Abstract
Objective
Heavy alcohol consumption can alter vitamin D status; however, the relationships between alcohol consumption and vitamin D concentrations in pregnant women have not been well studied. The aim of this study was to investigate the vitamin D status in a population of alcohol-exposed (N = 180) and low/unexposed control (N = 179) Ukrainian pregnant women.
Methods
Women who attended prenatal care facilities in 2 regions of Ukraine (Rivne and Khmelnytsky) for a routine prenatal visit were screened for the study. At the time of enrollment (20.4 ± 7.0 weeks of gestation), blood samples and alcohol consumption data (during a typical week around conception and the most recent 2 weeks) were collected. Vitamin D status was assessed by 25-hydroxyvitamin D [25(OH)D] concentrations.
Results
A high prevalence of suboptimal vitamin D status in pregnant Ukrainian women was observed. Overall, 50.1% and 33.4% of the women were classified as vitamin D deficient [25(OH)D < 20 ng/mL] or insufficient [25(OH)D ≥ 20 ng/mL and ≤30 ng/mL], respectively, based on 2011 Endocrine Society guidelines. Alcohol-exposed women had significantly lower 25(OH)D concentrations than low/unexposed women in Spring (p = 0.006) and Winter (p = 0.022). When vitamin D concentrations were grouped into sunny season (Summer + Fall) compared to not sunny season (Winter + Spring), there was a significant ethanol by season interaction (p = 0.0028), with alcohol-drinking women having lower circulating vitamin D compared to low/unexposed women in seasons of low sun availability.
Conclusions
These data suggest that when vitamin D concentrations are generally low (e.g., during seasons of low sun availability), alcohol consumption during pregnancy has a negative impact on vitamin D status.
Keywords: vitamin D, vitamin D deficiency, 25(OH)D, alcohol, pregnancy
INTRODUCTION
Vitamin D insufficiency is considered a significant global health issue [1,2]. Risk factors for vitamin D deficiency include low dietary intakes of vitamin D, obesity, dark skin, and low sunlight exposure due to multiple factors, including covered dressing styles, use of sunscreen, living at a high latitude, and reduced time outdoors [3,4]. Maternal vitamin D deficiency has been reported in many countries, with prevalence rates ranging from 5% to 89% depending on the population studied and the circulating 25(OH)D cutoff values used by investigators for defining vitamin D insufficiency [3,5–7]. Low vitamin D status during pregnancy has been associated with an increased maternal risk for preeclampsia, bacterial vaginosis, gestational diabetes, spontaneous abortion, and preterm birth [3,8–10]. Vitamin D can influence placental development and function, and the placenta can contribute to active vitamin D production via endogenous 1-α-hydroxylase (CYP27B1) activity [11].
Fetal vitamin D status is directly linked to the maternal status; thus, maternal vitamin D deficiency in pregnancy can lead to the infant being born vitamin D deficient [12,13]. Maternal vitamin D deficiency has been associated in some studies with an increased risk for a wide variety of neonatal and longer-term complications in the offspring, including low birth weight, diabetes, schizophrenia, asthma, and skeletal abnormalities [3,8,14].
In general, poor maternal nutritional status results in an in utero fetal environment that is suboptimal for development. Alcohol consumption is associated with poor nutritional status due in part to the effects alcohol can have on the ingestion, absorption, metabolism, and excretion of numerous nutrients [15–17]. The dietary intake of mothers of children with fetal alcohol spectrum disorder (FASD) compared to mothers of normal controls has been reported to be inadequate for a number of nutrients [18]. It is thought that suboptimal maternal nutritional status plays a role in the expression of certain alcohol-related disorders, including FASD [19]. Rodent studies have shown that offspring of dams fed iron-deficient diets throughout gestation and who were administered alcohol in the early postnatal period have poor neurocognitive outcomes compared to offspring from iron-sufficient dams [20]; maternal choline supplementation during the prenatal period can reduce the severity of FASD effects in the rat offspring [21]. A recent randomized, double-blind, placebo-controlled pilot study of choline supplementation or placebo in children with FASD suggests that choline may have beneficial effects on elicited imitation in younger children [22].
With regard to vitamin D, suboptimal vitamin D status and utilization secondary to the exposure to high amounts of alcohol has been noted in animal models [23–25] as well as in humans [26–28]. Low vitamin D concentrations (<10 ng/mL) were associated with increased mortality in patients with alcoholic liver disease [29]. In animal models, rat pups treated with alcohol via intragastric intubations during the equivalent of the human third-trimester brain growth spurt (postnatal days 4–9 in the rodent) committed significantly more errors in the spatial discrimination reversal learning task compared to controls; supplementation with vitamin D3 (1–5 mg/kg/day cholecalciferol) from postnatal day 2 to 30 improved their performance in a dose-related manner [30].
Though a few studies have investigated the effect of alcohol during pregnancy in animal models, there is a paucity of human studies assessing the relationship between alcohol consumption and maternal vitamin D status. The hypothesis that maternal nutritional status can modulate the risk for FASD in women who consume alcohol is a major focus of attention in our group. Given that alcohol has been shown to affect vitamin D metabolism in humans and animal models, in the current study we investigated the association of alcohol consumption and 25(OH)D levels in pregnant women who reported consuming little to no alcohol or moderate to heavy amounts of alcohol during pregnancy.
MATERIALS AND METHODS
Study Design and Setting
An ongoing cohort study was conducted among a sample of pregnant women in Ukraine as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). The CIFASD is supported by the U.S. National Institute on Alcohol Abuse and Alcoholism, and it is a multidisciplinary initiative conducted in several countries throughout the world (www.CIFASD.org). The primary goals of the CIFASD are to better characterize the spectrum of physical and neurodevelopmental outcomes resulting from prenatal alcohol exposure and to develop better diagnostic, prevention, and treatment approaches for FASD, including the investigation of maternal nutrition as a possible permissive or protective factor. The methods and scope of CIFASD studies have been described elsewhere [31,32].
In order to identify women eligible for recruitment in Ukraine, 2 centralized prenatal care facilities in 2 western regions (Rivne and Khmelnytsky) that are members of the Omni-Net Birth Defects Prevention Program were selected as sources of the sample. Each regional care facility serves both rural and urban residents with variable socioeconomic status and general health. The study was approved by the institutional review boards of the University of California San Diego, La Jolla, California; the University of California Davis, Davis, California; and the Lviv Medical University, Lviv, Ukraine. All study participants provided written informed consent.
Selection of the Sample
All women who came in for a routine prenatal visit to one of the 2 centers were eligible for screening for the study. Women were screened with a standard, brief set of questions on alcohol consumption [33], other exposures, demographics, and pregnancy history [34,35]. The screening process was incorporated into routine practice at both sites and conducted in person by a specially trained study nurse, and all women who were capable of responding to the screening questions (e.g., who were considered to have the capacity to consent and to answer questions) were included.
