Abstract
A soluble N-ethylmaleimide-sensitive factor-attachment protein alpha (αSNAP) is a multifunctional scaffolding protein that regulates intracellular vesicle trafficking and signaling. In cultured intestinal epithelial cells, αSNAP has been shown to be essential for cell survival, motility, and adhesion; however, its physiologic functions in the intestinal mucosa remain unknown. In the present study, we used a mouse with a spontaneous hydrocephalus with hop gait (hyh) mutation of αSNAP to examine the roles of this trafficking protein in regulating intestinal epithelial homeostasis in vivo. Homozygous hyh mice demonstrated decreased expression of αSNAP protein in the intestinal epithelium, but did not display gross abnormalities of epithelial architecture in the colon and ileum. Such αSNAP depletion attenuated differentiation of small intestinal epithelial enteroids ex vivo. Furthermore, αSNAP-deficient mutant animals displayed reduced formation of lysozyme granules in small intestinal crypts and decreased expression of lysozyme and defensins in the intestinal mucosa, which is indicative of defects in Paneth cell differentiation. By contrast, development of Goblet cells, enteroendocrine cells, and assembly of enterocyte apical junctions was not altered in hyh mutant mice. Our data revealed a novel role of αSNAP in the intestinal Paneth cell differentiation in vivo.
Keywords: intestinal mucosa, membrane fusion, tight junctions, adherens junctions, NAPA
INTRODUCTION
A single layer of intestinal epithelial cells separates the internal organs from the luminal content of the gut. This epithelial layer serves as an essential interface that mediates bidirectional transport of fluids and substances between the body interior and the environment. It also forms a critical protective barrier against luminal antigens and pathogens. The barrier and transport functions of the gut are regulated by the interplay between several types of intestinal epithelial cells, most notably, absorptive enterocytes, Goblet cells, and Paneth cells. Enterocytes represent the predominant type of epithelial cells that determines the barrier properties of the intestinal epithelium and regulates paracellular and transcellular fluxes of water and solutes across the gut wall. Goblet cells populating the small and large intestine are specialized for synthesis and secretion of mucins, high-molecular weight glycoproteins that create a non-cellular, mucus barrier in the gut. Paneth cells are abundant in the crypt region of the small intestine [1]. They play a key role in the host-microbe interactions by producing a variety of antimicrobial peptides and proteins.
It is increasingly being recognized that intracellular vesicle trafficking is an important mechanism that regulates intestinal epithelia homeostasis [2, 3]. Vesicle exocytosis is a key step in the release of mucins and antibacterial proteins from Goblet and Paneth cells. Furthermore, vesicle trafficking is essential for establishing the apico-basal epithelial cell polarity, which is characteristic feature of all differentiated epithelial cell lineages in the gut. Finally, vesicle endocytosis regulates host-pathogen interactions and antibacterial defense. Defects in epithelial vesicle trafficking machinery increase host susceptibility to luminal pathogens [2, 4]. Trafficking of membrane-coated vesicles is a tightly regulated process that depends on a number of lipid-binding, scaffolding, and signaling proteins. Members of the Rab family of small GTPases regulate the sorting of transported molecules into the correct vesicles and vesicle delivery to target membranes [5]. Vesicle fusion with the targeted membranes involves the assembly of a so-called SNARE (soluble N-ethylmaleimide-sensitive factor associated receptor) complexes [6]. Different SNARE proteins interacts with their specific partners on the opposing membrane, brining two membranes into close proximity and triggering their fusion [6]. A significant body of literature addresses the roles of different trafficking and fusion proteins in intestinal epithelial cell differentiation in vitro; however, the roles of vesicle trafficking in the regulation of gut homeostasis and functions in vivo remain poorly understood.
A soluble N-ethylmaleimide sensitive factor-attachment protein alpha (αSNAP) is a key regulator of SNARE-mediated vesicle fusion [7]. It acts as a scaffold to assist a specific ATPase N-ethylmaleimide sensitive factor (NSF) in disassembly of the post-fusion SNARE complexes that is critical for continuous vesicle fusion events [8, 9]. Furthermore, several NSF-independent cellular activities of αSNAP have been reported that involve different binding partners of this adaptor protein. For example, αSNAP inhibits cell apoptosis by binding to the ER-resident, pro-apoptotic protein BNIP1 [10]. Furthermore, αSNAP can regulate calcium homeostasis via its interaction with the Stim1-Orai1 calcium channel [11]. Finally, αSNAP has been shown to bind and dephosphorylate the AMP-activating protein kinase [12]. Multiple NSF-independent functions of αSNAP have been reported in cultured intestinal epithelial cells. They include regulation of epithelial junctions, cell migration, apoptosis, and autophagy [13–16]. However, no previous studies have addressed the roles of αSNAP in the regulation of intestinal epithelial homeostasis in vivo. A spontaneous single amino acid M105I substitution of αSNAP results in the development of the hydrocephalus with hop gait (hyh) phenotype in mice [17, 18]. Homozygous hyh animals are characterized by severe neurological abnormalities and defective sperm reactions [17–19], and they have decreased expression of αSNAP protein in the brain and testis tissues [17, 20]. In the present study, we used hyh mice to investigate the roles αSNAP in regulating intestinal epithelial homeostasis in vivo.
