Abstract
Tendons are dense connective tissues that attach muscles to bone with an indispensable role in locomotion because of their intrinsic properties of storing and releasing muscle- generated elastic energy. Tenomodulin (Tnmd) is a well-accepted gene marker for the mature tendon/ligament lineage and its loss-of -function in mice leads to a phenotype with distinct signs of premature aging on tissue and stem/progenitor cell levels. Based on these findings, we hypothesized that Tnmd might be an important factor in the functional performance of tendons. Firstly, we revealed that Tnmd is a mechanosensitive gene and that the C-terminus of the protein co-localize with collagen I-type fibers in the extracellular matrix. Secondly, using an endurance training protocol, we compared Tnmd knockout mice with wild types and showed that Tnmd deficiency leads to significantly inferior running performance that further worsens with training. In these mice, endurance running was hindered due to abnormal response of collagen I cross-linking and proteoglycan genes leading to an inadequate collagen I fiber thickness and elasticity. In sum, our study demonstrates that Tnmd is required for proper tendon tissue adaptation to endurance running and aids in better understanding of the structural-functional relationships of tendon tissues.
Keywords: Tendons, Tenomodulin, Knockout mouse model, Running tests, Atomic force microscopy, Collagen I crosslinking
Highlights
-
•
Tnmd is a mechanosensitive gene and its protein is co-localized with collagen I fibers in the ECM of tendons.
-
•
Tnmd knockout mice fail in endurance running tests, a phenotype that worsens with training.
-
•
Tnmd knockout tendons had significantly thicker and stiffer collagen I fibers and altered crosslinking gene expression.
We performed a multidisciplinary approach to decipher the role of tenomodulin, a gene marker for the mature tendon lineage, in tendon functional performance. Loss-of-function in mice led to significantly inferior endurance running and detailed analyses revealed that tenomodulin is involved in the regulation of collagen I fiber structural and biomechanical properties in response to exercise. Our study expands the current view on the complex structural-functional relationships of tendon tissues, and tenomodulin expression levels may indicate whether an individual is suitable for a certain sport.
1. Introduction
Tendon biology is under-investigated, yet tendon pathologies are a big burden for affected patients. The poor regenerative ability of tendon tissues (Benjamin and Ralphs, 1997) and unsuccessful surgical attempts to repair tendon tears (Pennisi, 2002) underline the need to develop superior therapies. Identifying key molecular markers would aid in defining the response to therapy (Morse and Gillies, 2010).
Tenomodulin (TNMD/Tnmd) is an established gene marker for the mature tendon/ligament lineage in vertebrates (Docheva et al., 2005). So far, limited studies have focused on deciphering its functions in tendon tissues and residing cells (Dex et al., 2016). The gene is abundantly expressed in tendons and ligaments (Brandau et al., 2001, Shukunami et al., 2001, Docheva et al., 2005). Its protein has a highly conserved cleavable C-terminal cysteine-rich domain (Brandau et al., 2001, Shukunami et al., 2001) that has been identified as an important regulator for tendon stem/progenitor cell (TSPC) proliferation and senescence as well as for tendon maturation (Alberton et al., 2015, and reviewed by Dex et al., 2016). A variety of tissues express Tnmd mRNA, however Tnmd cleavage and secretion is exclusive to tissues undergoing tension, suggestive of a possible mechano-regulation. The cleaved form is detected in the extracellular matrix (ECM) of mouse Achilles tendons (Docheva et al., 2005) and human chordae tendineae cordis (Kimura et al., 2008), but not in the eye (Oshima et al., 2003). Loss of Tnmd transcripts in cultivated human periodontal ligament cells, tenocytes (Itaya et al., 2009, Mazzocca et al., 2011) and in rat tendon fibroblasts has been reported (Jelinsky et al., 2010), suggesting its downregulation is a consequence of the lack of mechanical stimuli in vitro.