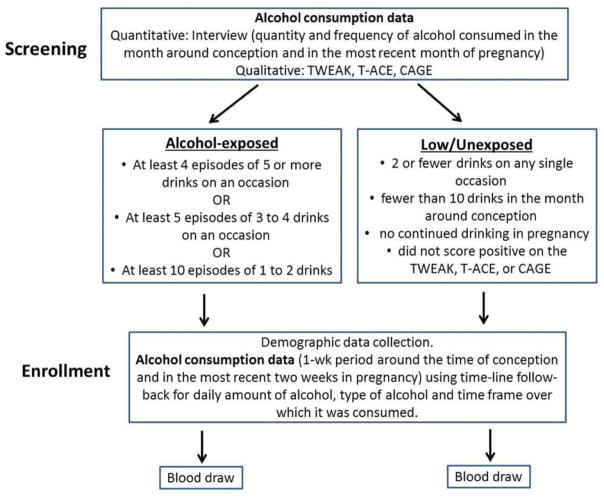
Women were asked to report on the quantity and frequency of alcohol consumed in the month around conception and in the most recent month of pregnancy (Fig. 1). To account for those who may have denied alcohol use in pregnancy, women were also asked to answer questions comprising a standard screening tool for harmful alcohol consumption, such as the TWEAK: T = tolerance (How many drinks can you hold?), W = worried (Have friends, family complained about your drinking?), E = eye-opener (Do you sometimes take a drink in the morning when you get up?), A = amnesia (Has a friend or family member ever told you about things you did while drinking that you can’t remember?), K = cut down (Do you sometimes feel the need to cut down on your drinking?), the T-ACE (T = tolerance, A = annoyed, C = cut down, E = eye-opener), or the CAGE (C = cut down, A = annoyed, G = guilty, E = eye-opener) encompassing the past year [36].
Fig. 1.
Study design.
The criteria for recruitment into the alcohol-exposed group was the report of at least 4 episodes of 5 or more drinks on an occasion, at least 5 episodes of 3 to 4 drinks on an occasion, or at least 10 episodes of one to 2 drinks in the month around conception or the most recent month. Women who scored positive (2 or more points) on the TWEAK or other standard screening tools were also considered eligible; however, these women also had to meet the criteria based on reported quantity and frequency of alcohol consumed. Those who reported 2 or fewer drinks on any single occasion and fewer than 10 drinks in the month around conception, reported no continued drinking in pregnancy, and did not score positive on the TWEAK, T-ACE, or CAGE were recruited and enrolled into the unexposed/low exposure comparison group in a 1:1 ratio.
Maternal Interviews and Measures of Alcohol Consumption
After enrollment, all women participated in a 1-hour in-person interview during which time data were collected on demographics (maternal and paternal age, site of interview, socioeconomic status), previous pregnancy history (gravidity, parity, previous spontaneous abortions or stillbirths, and previous elective terminations), use of vitamin/mineral supplements, prepregnancy body mass index (BMI), tobacco use, current weeks of gestation of the pregnancy, and alcohol use. Current weeks’ gestation was calculated based on maternal report of first day of last menstrual period and was adjusted by ultrasound measurements if discrepant by the standard length of variation; for example, by more than 7 days for a first-trimester ultrasound.
A timeline follow-back procedure [37] was used to enhance accuracy of recall regarding alcohol use. For each type of alcoholic beverage consumed each day in a 1-week period around the time of conception and in the most recent 2 weeks in pregnancy, the number of drinks and volume were recorded. These data were converted to ounces of absolute alcohol based on type of alcohol consumed and estimated alcohol content by volume. The data were then summarized in 4 variables representing the pattern of consumption at each time point in pregnancy: average number of ounces of absolute alcohol per day and average number of ounces of absolute alcohol per drinking day at conception and in the period just prior to the interview. The summary variables were calculated by summing the total ounces of absolute alcohol consumed over the time period and dividing by the time period of interest; that is, for ounces of absolute alcohol per day around conception, the sum was divided by 7 days, and for ounces of absolute alcohol per drinking day, the sum was divided by the number of days the mother reported any drinking during that 7-day period. The same calculations were performed for the most recent 2 weeks, using 14 days as the divisor for ounces of absolute alcohol per day, and number of days in the 14-day period that any alcohol was consumed as the divisor for ounces of absolute alcohol per drinking day. These alcohol measures (ounces of absolute alcohol per day at time of conception [AADO], ounces of absolute alcohol per drinking day at time of conception [AADDO], ounces of absolute alcohol per day at time of enrollment [AADXP], and ounces of absolute alcohol per drinking day at time of enrollment [AADDXP]) were used in determining the relationship between alcohol consumption and maternal vitamin D status in the current article.
Blood Sampling and Analysis
Following completion of the enrollment interview, a 25-mL sample of blood was drawn into EDTA or heparin-treated tubes, centrifuged at 1500 g for 10 minutes at 4°C, and plasma aliquoted into tubes and frozen at −80°C until analyzed for a number of nutritional measures. Concentrations of 25(OH)D in EDTA-treated plasma samples were analyzed by radioimmunoassay following the manufacturer’s instructions (DiaSorin Inc, Stillwater, MN) [38]. The coefficient of variation for the vitamin D assay for the low, high, and internal control samples (duplicates) was 8.56%, 8.99%, and 14.73%, respectively. Classification of vitamin D status was based on the 2011 Endocrine Society guidelines [39]:
Vitamin D adequate: >75 nmol/L (>30 ng/mL)
Vitamin D insufficient: 52.5–72.5 nmol/L (21–29 ng/mL)
Vitamin D deficient: <50 nmol/L (<20 ng/mL)
as well as according to the 2011 U.S. Institute of Medicine (IOM) guidelines [40]:
Vitamin D adequate: >50 nmol/L (>20 ng/mL)
Vitamin D insufficient: 30–50 nmol/L (12–20 ng/mL)
Vitamin D deficient: <30 nmol/L (<12 ng/mL)
Vitamin D can be converted as follows: 1 ng/mL = 2.5 nmol/L.
Statistical Analysis
Characteristics of the participants by alcohol exposure group were described using frequencies and percentages; comparisons between groups were performed by chi-square tests for independence for categorical variables and 2-sample t tests for continuous variables. Unadjusted comparisons between alcohol exposure groups of the mean values for vitamin D were performed using nonparametric 2-sample Wilcoxon tests. To evaluate the associations between dose of alcohol and vitamin D, linear regression models were used. Separate models were constructed for vitamin D regressed on each of the 4 measures of alcohol dose: Ounces of absolute alcohol per day and per drinking day around conception and ounces of absolute alcohol per day and per drinking day in the most recent 2 weeks. To identify covariates as confounders, we selected those that changed the estimated coefficient of alcohol consumption by 10% or more in a linear regression model containing the 2 explanatory variables alone. We fitted the final model with those covariates that met the criteria for confounders as well as other variables that were independently associated with vitamin D status. Variables considered included maternal age, smoking status (never, past smoker but quit before pregnancy, quit after realizing pregnant, and current smoker), number of cigarettes smoked, weeks of gestation at blood sampling, pre-pregnancy BMI, consumption of vitamin/mineral supplements (yes, no), socioeconomic status, study site, season at blood draw (Spring = March, April, May; Summer = June, July, August; Fall = September, October, November; Winter = December, January, February), and Season (sunny; e.g., Summer + Fall and not sunny; e.g., Winter + Spring) × Alcohol dose interaction. To further address nonnormal distribution of the vitamin D measure, ordinal logistic regression models were developed categorizing the outcome measure in 3 groups based on their vitamin D status using either Endocrine Society or IOM guidelines. Missing values for covariates resulted in exclusion of subjects on a case-by-case basis in each analysis. A 2-sided p-value ≤ 0.05 was considered to be statistically significant. All analyses were conducted using SAS Enterprise Guide Version 4.2 (SAS, Cary, NC) and R version 3.2.1 (https://www.r-project.org/, Vienna, Austria).