MATERIALS AND METHODS
Animals
Mice of the hyh mutant strain (B6C3Fe a/a-Napahyh/J) were originally obtained from Jackson Laboratory (Bar Harbor, ME). Breeding pairs of heterozygous animals used to establish a colony at the VCU Medical Center were provided by Dr. Antonio Jimenez (University of Malaga, Malaga, Spain). The animals were maintained under pathogen-free conditions with standard food and water available, ad libitum. Animal genotyping was carried out by a PCR-based technique as described previously [21]. Homozygous hyh mutant mice and their wild-type littermates of both sexes were used in the study. The animal experiments were conducted in compliance with the ARRIVE guidelines and in accordance with the National Institutes of Health Animal Care and Use Guidelines. All procedures were approved by the Virginia Commonwealth University Animal Care and Use Committee (protocol # AD10000452).
Antibodies
The following primary monoclonal (mAb) and polyclonal (pAb) antibodies were used: anti-p120-catenin, E-cadherin, and NSF mAbs (BD Biosciences, San Jose, CA); anti-occludin, JAM-A, ZO-1, Claudin-1 and 7 pAbs, and Claudin-4 mAb (Life Technologies, Waltham, MA); anti-GAPDH (14C10) (Cell Signaling, Beverly, MA); anti-β-catenin pAb (Sigma-Aldrich, Saint Lois, MO); goat anti-E-cadherin pAb (R&D Systems, Minneapolis, MN); anti-lysozyme, mucin-2 and Chromogranin A pAbs (Santa Cruz, Dallas, TX); anti-α-SNAP mAb (Abcam, Cambridge, MA). Alexa Fluor-488-conjugated donkey anti-rabbit and donkey anti-goat secondary antibodies and Alexa Fluor-555-conjugated donkey anti-mouse secondary antibodies were obtained from Life Technologies. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were acquired from Bio-Rad Laboratories (Hercules, CA).
Culture of intestinal enteroids
Enteroids were generated from isolated small intestinal crypts of wild type and hyh mutant mice as described previously [22]. Briefly, mice were euthanized and then their small intestine was dissected, longitudinally opened, and washed with ice-cold PBS. Crypts were released from the intestine by 30 min incubation with PBS containing 2 mM of EDTA at 4°C with constant agitation followed by mechanical shaking. Debris and villous fragments were discarded, and the resulting crypt fraction was collected by centrifugation and resuspended in growth factor reduced Matrigel (BD Bioscience). After Matrigel polymerization, DMEM/F12 medium containing HEPES, glutamine, N2 and B27 supplements, and growth factors [50 ng/ml epidermal growth factor, 500 ng/ml R-spondin 1, and 100 ng/ml Noggin (R&D Systems)] was added. Intestinal enteroids were allowed to differentiate for 7 days and were observed using a bright field microscope (Olympus BX41, Japan). Depending on their differentiation (budding) stage, they were assigned to three different differentiation stages: I – non-differentiated spherical enteroids; II- intermediate structures with 2–3 buds; III – well-differentiated enteroids with multiple buds. The number of enteroids at each differentiated stage was counted manually and was expressed as percentage of the total enteroid number.
Immunoblotting analysis
Animals were euthanized, and then the proximal colonic segments were collected, opened longitudinally, and washed with ice-cold PBS. Colonic epithelium was collected by scraping with a glass slide and snap-frozen in liquid nitrogen for further analysis. Colonic epithelial scrapes were homogenized in RIPA buffer containing a protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). SDS gel electrophoresis and immunobloting analysis of tissue lysates were conducted using standard protocols as previously described [23]. Protein expression was quantified by densitometry using Image J software (National Institutes of Health, Bethesda MD).
