 90, 357-371. [Google Scholar]
- 4.Tilman, D., Wedin, D. & Knops, J. (1996) Nature 379, 718-720. [Google Scholar]
- 5.Hooper, D. U. & Vitousek, P. M. (1997) Science 277, 1302-1305. [Google Scholar]
- 6.Wardle, D. A., Zackrisson, O., Hörnberg, G. & Gallet, C. (1997) Science 277, 1296-1299. [Google Scholar]
- 7.Binkley, D. & Giardina, C. (1998) Biogeochemistry 42, 89-106. [Google Scholar]
- 8.Prescott, C. E. (2002) Tree Phys. 22, 1193-1200. [DOI] [PubMed] [Google Scholar]
- 9.Bardgett, R. D. & Shine, A. (1999) Soil Biol. Biochem. 31, 317-321. [Google Scholar]
- 10.McTiernan, K. B., Ineson, P. & Coward, P. A. (1997) Oikos 78, 527-538. [Google Scholar]
- 11.Wardle, D. A., Bonner, K. I. & Nicholson, K. S. (1997) Oikos 79, 247-258. [Google Scholar]
- 12.Gartner, T. B. & Cardon, Z. G. (2004) Oikos 104, 230-246. [Google Scholar]
- 13.Hättenschwiler, S. (2005) in Forest Diversity and Function: Temperate and Boreal Systems, eds. Scherer-Lorenzen, M., Körner, C. & Schulze, E.-D. (Springer, Berlin), Vol. 176, pp. 149-164. [Google Scholar]
- 14.Hoorens, B., Aerts, R. & Stroetenga, M. (2003) Oecologia 442, 578-586. [DOI] [PubMed] [Google Scholar]
- 15.Salamanca, E. F., Kaneko, N. & Katagiri, S. (1998) Ecol. Engineer. 10, 53-73. [Google Scholar]
- 16.Briones, M. J. I. & Ineson, P. (1996) Soil Biol. Biochem. 28, 1381-1388. [Google Scholar]
- 17.Càrcamo, H. A., Abe, T. A., Prescott, C. E., Holl, F. B. & Chanway, C. P. (2000) Can. J. For. Res. 30, 817-826. [Google Scholar]
- 18.Hättenschwiler, S. & Bretscher, D. (2001) Global Change Biol. 7, 565-579. [Google Scholar]
- 19.Seastedt, T. R. (1984) Annu. Rev. Entomol. 29, 25-46. [Google Scholar]
- 20.Anderson, J. M. (1988) Biol. Fert. Soils 6, 216-227. [Google Scholar]
- 21.Wardle, D. A. & Lavelle, P. (1997) in Driven by Nature: Plant Litter Quality and Decomposition, eds. Cadish, G. & Giller, K. E. (CAB International, Wallingford, U.K.), pp. 107-124.
- 22.David, J.-F. & Gillon, D. (2002) Pedobiologia 46, 42-52. [Google Scholar]
- 23.Bonkowski, M., Scheu, S. & Schaefer, M. (1998) Appl. Soil Ecol. 9, 161-166. [Google Scholar]
- 24.Bruce, J. R. & West, C. A. (1989) Plant Physiol. 91, 889-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschel, G., Körner, C. & Arnone, J. A., III (1997) Oecologia 110, 387-392. [DOI] [PubMed] [Google Scholar]
- 26.Sokal, R. R. & Rohlf, F. J. (1981) Biometry (Freeman, New York), 2nd Ed.
- 27.Aber, J. D., Melillo, J. M. & McClaugherty, C. A. (1990) Can. J. Bot. 68, 2201-2208. [Google Scholar]
- 28.Coûteaux, M.-M., Bottner, P. & Berg, B. (1995) Trends Ecol. Evol. 10, 63-66. [DOI] [PubMed] [Google Scholar]
- 29.Cadish, G. & Giller, K. E., eds. (1997) Driven by Nature: Plant Litter Quality and Decomposition (CAB International, Wallingford, U.K.).
- 30.Gholz, H. L., Wedin, D. A., Smitherman, S. M., Harmon, M. E. & Parton, W. J. (2000) Global Change Biol. 6, 751-765. [Google Scholar]
- 31.Parton, W. J., Schimel, D. S., Ojima, D. S. & Cole, D. V. (1994) in Sensitivity to Litter Chemistry, Texture and Management, eds. Bryant, R. B. & Arnold, R. W. (Soil Sci. Soc. Am., Madison, WI), Special Publication No. 38, pp. 137-167.
- 32.Kirschbaum, M. U. F. & Paul, K. I. (2002) Soil Biol. Biochem. 34, 341-354. [Google Scholar]
- 33.Nicolardot, B., Recous, S. & Mary, B. (2001) Plant Soil 228, 83-103. [Google Scholar]
- 34.Chapman, K., Whittaker, J. B. & Heal, O. W. (1988) Agric. Ecosyst. Environ. 24, 33-40. [Google Scholar]
- 35.Wardle, D. A., Nilsson, M.-C., Zackrisson, O. & Gallet, C. (2003) Soil Biol. Biochem. 35, 827-835. [Google Scholar]
- 36.Scheu, S. (1987) Biol. Fert. Soils 5, 230-234. [Google Scholar]
- 37.Maraun, M. & Scheu, S. (1996) Oecologia 107, 131-140. [DOI] [PubMed] [Google Scholar]
- 38.Finzi, A. C. & Canham, C. D. (1998) For. Ecol. Manage. 105, 129-136. [Google Scholar]
- 39.Nilsson, M.-C., Wardle, D. A. & Dahlberg, A. (1999) Oikos 86, 16-26. [Google Scholar]