RESULTS
A total of 359 subjects were available for the analysis (180 alcohol-exposed and 179 low/unexposed). All women in the alcohol-exposed group met the criteria for enrollment based on reported quantity and frequency of alcohol consumed; among those who were eligible for enrollment based on the TWEAK, CAGE, or T-ACE score, all women also met the criteria based on reported quantity and frequency of alcohol consumed. Women in the alcohol-exposed group were significantly younger, had lower socioeconomic status, had fewer years of education, were more likely to be past or current smokers, had less vitamin/mineral usage, and were enrolled later in gestation than women in the low/unexposed group (Table 1). Consistent with the group selection criteria, indicators of risky alcohol consumption (as reported over the previous 12 months using the TWEAK; Table 1) and alcohol consumption data (Table 2) were markedly higher in the alcohol-exposed subjects than in the low/unexposed women.
Table 1.
Maternal Characteristics by Alcohol Groupa
| Characteristic | Low/Unexposedb N = 179 % (n) |
Alcohol-Exposedb N = 180 % (n) |
P-Valuec |
|---|---|---|---|
| Maternal age (years)(range 15–41) | |||
| <21 | 14 (24) | 22 (39) | 0.015 |
| 21–34 | 84 (147) | 71 (128) | |
| >34 | 3 (5) | 7 (13) | |
| Gravidity | |||
| 1 | 45 (80) | 54 (97) | 0.112 |
| >1 | 55 (96) | 46 (83) | |
| Parity | |||
| 0 | 61 (107) | 64 (115) | 0.547 |
| >0 | 39 (69) | 36 (65) | |
| Socioeconomic statusd | |||
| 1 | 11 (19) | 6 (10) | <0.001 |
| 2 | 38 (67) | 21 (38) | |
| 3 | 36 (64) | 41 (74) | |
| 4 | 14 (24) | 23 (42) | |
| 5 | 1 (2) | 8 (15) | |
| Highest maternal education level | |||
| 9 Years or less | 2 (4) | 9 (16) | <0.001 |
| High school diploma/trade school | 36 (64) | 57 (102) | |
| College degree/unfinished university | 16 (28) | 12 (22) | |
| University graduate | 45 (80) | 22 (40) | |
| Smoking status | |||
| Never smoked | 87 (151) | 33 (59) | <0.001 |
| Past smoker (quit before pregnancy) | 8 (14) | 12 (21) | |
| Past smoker (quit after realized that pregnant) | 3 (6) | 32 (57) | |
| Current smoker | 2 (3) | 24 (43) | |
| Multivitamin/mineral prenatal supplement use at enrollment | |||
| No | 20 (36) | 33 (59) | 0.009 |
| Yes | 80 (140) | 67 (121) | |
| Prepregnancy body mass index | |||
| Underweight (<18) | 11 (19) | 13 (23) | 0.697 |
| Normal (18–24.99) | 78 (136) | 73 (130) | |
| Overweight (25–29.99) | 9 (15) | 10 (18) | |
| Obese (30+) | 3 (5) | 4 (8) | |
| Weeks of gestation at enrollment (range 2.6–39 weeks; mean (SD) = 18.9 (6.4)) | |||
| <11 | 5 (8) | 6 (10) | 0.046 |
| 11–24 | 82 (145) | 72 (129) | |
| >24 | 13 (23) | 23 (41) | |
| Season at blood draw | |||
| Sunny (Summer and Fall) | 50 (90) | 52 (94) | 0.713 |
| Not sunny (Winter and Spring) | 50 (89) | 48 (86) | |
| Study site | |||
| Rivne | 68 (121) | 70 (126) | 0.623 |
| Khmelnytsky | 32 (58) | 30 (54) | |
| TWEAK | |||
| 0 | 98 (164) | 21 (37) | <0.001 |
| 1 | 1 (2) | 3 (5) | |
| 2 or more | 1 (2) | 76 (135) | |
Numbers after percentages are frequencies.
Missing values: 3 in the low/unexposed group and 1 in the exposed group for age, gravidity, parity, education, vitamin use, weeks of gestation; 3 in the low/unexposed group and 2 in the exposed group for socioeconomic status, absolute alcohol per day at time of enrollment, absolute alcohol per drinking day at time of enrollment; 4 in the low/unexposed group and 1 in the exposed group for absolute alcohol per day at time of conception; 4 in the low/unexposed group and 2 in the exposed group for body mass index, TWEAK; 5 in the low/unexposed group and 1 in the exposed group for smoking, absolute alcohol per drinking day at time of conception.
Pearson test.
Socioeconomic status; based on Hollingshead score calculated from maternal and paternal education and occupation; 1 is highest and 5 is lowest.
Table 2.
Maternal Alcohol Consumption by Alcohol Group
| Low/Unexposeda N = 179 |
Alcohol- Exposeda N = 180 |
P-Valueb | |
|---|---|---|---|
| Ounces absolute alcohol per day at time of conception (ounces/day) | |||
| Mean ± SE | 0.002 ± 0.016 | 0.574 ± 0.422 | <0.001 |
| Minimum | 0 | 0 | |
| Maximum | 0.145 | 2.94 | |
| Ounces absolute alcohol per drinking day at time of conception (ounces/drinking day) | |||
| Mean ± SE | 0.014 ± 0.112 | 1.607 ± 1.146 | <0.001 |
| Minimum | 0 | 0 | |
| Maximum | 1.014 | 6.767 | |
| Ounces absolute alcohol per day at time of enrollment (ounces/day) | |||
| Mean ± SE | 0.00003 ± 0.00044 | 0.138 ± 0.792 | <0.001 |
| Minimum | 0 | 0 | |
| Maximum | 0.006 | 10.328 | |
| Ounces absolute alcohol per drinking day at time of enrollment (ounces/drinking day) | |||
| Mean ± SE | 0.0005 ± 0.0061 | 0.475 ± 1.07 | <0.001 |
| Minimum | 0 | 0 | |
| Maximum | 0.081 | 12.05 | |
Missing values: 3 in the low/unexposed group and 1 in the exposed group for age, gravidity, parity, education, vitamin use, weeks of gestation; 3 in the low/unexposed group and 2 in the exposed group for socioeconomic status, absolute alcohol per day at time of enrollment, absolute alcohol per drinking day at time of enrollment; 4 in the low/unexposed group and 1 in the exposed group for absolute alcohol per day at time of conception; 4 in the low/unexposed group and 2 in the exposed group for body mass inex, TWEAK; 5 in the low/unexposed group and 1 in the exposed group for smoking, absolute alcohol per drinking day at time of conception.
t Test.