Quantitative real-time RT-PCR
Total RNA was isolated from the colonic and ileac segments using an RNeasy mini kit (QIAGEN, Valencia, CA). Total RNA (1 μg) was reverse transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative real-time RT-PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories) and the following pair of primers: GAPDH 5′-CATGTTTGTGATGGGTGTGAACCA-3′ and 5′-AGTGATGGCATGGACTGTGGTCAT-3′; Lysozyme 5′-GTCACTGCCCAGGCCAAGGT-3′ and 5′-CGGTGCTTCGGTCTCCACGG-3′; Defensin 1 5′-TGCCCTCGTTCTGCTGGCCT-3′ and 5′-AGCAGAGCCTTCTGTGCCTCCA-3′; Defensin 2 5′-TGGCCTTCCAGGTCCAGGCT-3′ and 5′-CCTGGTCCTCCTCCCCTGGC-3′; Notch-1 5′-CATGGGCGCACAGGTCTGCT-3′ and 5′-AGGGGCAGGTGCAGATGGCT-3′; Math1 5′-AGCTGTCCAAATATGAGACCCTACA-3′; Hes1 5′-CAGCTCCGGGAAAGCAAGCCC-3′ and 5′-GACATTGGGAGTCTGCAGCAA-3′; and Mucin-2 5′-GGGAGGGTGGAAGTGGCATTGT3′ and 5′-TGCTGGGGTTTTTGTGAATCTC-3′. Gene amplification was performed using a 7900HT Fast Real-Time PCR System (A&B Applied Biosystems, Carlsbad, CA). Relative expression of each gene was calculated by a comparative threshold cycle number (Ct) method that is based on the inverse proportionality between Ct and the initial template concentration (2−ΔΔCt) as previously described [24]. This method is based on two-step calculations of ΔCt = Ct(target gene) − Ct(GAPDH) and ΔΔCt = ΔCte − ΔCtc, where index e refers to either hyh mutant or wild type mice, and index c refers to a selected wild type animal sample assigned as an internal control.
Immunohistochemistry
Formalin-fixed, paraffin-embedded colonic and ileac tissues were subjected to immunohistochemistry. After deparaffinization and rehydration, antigens were exposed through heat-induced epitope retrieval for 15 min in the R-Universal epitope recovery buffer (Electron Microscopy Sciences, Fort Washington, PA). The slides were blocked for 60 minutes in Hanks’ buffered salt solution containing 1% bovine serum albumin, followed by overnight incubation at 4°C with primary antibodies. Washed samples were incubated with Alexa Fluor (488 or 555)-conjugated secondary antibodies for 60 minutes at room temperature, rinsed with blocking buffer, and mounted on slides with ProLong antifade mounting reagent (Life Technologies). Images were obtained on Zeiss LSM 700 Laser Scanning Confocal Microscope (Carl Zeiss Microscopy LCC, Peabody, MA).
Hematoxylin-eosin and Periodic Acid-Schiff-Alcian Blue staining
Tissue sections were deparaffinized and stained with hematoxylin and eosin according to standard protocols. The histochemical visualization of Goblet cells was performed using a combined Periodic Acid-Schiff and Alcian Blue staining kit (Sigma-Aldrich) according to the manufacturer’s instructions.
RESULTS
Decreased αSNAP expression attenuates differentiation of small intestinal enteroids
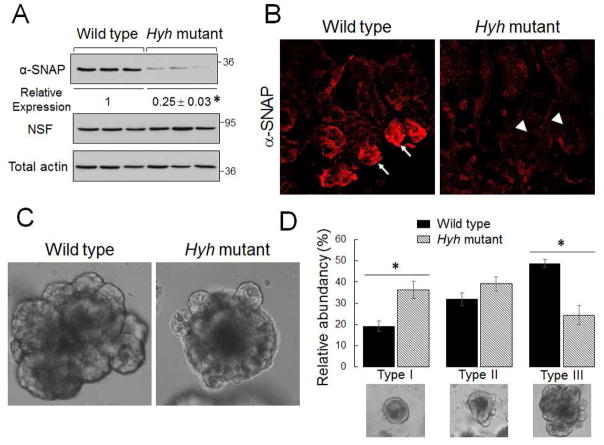
Homozygous hyh mice exhibited marked neurological abnormalities such as hydrocephalus, which is consistent with a previously reported phenotype of these animals [17, 19]. Furthermore, hyh mice demonstrated delayed growth and a shortened colon when compared to their wild-type littermates (Table 1), which could reflect diminished food intake and/or nutrient absorption. In our colony, homozygous hyh mice did not survive after weaning and were euthanized at day 21 after birth. Colonic segments were harvested, and epithelial cells were isolated to examine αSNAP expression. Immunoblotting analysis demonstrated an approximately 75% decrease in αSNAP protein level in the colonic epithelium of homozygous hyh mice as compared to their wild-type littermates (Fig. 1A). In wild-type animals, αSNAP appeared to be enriched in small intestinal crypts (Fig. 1B, arrows). Its crypt labeling was significantly diminished in hyh mice (Fig. 1B, arrowheads). Interestingly, histological analysis did not find gross abnormalities of the epithelial architecture in the ileac and colonic sections of hyh mutants (Suppl. Fig. 1). This indicates that the remaining pool of αSNAP is sufficient to maintain intestinal epithelial cell survival and proliferation. Next, we sought to elucidate the effects of αSNAP depletion on intestinal epithelial differentiation. Stem cell-containing epithelial crypts were isolated from the murine ileum, embedded in Matrigel, and allowed to differentiate ex vivo into intestinal enteroids. Such enteroid differentiation involved initial formation of spherical cysts, which gradually developed a budding pattern with increased complexity (Fig. 1C,D). Enteroids originated from αSNAP-depleted intestinal tissue exhibited significantly lower budding ability (Fig. 1C,D), which indicates defects of their differentiation.