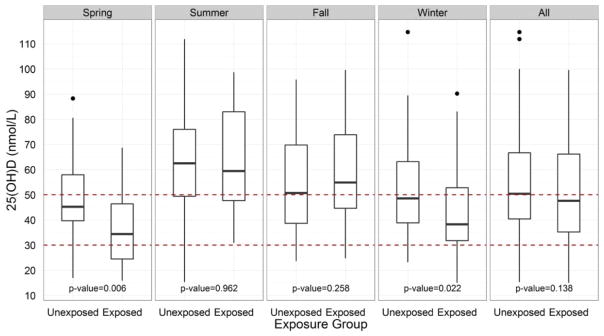
At the group level, there was no statistically significant difference in the mean circulating concentrations of 25(OH)D in alcohol-exposed (20.6 ± 0.63 ng/mL) compared to low/unexposed (21.8 ± 0.59 ng/mL) subjects (p = 0.138) in unadjusted analysis using a nonparametric 2-sample Wilcoxon test. Similar results were obtained in ordered logistic regression models when subjects were categorized based on their vitamin D status using either the 2011 Endocrine Society or IOM guidelines (data not shown). However, when subjects were stratified by seasons based on month of blood draw—that is, Spring (March, April, May), Summer (June, July, August), Fall (September, October, November), and Winter (December, January, February), comparisons by alcohol group showed that alcohol-exposed women had significantly lower 25(OH)D concentrations compared to low/unexposed women in Spring (p = 0.006) and Winter (p = 0.022; Fig. 2). When the seasons were split into 2 categories—sunny season (Summer + Fall) and not sunny season (Winter + Spring)—and the data were analyzed by 2-way analysis of variance, we observed a significant interaction for Alcohol group × Season (p = 0.0028); vitamin D concentrations were similar between low/unexposed and exposed women during the sunny season but were markedly lower in alcohol-exposed women compared to controls in the not sunny season.
Fig. 2.
Plasma vitamin D concentrations of pregnant Ukrainian women by season and by alcohol exposure group. Concentrations of plasma vitamin D during Spring (March, April, May), Summer (June, July, August), Fall (September, October, November), Winter (December, January, February) and all seasons combined in alcohol-exposed and low/unexposed pregnant Ukrainian women. Black lines within each bar represents the median vitamin D concentration, with the 25th and 75th percentiles represented by the bottom and top of the box, respectively. Data above 1.5 interquartile range are represented by dots. Dotted lines represent vitamin D deficiency cutoff values using the 2011 Endocrine Society guidelines (25(OH)D concentrations <50 nmol/L) [39] and 2011 IOM guidelines (25(OH)D concentrations <30 nmol/L) [40]. Comparisons were performed using Wilcoxon rank sum tests. (Color figure available online.)
In multivariate analyses of alcohol dose in relation to vitamin D, there was no significant association between alcohol consumption in a 1-week period around the time of conception or between alcohol consumption in the most recent 2 weeks in pregnancy, expressed as average ounces of absolute alcohol per day or as average ounces of absolute alcohol per drinking day and plasma vitamin D concentrations, after adjustment for weeks of gestation at blood draw, maternal age, socioeconomic status, prepregnancy BMI, site, vitamin use, smoking, and season (Table 3).
Table 3.
Relationship of Covariates to Vitamin D Levels (nmol/L) by Maternal Alcohol Dosea
| Model 1 Average Ounces of Absolute Alcohol per Day around the Time of Conception (AAD0) | Model 2 Average Ounces of Absolute Alcohol per Drinking Day around the Time of Conception (AADD0) | Model 3 Average Ounces of Absolute Alcohol per Day in the Most Recent 2 Weeks in Pregnancy (AADXP) | Model 4 Average Ounces of Absolute Alcohol per Drinking Day in the Most Recent 2 Weeks in Pregnancy (AADDXP) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Estimated Coefficient (SE) | p-Value | Estimated Coefficient (SE) | p-Value | Estimated Coefficient (SE) | p-Value | Estimated Coefficient (SE) | p-Value | |
| Alcohol consumption | −0.55 (3.80) | 0.887 | 0.76 (1.46) | 0.605 | −2.67 (1.91) | 0.164 | −2.27 (1.58) | 0.152 |
|
| ||||||||
| Weeks of gestation at blood draw (weeks) | 0.36 (0.17) | 0.028 | 0.35 (0.17) | 0.036 | 0.46 (0.17) | 0.008 | 0.42 (0.17) | 0.013 |
|
| ||||||||
| Maternal age (years): | ||||||||
| 21–34 | Reference | Reference | Reference | Reference | ||||
| <21 | −4.53 (2.83) | 0.111 | −4.67 (2.84) | 0.102 | −4.92 (2.81) | 0.082 | −4.30 (2.81) | 0.127 |
| >35 | 0.68 (4.74) | 0.886 | 0.96 (4.80) | 0.843 | 0.44 (4.71) | 0.926 | 1.09 (4.73) | 0.818 |
|
| ||||||||
| Body mass index: | ||||||||
| Normal | Reference | Reference | Reference | Reference | ||||
| Underweight | −2.27 (3.25) | 0.487 | −1.99 (3.27) | 0.543 | −2.39 (3.24) | 0.462 | −2.42 (3.24) | 0.457 |
| Overweight | −1.37 (3.64) | 0.707 | −1.43 (3.66) | 0.697 | −0.78 (3.66) | 0.833 | −0.63 (3.68) | 0.864 |
| Obese | −0.75 (5.55) | 0.892 | −0.80 (5.58) | 0.886 | −1.59 (5.51) | 0.774 | −1.47 (5.51) | 0.791 |
|
| ||||||||
| Socioeconomic status: | ||||||||
| 1 | −5.58 (6.25) | 0.373 | −4.97 (6.27) | 0.429 | −7.78 (6.35) | 0.222 | −6.29 (6.25) | 0.315 |
| 2 | 2.14 (5.49) | 0.697 | 2.54 (5.52) | 0.647 | 0.43(5.57) | 0.940 | 1.86 (5.47) | 0.734 |
| 3 | −1.55 (5.36) | 0.773 | −1.59 (5.40) | 0.769 | −3.17 (5.43) | 0.561 | −1.73 (5.35) | 0.748 |
| 4 | 2.05 (5.55) | 0.712 | 1.65 (5.58) | 0.768 | −0.51 (5.64) | 0.928 | 1.16 (5.54) | 0.835 |
| 5 | Reference | Reference | Reference | Reference | ||||
|
| ||||||||
| Site | ||||||||
| Khmelnytsky | −0.85 (2.28) | 0.709 | −1.12 (2.30) | 0.626 | 0.71 (2.34) | 0.762 | 1.02 (2.38) | 0.668 |
| Rivne | Reference | Reference | Reference | Reference | ||||
|
| ||||||||
| Vitamin use: | ||||||||
| Yes vs. no | 5.29 (2.47) | 0.033 | 5.74 (2.46) | 0.021 | 5.36 (2.45) | 0.029 | 5.33 (2.45) | 0.031 |
|
| ||||||||
| Smoking: | ||||||||
| Never smoked/quit before pregnancy | Reference | Reference | Reference | Reference | ||||
| Past smoker, quit after realized that pregnant | −3.31 (2.91) | 0.258 | −3.31 (2.91) | 0.173 | −4.79 (2.82) | 0.091 | −1.88 (3.44) | 0.587 |