Table 1.
Body weight and colon length of mice at 21 days after birth
| Mice | Weight (grams) | Colon length (centimeters) |
|---|---|---|
|
| ||
| Wild type | 9.75 ±0.39 | 12.1 ± 0.1 |
| Hyh mutant | 5.25 ± 0.24* | 10.5±0.1* |
|
| ||
| Animal number per group | 12 | 7 |
P < 0.001 as compared to wild type animals (unpaired Student’s t-test).
Figure 1. Hyh mutation decreases expression of αSNAP in the intestinal epithelium and attenuates differentiation of isolated enteroids.
Homozygous hyh mice and their wild-type littermates were sacrificed 21 days after birth. (A) Expression of αSNAP and NSF in colonic epithelial cell lysates was determined by immunoblotting. Data is presented as mean ± SE (n = 6); *P<0.01 (unpaired Student’s t-test). (B) Immunofluorescence analysis shows strong αSNAP labeling in small intestinal epithelial crypts of wild-type animals (arrows) and disappearance of such crypt labeling in hyh mutant mice (arrowheads). (C,D) Isolated small intestinal crypts were embedded in Matrigel to generate intestinal enteroids. (C) Representative images of the most abundant morphological type of enteroids. (D) The relative abundance of different stage of enteroid differentiation (see Material and Methods for the explanation). Data is presented as mean ± SE (n = 6); *P<0.01 (unpaired Student’s t-test).
Decreased αSNAP expression selectively inhibits Paneth cell development
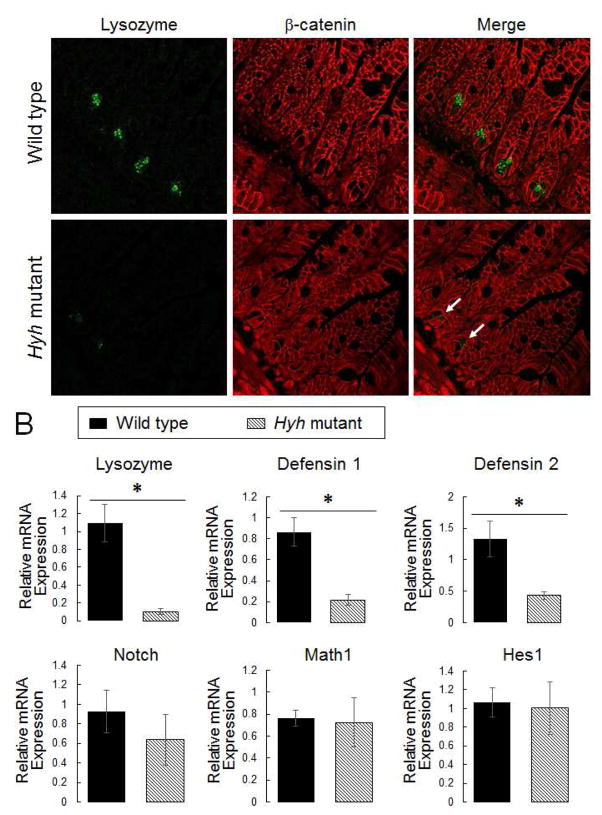
Since the ex vivo budding of enteroids recapitulates in vivo development of the intestinal epithelial crypts [25], attenuation of this process in αSNAP-depleted enteroids suggests inhibition of crypt morphogenesis. Paneth cells represent the most important type of differentiated epithelial cells in small intestinal epithelial crypts [1]. Therefore, we examined if αSNAP depletion affects Paneth cell differentiation by performing immunolabeling of lysozyme as a selective Paneth cell marker. Figure 2 shows a dramatic loss of lysozyme labeling in small intestinal crypts of homozygous hyh mice (arrows). Furthermore, quantitative RT-PCR analysis revealed a significant decrease in lysozyme mRNA expression in the ileum of these animals as compared to their wild-type littermates (Fig 2B). Likewise, mRNA expression of antibacterial peptides defensins 1 and 2, selectively produced by Paneth cells, was significantly decreased in the small intestinal mucosa of hyh mutant mice (Fig. 2B). Together, these data indicate that αSNAP depletion inhibits Paneth cell differentiation.
Figure 2. Decreased αSNAP expression attenuates Paneth cell differentiation.
(A) Dual-immunolabeling of a selective Paneth cell marker, lysozyme (red), and a general epithelial marker, β-catenin (green), in the ileac sections of wild-type and αSNAP-depleted homozygous hyh mice. Arrows indicate decreased lysozyme expression in the intestinal mucosa of αSNAP-depleted animals. (B) mRNA expression of Paneth cell markers, and regulators of Paneth cell differentiation in the whole ileac segments of these animals. Data is presented as mean ± SE (n = 6); *P<0.01 (unpaired Student’s t-test).
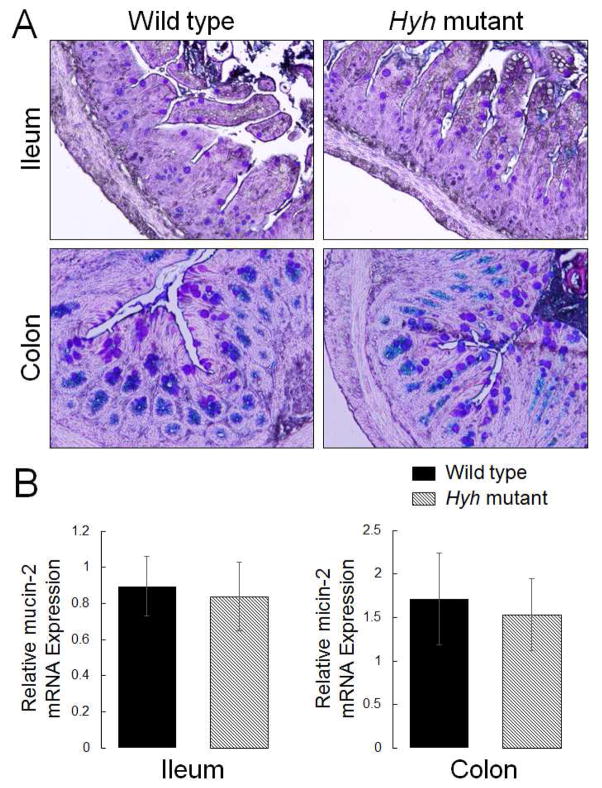
Next we sought to elucidate if such inhibition of differentiation is specific for Paneth cells or it is common for other secretory intestinal epithelial cell lineages. We analyzed expression of transcriptional factors Math1 and Hes1, as well as Notch receptor, which are responsible for differentiation of different types of secretory intestinal epithelial cells [26]. Furthermore, we examined the expression of mucins and chromogranin A, which are selective markers of Goblet cells and enteroendocrine cells, respectively. The quantitative RT-PCR analysis did not detect any effects of αSNAP depletion on the ileac mRNA levels of Math1, Hes1, and Notch (Fig. 2B). Furthermore, Periodic Acid-Schiff-Alcian Blue pan-mucin labeling, and RT-PCR analysis of mucin-2 expression did not demonstrate difference in ileac and colonic Goblet cell differentiation between hyh homozygous and wild-type mice (Fig. 3). Finally, chromogranin A immunolabeling showed no effect of αSNAP depletion on the abundance of enteroendocrine cells in the intestinal mucosa (Suppl. Fig. 2). Together, these data suggest that downregulation of αSNAP expression selectively inhibits Paneth cell differentiation without affecting other types of secretory epithelial cells in the gut.
Figure 3. Decreased αSNAP expression does not affect intestinal Goblet cell differentiation.
(A) Periodic acid-Schiff-Alcian Blue staining of Goblet cells in the ileac and colonic mucosa of control and homozygous hyh mice. (B) mRNA expression of mucin-2 in the intestinal segments of these animals. Data is presented as mean ± SE (n = 5).