| Current smoker | −2.14 (3.59) | 0.552 | −3.10 (3.55) | 0.384 | −2.34 (3.42) | 0.495 | −1.88 (3.44) | 0.587 |
|
| ||||||||
| Season: | ||||||||
| Sunny (Summer + Fall) | Reference | Reference | −Reference | Reference | ||||
| Not sunny (Winter + Spring) | −13.50 (2.57) | <0.0001 | −13.68 (2.64) | <0.0001 | −15.00 (2.14) | <0.0001 | −14.78 (2.21) | <0.0001 |
| Interaction term: Alcohol consumption × Season | −6.53 (5.12) | 0.203 | −2.06 (1.88) | 0.273 | −16.01 (8.72) | 0.068 | −3.50 (3.08) | 0.257 |
| Constant | 52.20 (6.33) | <0.0001 | 51.53 (6.41) | <0.0001 | 52.26 (6.31) | <0.0001 | 51.33 (6.30) | <0.0001 |
|
| ||||||||
| Observations | 350 | 349 | 350 | 350 | ||||
| R2 | 0.198 | 0.193 | 0.205 | 0.204 | ||||
| Adjusted R2 | 0.157 | 0.152 | 0.164 | 0.163 | ||||
| Residual SE | 19.124 (df = 332) | 19.205 (df = 331) | 19.036 (df = 332) | 19.043 (df = 332) | ||||
| F statistic | 4.818 (df = 17; 332) | <0.01 | 4.655 (df = 17; 331) | <0.01 | 5.023 (df = 17; 332) | <0.01 | 5.005 (df = 17; 332) | <0.01 |
| Comparison of nested modelsb | χ2 = 3.82 | 0.148 | χ2 = 1.46 | 0.482 | χ2 = 6.71 | 0.035 | χ2 = 6.46 | 0.040 |
Estimated by linear regression: each covariate in the model was adjusted for all other covariates in the model; 9 subjects excluded due to missing values in models 1, 3, and 4; 10 subjects excluded due to missing values in model 2.
Likelihood ratio test from a nested model approach comparison.
We were interested in testing whether alcohol consumption, season, and the interaction of alcohol by season were associated with vitamin D levels. As expected, the main effect of season was highly associated with vitamin D levels. Neither the main effect of alcohol consumption nor the interaction term between alcohol and season were significantly associated with vitamin D levels. In order to simultaneously test the effect of these 2 terms together, we used the likelihood ratio test from a nested model comparison of the full model (Table 3) with the reduced model (dropping the alcohol consumption main effect and the alcohol by season interaction term). We observed that alcohol dose together with the interaction between dose and season was significant. Adding these 2 predictors to the reduced model (which gives the full model) provided a statistically significant improvement to model fit when dose reflected recent alcohol consumption (in the most recent 2 weeks of pregnancy) expressed as average ounces of absolute alcohol per day or per drinking day (Table 3; p = 0.035 for model 3 and p = 0.040 for model 4).
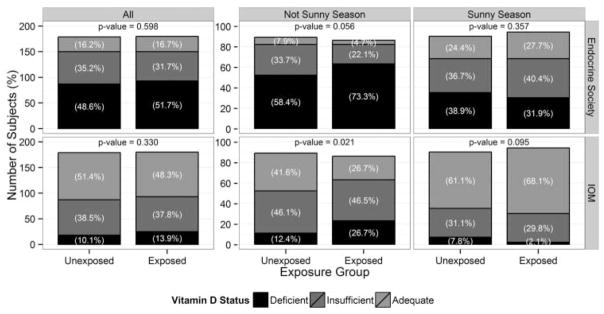
As expected, the percentage of alcohol-exposed and low/unexposed subjects classified as vitamin D deficient using either the IOM or Endocrine Society criteria was larger in the not sunny season (Winter + Spring) compared to the sunny season (Summer + Fall; Fig. 3). Using the 2011 Endocrine Society criteria [39], the differences between alcohol groups in vitamin D status category (deficiency/insufficiency/adequacy) were not statistically significant whether expressed combining all seasons or by sunny or not sunny seasons (Fig. 3, top panels) although the differences in vitamin D status category between exposed and low/unexposed women were marginally significant in the not sunny season (p = 0.056). However, using the 2011 IOM guidelines [40], the percentage of alcohol-exposed women in the not sunny season (Winter + Spring) who were classified as vitamin D deficient compared to low/unexposed women was significantly higher (p = 0.021), whereas there were no significant differences when all seasons were combined or in the sunny season alone (Summer + Fall; Fig. 3, bottom panels).
Fig. 3.
Vitamin D status of pregnant Ukrainian women by alcohol exposure group and by season. Number and percentages of pregnant Ukrainian subjects during the not sunny season (Winter + Spring; includes December through May) and during the sunny season (Summer + Fall; includes June through November) and all seasons combined whose plasma 25(OH)D concentrations are classified as vitamin D deficient, insufficient, and adequate based on the 2011 Endocrine Society [39] and the 2011 Institute of Medicine (IOM) [40] guidelines. Comparisons were performed using Fisher’s exact tests comparing vitamin D deficient to not deficient.
The predicted probability that a woman would be characterized by a particular vitamin D status category was determined based on the ordered logit model for each combination of exposure group and season (Table 4). The predicted probability that a woman would fall into the deficient, insufficient, and adequate vitamin D status categories as defined by the IOM (top section of Table 4) were fairly equivalent between unexposed (5%, 26%, and 69%, respectively) and low/unexposed women (7%, 33%, and 61%, respectively) in the sunny season. In contrast, the predicted probability that a woman who consumed alcohol during pregnancy in the not sunny season would fall into the deficient vitamin D category was nearly double compared to low/unexposed women (25% versus 13%, respectively). The Endocrine Society guidelines have a higher cutoff of vitamin D concentrations for categorization into the vitamin D–deficient category. Though the predicted probability for vitamin D deficiency was twice as high in the not sunny compared to the sunny season using Endocrine Society guidelines, there were no differences between the alcohol-exposed and low/unexposed women in either of the 2 season categories (bottom section of Table 4).
Table 4.
Predicted Probabilities for Vitamin D Status Categories from Ordered Logit Model
| Exposure Group | Seasona | Vitamin D Deficient | Vitamin D Insufficient | Vitamin D Adequate | |
|---|---|---|---|---|---|
| IOM guidelines | Low/unexposed | Sunny | 0.07 | 0.33 | 0.61 |
| Alcohol-exposed | Sunny | 0.05 | 0.26 | 0.69 | |
| Low/unexposed | Not sunny | 0.13 | 0.45 | 0.42 | |
| Alcohol-exposed | Not sunny | 0.25 | 0.50 | 0.25 | |
| Endocrine Societyguidelines | Low/unexposed | Sunny | 0.37 | 0.41 | 0.22 |
| Alcohol-exposed | Sunny | 0.31 | 0.42 | 0.27 | |
| Low/unexposed | Not sunny | 0.60 | 0.30 | 0.10 | |
| Alcohol-exposed | Not sunny | 0.74 | 0.21 | 0.06 |
Sunny season (June–November), not sunny season (December–May).
When combining the subjects over all seasons, using Endocrine Society guidelines, the percentages of alcohol-exposed and low/unexposed population that would be classified as vitamin D deficient (25(OH)D with concentrations < 50 nmol/L) or insufficient (25(OH)D with concentrations 52.5–72.5 nmol/L) were 50.1% and 33.4%, respectively. Using the IOM guidelines [40] and combining the subjects over all seasons, the percentages of alcohol-exposed and low/unexposed population that would be classified as vitamin D deficient (25(OH)D concentrations <30 nmol/L) or insufficient (25(OH)D concentrations 30–50 nmol/L) were 12.0% and 38.2%, respectively.
DISCUSSION
Alcohol and Vitamin D
Using a likelihood ratio test and comparison of nested models, our data showed that in the not sunny seasons (Winter + Spring), recent alcohol consumption (average absolute ounces of alcohol per day or per drinking day) in the 2 weeks prior to enrollment was associated with lower vitamin D concentrations compared to low/unexposed women. Similar to our study, vitamin D concentrations were reported to be lower in patients with alcohol use disorders whose last alcohol intake was within the last 30 days compared to those who had abstained 30+ days from drinking alcohol [41]. In our study, during the Winter and Spring, when overall vitamin D concentrations were low (compared to vitamin D concentrations during the Summer and Fall), alcohol-exposed pregnant women had significantly lower vitamin D concentrations than low/unexposed pregnant women. Similarly, the distribution of the percentage of alcohol-exposed women in IOM-defined vitamin D status categories (deficient, insufficient, adequate) was significantly different than low/unexposed women in the not sunny season (p = 0.021).
There is a paucity of studies concerning the effects of alcohol on vitamin D during pregnancy. In rats, maternal alcohol consumption has been associated with low fetal hepatic 25 (OH)D concentrations [42]. In nonpregnant rats, chronic alcohol consumption can lead to depletion of vitamin D stores [43]. Multiple nonpregnant human studies have shown that chronic alcohol consumption can be associated with a high frequency of low circulating concentrations of 25(OH)D [26,28,44]. It was reported that 25(OH)D, 1,25(OH)2D, and 24,25-dihydroxyvitamin D3 were lower in noncirrhotic male alcoholics by 40%, 23%, and 48%, respectively, when compared to nonalcoholic controls [26]. Similarly, alcoholic patients demonstrated a high prevalence of suboptimal vitamin D status [27,45]. 25(OH)D has been reported to be low in patients with alcoholic liver disease [44], although Belgian women who drank alcohol during pregnancy were reported to have no increase in risk for severe vitamin D deficiency (defined as 25 (OH)D concentration < 10 ng/mL) [46].
Studies have suggested that a low vitamin D concentration in alcoholics can be due to multiple factors, including malabsorption, poor diets, limited sunlight exposure, and perhaps a direct effect of alcohol on vitamin metabolism. For example, metabolically, it has been reported that alcohol can impair hepatic protein synthesis, leading to low levels of vitamin D binding protein [47], which may result in lower circulating levels of vitamin D. Alcohol may also increase the turnover of the active form of vitamin D (1,25 dihydroxyvitamin D3; 1,25(OH)2D3) by induction of 1,25 dihydroxyvitamin D3-24-hydroxylase (CYP24A1) [25]. To our knowledge, the current article is among the first to investigate the 25(OH)D levels in moderate to heavy alcohol-exposed human mothers during pregnancy.
Maternal Vitamin D Status
The frequency of suboptimal vitamin D status depends on the criteria one uses to determine vitamin D status. Of the pregnant Ukrainian women in our study, 41.7% of the low/unexposed women and 41.8% of the alcohol-exposed women had suboptimal 25(OH)D status according to the 2011 Endocrine Society guidelines, but using the 2011 IOM guidelines, suboptimal vitamin D status characterized 24.2% and 25.9% of low/unexposed and alcohol-exposed women, respectively. The insufficient vitamin D levels observed in these pregnant subjects adds to the data of populations at risk for vitamin D deficiency worldwide [1]. A recent study in Ukraine that analyzed serum from 1575 men and women (20–95 years old) showed a greater than 80% prevalence of vitamin D deficiency (defined by the authors as 25(OH)D concentrations below 20 ng/mL) [48].
Our data showed a significant interaction between alcohol and season in that women who drank alcohol had a higher percentage of vitamin D deficiency compared to controls during the low sunlight seasons (Winter + Spring) but not during more abundant sunlight seasons (Summer + Fall). When predicted probability tables were constructed, women who consumed alcohol during the not sunny season were twice as likely to be in the vitamin D–deficient category compared to low/unexposed women. These data indicate that alcohol consuming women are at a higher risk of vitamin D deficiency in seasons when vitamin D levels are relatively low, but this effect may be blunted in sunny seasons where more vitamin D may be synthesized. Given the high prevalence of vitamin D deficiency, we suggest that new public health approaches to improving the vitamin D status of this population are warranted.
Maternal vitamin D status is correlated with vitamin D in the neonate and breast milk; thus, low maternal vitamin D status during pregnancy and lactation can predispose the infant to vitamin D deficiency, particularly if the infant is exclusively breastfed [3,49]. Suboptimal vitamin D status during pregnancy can be associated with several potential negative health consequences for both the mother and child [3,50,51]. Mothers with 25(OH)D ≥ 50 nmol/L had a 40% reduction in risk for severe preeclampsia compared to mothers with 25(OH)D levels < 50 nmol/L [52]. Inverse associations between 25(OH) D and bacterial vaginosis have been reported [53]. In utero and early postnatal vitamin D deficiency has been associated with an increased risk of skeletal abnormalities, impaired immune function, and increased risk for certain diseases [3,54].