Decreased αSNAP expression does not affect the integrity of intestinal epithelial junctions
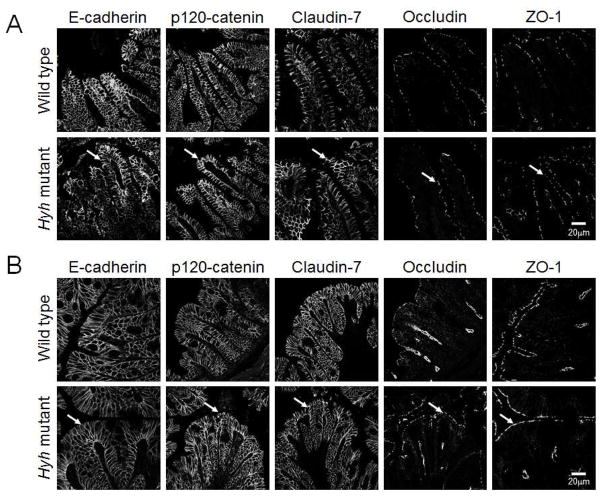
Previous in vitro studies revealed that loss of αSNAP markedly inhibits the assembly and functions of intercellular junctions in model intestinal epithelium [13]. Based on these data, we sought to examine the integrity of epithelial junction in the intestinal mucosa of αSNAP-depleted hyh mutant mice. Immunolabeling and confocal microscopy was used to examine localization of major AJ proteins (E-cadherin and p120 catenin) and TJ proteins (claudin-7, occludin, and ZO-1). Figure 4 demonstrates that αSNAP depletion did not affect localization of the tested AJ and TJ proteins in either ileac or colonic sections (arrows). Furthermore, immunoblotting analysis did not detect any effect of the decreased αSNAP expression on the levels of different AJ and TJ proteins in the colonic epithelium (Suppl. Fig. 3). Together, these results suggest that αSNAP depletion does not affect the integrity and molecular composition of intestinal epithelial junctions.
Figure 4. Decreased αSNAP expression does not affect the architecture of intestinal epithelial junctions.
Immunofluorescence labeling and confocal microscopy of AJ (E-cadherin, p120 catenin) and TJ (claudin-7, occludin, ZO-1) proteins in ileac (A) and colonic (B) sections of wild type and homozygous hyh mutant mice. Arrows show normal localization of different AJ and TJ proteins in αSNAP-depleted intestinal mucosa.
DISCUSSION
Our study represents the first attempt to investigate the roles of a key membrane trafficking protein, αSNAP, in the regulation of intestinal epithelial homeostasis in vivo. We observed that the M105I αSNAP mutation in hyh mice results in marked downregulation of its protein level in the intestinal epithelium and selectively attenuates Paneth cell differentiation. Interestingly, the observed cellular defects in the intestinal mucosa of hyh mutant mice were much less evident as compared to the severe abnormalities caused by the αSNAP knockdown in model human intestinal epithelial cell monolayers. Indeed, in cultured epithelial cells, loss of αSNAP resulted in dramatic disorganization of the epithelial architecture manifested by the loss of cell-cell and cell-matrix adhesion and significant cell death [13–16]. None of these effects was detected in the small or large intestine of hyh mutant mice where epithelial cells retained robust apical junctions and showed no signs of excessive cell detachment or death (Fig. 4, Suppl. Figs 1 & 3, and data not shown). This disparity in the intestinal epithelial responses could be explained by different levels of αSNAP depletion in vitro and in vivo. While siRNA-mediated knockdown of αSNAP in cultured intestinal epithelial cells caused more than a 90% decrease in its protein expression [13], only a 75% depletion was observed in the colonic epithelium of hyh mutants mice (Fig. 1A). The remaining fraction of αSNAP in the murine intestinal mucosa is likely to be sufficient to mediate the major cellular functions of this protein.
Intestinal epithelium appears to be more resistant to αSNAP depletion as compared to the ventricular neiroepithelium. The neiroepithelium was shown to be significantly disrupted in homozygous hyh mice, which included vivid abnormalities in the AJ structure, defective apico-basal cell polarity, and focal loss of epithelial cells [17, 19]. By contrast, hyh mutant mice did not have noticeable defects in the intestinal epithelial integrity or assembly of enterocyte AJ and TJ (Fig. 4, Suppl. Fig. 1). This is consistent with the phenotype of these animals, which rapidly develop hydrocephalus, but do not display any symptoms associated with gut barrier disruption, such as diarrhea or rectal bleeding. The reasons for such tissue-specific epithelial responses remain unknown. It is possible that the level of αSNAP depletion is deeper in the neuroepithelium or that development of this epithelium requires more robust vesicle trafficking to maintain tissue integrity and remodeling.
Surprisingly, our data revealed selective inhibitory effect of αSNAP depletion on Paneth cell differentiation in hyh mice (Fig. 2). This is not a simple consequence of the abnormal secretory granule biogenesis since no defects in the sorting and packaging of secreted proteins by Goblet cells and enteroendocrine cells was detected in αSNAP-depleted intestinal mucosa (Fig. 3 and Suppl. Fig. 2). Furthermore, the observed decrease in mRNA expression of protein markers of mature Paneth cells suggests that αSNAP depletion attenuates Paneth cell differentiation. This finding is in line with a previous report of inhibited neuronal differentiation in the cerebral cortex of hyh mutants [17]. It is also consistent with recent studies that implicated intracellular vesicle trafficking in Paneth cell differentiation. For example, attenuation of Paneth cell development was observed after depletion of either Rab2a or Rab8a in murine intestinal epithelium [27, 28]. These defects were attributed to either impaired trafficking/signaling of the Wnt morphogen or to defective cellular responses to the commensal bacteria in the gut [27, 28]. It remains to be investigated if similar mechanisms could be responsible for the defective Paneth cell development in αSNAP-depleted intestinal mucosa.
In conclusion, the present study represents the first step toward understanding physiologic functions of αSNAP in the gut. It reveals a novel and important role of αSNAP in intestinal Paneth cell differentiation. Our study most likely missed many other important functions of this membrane trafficking protein because of incomplete depletion of αSNAP in the intestinal epithelium of hyh mutant animals. Since total αSNAP knockout in mice is embryonically lethal [17], creation of the intestinal-epithelial specific knockout of this protein is needed to fully elucidate the roles of αSNAP in the regulation of intestinal epithelial homeostasis in vivo.
Supplementary Material
Ileac (A) and colonic (B) sections of homozygous hyh mutant mice and their wild-type controls were stained with hematoxylin and eosin to visualize the general architecture of the intestinal mucosa.
Dual-immunolabeling of the selective enteroendocrine cell marker Chromogranin A (red) and E-cadherin (green) in the ileac (A) and colonic (B) mucosa of wild-type and homozygous hyh mice.
Immunoblotting analysis of different AJ and TJ proteins in the colonic epithelium of control and homozygous hyh mice.
HIGHLIGHTS.
αSNAP protein with a M105I mutation has decreased expression in the murine intestinal epithelium
Depletion of αSNAP depletion protein attenuates differentiation of intestinal enteroids
Depletion of αSNAP protein selectively inhibits Paneth cell differentiation in vivo
Acknowledgments
Services in support of this study were provided by the VCU Massey Cancer Center, supported in part with funding from NIH-NCI core grant P30CA016059. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from the NIH-NINDS Center core grant 5P30NS047463. The authors thank Kevin Hogan for editorial assistance with the manuscript.
Abbreviation
- α-SNAP
soluble N-ethylmaleimide sensitive factor attachment protein alpha
- NSF
N-ethylmaleimide sensitive factor
- SNARE
soluble N-ethylmaleimide sensitive factor associated receptor
- ZO-1
zonula occludens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 2.Guichard A, Nizet V, Bier E. RAB11-mediated trafficking in host-pathogen interactions. Nat Rev Microbiol. 2014;12:624–634. doi: 10.1038/nrmicro3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirtz-Peitz F, Zallen JA. Junctional trafficking and epithelial morphogenesis. Curr Opin Genet Dev. 2009;19:350–356. doi: 10.1016/j.gde.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 6.Jahn R, Scheller RH. SNAREs - engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 7.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. A ubiquitous membrane fusion protein alpha SNAP: a potential therapeutic target for cancer, diabetes and neurological disorders? Expert Opin Ther Targets. 2006;10:723–733. doi: 10.1517/14728222.10.5.723. [DOI] [PubMed] [Google Scholar]