Factors Contributing to Low Maternal Vitamin D Status
Unlike many nutrients whose concentration in the blood decreases as pregnancy progresses, in part due to hemodilution, serum 25(OH)D concentrations are largely unaffected or are reported to be increased by pregnancy duration [40,55,56]. Our multivariate regression analyses showed that week of gestation was associated with a small but significant increase in vitamin D concentrations (0.4 nmol/L; Table 3); thus, other factors may have contributed to the high incidence of suboptimal 25(OH)D status that was observed in our subjects. The population studied may have had less sun exposure than needed to attain adequate circulating levels of 25(OH)D. Rivne and Khmelnytsky oblasts are at 50.6° N and 49.4° N latitude, respectively. Other factors such as the use of sunscreen and protective clothing can affect UV exposure and reduce subsequent vitamin D production. Though few foods naturally contain vitamin D [57], vitamin D–fortified milk can be a good source of the nutrient. In Ukraine, it is estimated that 81% of milk production is manufactured in private households and small farms [58]. Thus, cultural differences may have contributed to this population’s low 25(OH)D status because many individuals may not drink milk from commercial stores where the milk may have gone through processing and vitamin D fortification. Seasonal changes in plasma vitamin D levels are well documented. A significant finding in our study is that 25(OH)D was negatively associated with recent alcohol consumption when vitamin D concentrations were generally low (e.g., in Winter and Spring).
Low exposure to sunlight can result in a low endogenous production of vitamin D. In such situations, vitamin D supplementation may be particularly important. In our study, 79.6% of low/unexposed and 67.6% of alcohol-exposed subjects reported consumption of multivitamin/mineral supplements. Though multivitamin supplement use was associated with an approximately 5 nmol/L increase in 25(OH)D concentrations (Table 3), over 80% of the subjects still had suboptimal vitamin D status by Endocrine Society guidelines. The IOM defines sufficient vitamin D status as >20 ng/mL and recommends 600 IU/day for pregnant and lactating mothers [59], whereas the Endocrine Society recommends at least 600 IU/day for pregnant and lactating women and suggests that for those at risk for vitamin D deficiency, 1500–2000 IU/day may be needed to maintain 25(OH)D above 30 ng/mL [39]. Although the specific brand of supplement was occasionally not reported, the majority of multivitamin products available over the counter in Ukraine contain 200–400 IU of vitamin D3.
Limitations
This study had several strengths and limitations. We did not collect measures of sun exposure, habitual time outdoors, degree of skin pigmentation, or dietary vitamin D intake, exclusive of nutrient supplements, and the sample was not population based but rather selected to represent moderate to heavy drinkers and low consumers or abstainers. The study did have detailed information on quantity and frequency of alcohol and a wide range of other covariates to be considered, including the important seasonal factor.
CONCLUSION
In conclusion, our findings suggest a general need for vitamin D supplementation for pregnant Ukrainian women. Although the exact mechanism(s) by which alcohol affects vitamin D levels in pregnancy is unknown, data obtained in the current study support the concept that individuals consuming alcohol have an increased risk of vitamin D deficiency in low sun availability seasons, which could negatively impact pregnancy outcomes. We suggest that vitamin D supplementation is likely to be particularly important for women characterized by high alcohol intakes.
Acknowledgments
The authors sincerely thank all of the participants for their dedication.
FUNDING
This work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA) and the Office of Dietary Supplements. Additional information about CIFASD can be found at www.cifasd.org. Specific funding for this study was provided by NIH Research Grant U01AA014835 funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the NIH Office of Dietary Supplements (ODS).
References
- 1.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 3.Uriu-Adams JY, Obican SG, Keen CL. Vitamin D and maternal and child health: overview and implications for dietary requirements. Birth Defects Res C Embryo Today. 2013;99:24–44. doi: 10.1002/bdrc.21031. [DOI] [PubMed] [Google Scholar]
- 4.Sowell KD, Keen CL, Uriu-Adams JY. Vitamin D and reproduction: from gametes to childhood. Healthcare. 2015;3:1097–1120. doi: 10.3390/healthcare3041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darling AL, Hart KH, Macdonald HM, Horton K, Kang’ombe AR, Berry JL, Lanham-New SA. Vitamin D deficiency in UK South Asian Women of childbearing age: a comparative longitudinal investigation with UK Caucasian women. Osteoporos Int. 2013;24:477–488. doi: 10.1007/s00198-012-1973-2. [DOI] [PubMed] [Google Scholar]
- 6.Dawodu A, Davidson B, Woo JG, Peng YM, Ruiz-Palacios GM, de Lourdes Guerrero M, Morrow AL. Sun exposure and vitamin D supplementation in relation to vitamin D status of breastfeeding mothers and infants in the Global Exploration of Human Milk Study. Nutrients. 2015;7:1081–1093. doi: 10.3390/nu7021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flood-Nichols SK, Tinnemore D, Huang RR, Napolitano PG, Ippolito DL. Vitamin D deficiency in early pregnancy. PloS ONE. 2015;10:e0123763. doi: 10.1371/journal.pone.0123763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010;68:465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 9.Olmos-Ortiz A, Avila E, Durand-Carbajal M, Diaz L. Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients. 2015;7:443–480. doi: 10.3390/nu7010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodnar LM, Platt RW, Simhan HN. Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes. Obstet Gynecol. 2015;125:439–447. doi: 10.1097/AOG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental–decidual function. J Soc Gynecol Investig. 2004;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Kimball S, Fuleihan Gel H, Vieth R. Vitamin D: a growing perspective. Crit Rev Clin Lab Sci. 2008;45:339–414. doi: 10.1080/10408360802165295. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW, Pittard WB., III Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 14.Hart PH, Lucas RM, Walsh JP, Zosky GR, Whitehouse AJ, Zhu K, Allen KL, Kusel MM, Anderson D, Mountain JA. Vitamin D in fetal development: findings from a birth cohort study. Pediatrics. 2015;135:e167–e173. doi: 10.1542/peds.2014-1860. [DOI] [PubMed] [Google Scholar]
- 15.Halsted CH, Medici V. Vitamin-dependent methionine metabolism and alcoholic liver disease. Adv Nutr. 2011;2:421–427. doi: 10.3945/an.111.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815–820. doi: 10.1111/j.1530-0277.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devgun MS, Fiabane A, Paterson CR, Zarembski P, Guthrie A. Vitamin and mineral nutrition in chronic alcoholics including patients with Korsakoff’s psychosis. Br J Nutr. 1981;45:469–473. doi: 10.1079/bjn19810125. [DOI] [PubMed] [Google Scholar]
- 18.May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais AS, Brooke LE, Blankenship J, Hoyme HE, Gossage JP. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod Toxicol. 2014;46:31–39. doi: 10.1016/j.reprotox.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors. 2010;36:125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huebner SM, Tran TD, Rufer ES, Crump PM, Smith SM. Maternal iron deficiency worsens the associative learning deficits and hippocampal and cerebellar losses in a rat model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:2097–2107. doi: 10.1111/acer.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2015;102:1113–1125. doi: 10.3945/ajcn.114.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, Chen JR, Mason AZ, Badger TM, Ronis MJ. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J Pharmacol Exp Ther. 2012;343:401–412. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res. 1988;12:159–162. doi: 10.1111/j.1530-0277.1988.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 25.Shankar K, Liu X, Singhal R, Chen JR, Nagarajan S, Badger TM, Ronis MJ. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3-24-hydroxylase (CYP24A1) Endocrinology. 2008;149:1748–1756. doi: 10.1210/en.2007-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laitinen K, Valimaki M, Lamberg-Allardt C, Kivisaari L, Lalla M, Karkkainen M, Ylikahri R. Deranged vitamin D metabolism but normal bone mineral density in Finnish noncirrhotic male alcoholics. Alcohol Clin Exp Res. 1990;14:551–556. doi: 10.1111/j.1530-0277.1990.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 27.Wijnia JW, Wielders JP, Lips P, van de Wiel A, Mulder CL, Nieuwenhuis KG. Is vitamin D deficiency a confounder in alcoholic skeletal muscle myopathy? Alcohol Clin Exp Res. 2013;37(Suppl 1):E209–E215. doi: 10.1111/j.1530-0277.2012.01902.x. [DOI] [PubMed] [Google Scholar]
- 28.Bjorneboe GA, Johnsen J, Bjorneboe A, Morland J, Drevon CA. Effect of heavy alcohol consumption on serum concentrations of fat-soluble vitamins and selenium. Alcohol Alcohol. 1987;1(Suppl):533–537. [PubMed] [Google Scholar]
- 29.Trepo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, Gustot T, Degre D, Vercruysse V, Deltenre P, Verset L, Gulbis B, Franchimont D, Deviere J, Lemmers A, Moreno C. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344–350. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Idrus NM, Happer JP, Thomas JD. Cholecalciferol attenuates perseverative behavior associated with developmental alcohol exposure in rats in a dose-dependent manner. J Steroid Biochem Mol Biol. 2013;136:146–149. doi: 10.1016/j.jsbmb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arenson AD, Bakhireva LN, Chambers CD, Deximo CA, Foroud T, Jacobson JL, Jacobson SW, Jones KL, Mattson SN, May PA, Moore ES, Ogle K, Riley EP, Robinson LK, Rogers J, Streissguth AP, Tavares MC, Urbanski J, Yezerets Y, Surya R, Stewart CA, Barnett WK. Implementation of a shared data repository and common data dictionary for fetal alcohol spectrum disorders research. Alcohol. 2010;44:643–647. doi: 10.1016/j.alcohol.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Ramo I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr HM, Streissguth AP. Identifying maternal self-reported alcohol use associated with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2001;25:283–287. [PubMed] [Google Scholar]
- 34.Chambers CD, Kavteladze L, Joutchenko L, Bakhireva LN, Jones KL. Alcohol consumption patterns among pregnant women in the Moscow region of the Russian Federation. Alcohol. 2006;38:133–137. doi: 10.1016/j.alcohol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, Chan PH, Xu R, Wertelecki W. Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcohol Clin Exp Res. 2014;38:1012–1019. doi: 10.1111/acer.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell M, Martier SS, Sokol RJ, Mudar P, Jacobson S, Jacobson J. Detecting risk drinking during pregnancy: a comparison of four screening questionnaires. Am J Public Health. 1996;86:1435–1439. doi: 10.2105/ajph.86.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol RJ, Maritier S, Ernhart C. Identification of Alcohol Abuse in the Prenatal Clinic. Rockville, MD: Alcohol, Drug Abuse, and Mental Health Administration Research; 1983. [Google Scholar]
- 38.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 39.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 40.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. pp. 75–522. [PubMed] [Google Scholar]
- 41.Neupane SP, Lien L, Hilberg T, Bramness JG. Vitamin D deficiency in alcohol-use disorders and its relationship to comorbid major depression: a cross-sectional study of inpatients in Nepal. Drug Alcohol Depend. 2013;133:480–485. doi: 10.1016/j.drugalcdep.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Milne ML, Baran DT. End product inhibition of hepatic 25-hydroxyvitamin D production in the rat: specificity and kinetics. Arch Biochem Biophys. 1985;242:488–492. doi: 10.1016/0003-9861(85)90234-6. [DOI] [PubMed] [Google Scholar]
- 43.Gascon-Barre M. Interrelationships between vitamin D3 and 25-hydroxyvitamin D3 during chronic ethanol administration in the rat. Metabolism. 1982;31:67–72. doi: 10.1016/0026-0495(82)90028-2. [DOI] [PubMed] [Google Scholar]
- 44.Hepner GW, Roginsky M, Moo HF. Abnormal vitamin D metabolism in patients with cirrhosis. Am J Dig Dis. 1976;21:527–532. doi: 10.1007/BF01464758. [DOI] [PubMed] [Google Scholar]
- 45.Bang UC, Semb S, Nordgaard-Lassen I, Jensen JE. A descriptive cross-sectional study of the prevalence of 25-hydroxyvitamin D deficiency and association with bone markers in a hospitalized population. Nutr Res. 2009;29:671–675. doi: 10.1016/j.nutres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Vandevijvere S, Amsalkhir S, Van Oyen H, Moreno-Reyes R. High prevalence of vitamin D deficiency in pregnant women: a national cross-sectional survey. PloS ONE. 2012;7:e43868. doi: 10.1371/journal.pone.0043868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Investig. 1986;78:748–752. doi: 10.1172/JCI112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pororoznyuk VV, Muts VY, Klymovytsky FV, Synenky OV. Vitamin D deficiency in Ukraine: a demographic and seasonal analysis. Gerontologija. 2012;13:191–198. [Google Scholar]
- 49.Bergstrom I, Blanck A, Savendahl L. Vitamin D levels in children born to vitamin D–deficient mothers. Horm Res Paediatr. 2013;80:6–10. doi: 10.1159/000351809. [DOI] [PubMed] [Google Scholar]
- 50.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4:208–230. doi: 10.3390/nu4030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawodu A, Akinbi H. Vitamin D nutrition in pregnancy: current opinion. Int J Womens Health. 2013;5:333–343. doi: 10.2147/IJWH.S34032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, Klebanoff MA. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014;25:207–214. doi: 10.1097/EDE.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23:45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Weinert LS, Silveiro SP. Maternal–fetal impact of vitamin D deficiency: a critical review. Matern Child Health J. 2015;19:94–101. doi: 10.1007/s10995-014-1499-7. [DOI] [PubMed] [Google Scholar]
- 55.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvi-tamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 56.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 57.Blumfield ML, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. 2013;71:118–132. doi: 10.1111/nure.12003. [DOI] [PubMed] [Google Scholar]
- 58.Pro Agro. Ukraine: Dairy cattle-breeding and milk industry; 2007. Available at: http://www.proagro.com.ua/eng/news/market/45928.html. [Google Scholar]
- 59.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]