- 8.Bombardier JP, Munson M. Three steps forward, two steps back: mechanistic insights into the assembly and disassembly of the SNARE complex. Curr Opin Chem Biol. 2015;29:66–71. doi: 10.1016/j.cbpa.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteheart SW, Schraw T, Matveeva EA. N-ethylmaleimide sensitive factor (NSF) structure and function. Int Rev Cytol. 2001;207:71–112. doi: 10.1016/s0074-7696(01)07003-6. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima K, Hirose H, Taniguchi M, Kurashina H, Arasaki K, Nagahama M, Tani K, Yamamoto A, Tagaya M. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23:3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao Y, Miner C, Zhang L, Hanson PI, Dani A, Vig M. An essential and NSF independent role for alpha-SNAP in store-operated calcium entry. Elife. 2013;2:e00802. doi: 10.7554/eLife.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Brautigan DL. alpha-SNAP inhibits AMPK signaling to reduce mitochondrial biogenesis and dephosphorylates Thr172 in AMPKalpha in vitro. Nat Commun. 2013;4:1559. doi: 10.1038/ncomms2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naydenov NG, Brown B, Harris G, Dohn MR, Morales VM, Baranwal S, Reynolds AB, Ivanov AI. A membrane fusion protein alphaSNAP is a novel regulator of epithelial apical junctions. PLoS ONE. 2012;7:e34320. doi: 10.1371/journal.pone.0034320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naydenov NG, Feygin A, Wang L, Ivanov AI. N-ethylmaleimide-sensitive factor attachment protein alpha (alphaSNAP) regulates matrix adhesion and integrin processing in human epithelial cells. J Biol Chem. 2014;289:2424–2439. doi: 10.1074/jbc.M113.498691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naydenov NG, Harris G, Brown B, Schaefer KL, Das SK, Fisher PB, Ivanov AI. Loss of soluble N-ethylmaleimide-sensitive factor attachment protein alpha (alphaSNAP) induces epithelial cell apoptosis via down-regulation of Bcl-2 expression and disruption of the Golgi. J Biol Chem. 2012;287:5928–5941. doi: 10.1074/jbc.M111.278358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naydenov NG, Harris G, Morales V, Ivanov AI. Loss of a membrane trafficking protein alphaSNAP induces non-canonical autophagy in human epithelia. Cell Cycle. 2012;11:4613–4625. doi: 10.4161/cc.22885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–270. doi: 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- 18.Hong HK, Chakravarti A, Takahashi JS. The gene for soluble N-ethylmaleimide sensitive factor attachment protein alpha is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci U S A. 2004;101:1748–1753. doi: 10.1073/pnas.0308268100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferland RJ, Batiz LF, Neal J, Lian G, Bundock E, Lu J, Hsiao YC, Diamond R, Mei D, Banham AH, Brown PJ, Vanderburg CR, Joseph J, Hecht JL, Folkerth R, Guerrini R, Walsh CA, Rodriguez EM, Sheen VL. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009;18:497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batiz LF, De Blas GA, Michaut MA, Ramirez AR, Rodriguez F, Ratto MH, Oliver C, Tomes CN, Rodriguez EM, Mayorga LS. Sperm from hyh mice carrying a point mutation in alphaSNAP have a defect in acrosome reaction. PLoS One. 2009;4:e4963. doi: 10.1371/journal.pone.0004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batiz LF, Roales-Bujan R, Rodriguez-Perez LM, Matas IM, Paez P, Roque M, Jimenez AJ, Ramos C, Perez-Figares JM. A simple PCR-based genotyping method for M105I mutation of alpha-SNAP enhances the study of early pathological changes in hyh phenotype. Mol Cell Probes. 2009;23:281–290. doi: 10.1016/j.mcp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 23.Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA, Adelstein RS, Ivanov AI. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep. 2016;6:24161. doi: 10.1038/srep24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E(2)-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1104–1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- 25.In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol. 2016;13:633–642. doi: 10.1038/nrgastro.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010;96:207–229. doi: 10.1016/B978-0-12-381280-3.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das S, Yu S, Sakamori R, Vedula P, Feng Q, Flores J, Hoffman A, Fu J, Stypulkowski E, Rodriguez A, Dobrowolski R, Harada A, Hsu W, Bonder EM, Verzi MP, Gao N. Rab8a vesicles regulate Wnt ligand delivery and Paneth cell maturation at the intestinal stem cell niche. Development. 2015;142:2147–2162. doi: 10.1242/dev.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, Li W, Wei H, Liu Z. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol. 2015;16:918–926. doi: 10.1038/ni.3233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ileac (A) and colonic (B) sections of homozygous hyh mutant mice and their wild-type controls were stained with hematoxylin and eosin to visualize the general architecture of the intestinal mucosa.
Dual-immunolabeling of the selective enteroendocrine cell marker Chromogranin A (red) and E-cadherin (green) in the ileac (A) and colonic (B) mucosa of wild-type and homozygous hyh mice.
Immunoblotting analysis of different AJ and TJ proteins in the colonic epithelium of control and homozygous hyh mice.