Through a multidisciplinary approach, we aimed to reveal further features and functions of Tnmd in tendon tissue. First, we tested whether Tnmd gene promotor activity and transcription are responsive to in vitro mechanical stretching, which was followed by subjecting wildtype (WT) and Tnmd knockout (KO) mice to forced endurance and voluntary running protocols. We performed an ultrastructural analysis using atomic force microscopy (AFM) technology and investigated collagen I fiber topography, diameter distribution, average diameter, and average stiffness from sedentary and trained Achilles tendons. Lastly, we studied the localization of TNMD protein in human tendon and TSPC specimens and examined the effect of Tnmd loss-of-function on the expression of collagen I cross-linking genes.
In sum, we report that TNMD/Tnmd is a mechanosensitive gene vital for optimal running performance, proper tendon adaptation to running and maintenance of structurally and functionally integral collagen fibrils.
2. Materials and Methods
2.1. Cell Culture
Human Achilles tendon TSPC (3 young healthy donors) were previously isolated (under grant No.: 166-08 of the Ethical Commission of the Medical Faculty of the LMU), and validated to possess multipotential differentiation capacity, self-renewability and clonogenicity, and to express stem cell-, and tendon related gene markers (Kohler et al., 2013, Popov et al., 2015). TSPC were cultured as described in (Kohler et al., 2013, Popov et al., 2015). Cells in passages 1–3 were used for experiments.
2.2. Mouse Strain
The Tnmd KO mouse line was established by Docheva et al. (2005). Mouse husbandry, handling and euthanasia were strictly carried out according to the guidelines of the Bavarian authorities. Animals were euthanized with CO2 and dissected for collection of whole foot and tendon tissues.
2.3. Semiquantitative and Quantitative PCR
Total RNA from human TSPC was isolated with Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany) and used for standard semiquantitative PCR (protocol in Supp. Info.). Total RNA/cDNA from WT and Tnmd KO tendons (pool of three animals) was prepared from littermates subjected or not to the forced endurance running protocol. Quantitative PCR of collagen I regulatory and cross-linking genes (asporin, biglycan, decorin, fibromodulin, fibronectin, lysyl hydroxylase, lysyl oxidase and lumican) was performed using RealTime Ready Custom Panel 96 – 32 + plates (https://configurator.realtimeready.roche.com) according to the manufacturer's instructions (Roche, Penzberg, Germany). Briefly, PCR reactions were pipetted on ice and each well contained 10 μl LightCycler 480 probes master mix, 0,2 μl cDNA (diluted 1:5) and 9,8 μl PCR grade water. Plates were subsequently sealed and centrifuged down for 15 s. at 2100 rpm. Crossing points for each sample were determined by the second derivative maximum method and relative quantification was performed using the comparative ΔΔCt method according to the manufacturer's protocol. Four PCR independent repeats were carried out (n = 4).
2.4. Western Blot Analysis
Biopsies from cadaveric human tissue complex gastrocnemius muscle-Achilles tendon-calcaneus (from donors donated to the Department of Anatomy, LMU in Munich) as well as mouse tendons from WT and Tnmd KO animals were lysed with 8 M urea, 50 mM Tris-HCl [pH 8.0], 1 mM dithiothreitol, 1 mM EDTA). Protein (25 μg) aliquots were loaded on a 15% SDS- polyacrylamide gel and transferred to Hybond-P membrane (Amersham, GE Healthcare, Waukesha, USA). The membranes were pre-incubated for 4 h at 4 °C in blocking buffer and probed with rabbit polyclonal anti-C-terminal Tnmd antibody, raised against a synthetic polypeptide conforming to amino acids 245 to 252 in mouse and human Tnmd (Docheva et al., 2005). A mouse anti-beta-actin antibody cross-reacting with mouse and human beta-actin (Abcam, Cambridge, United Kingdom) was used as a loading control. Following overnight incubation at 4 °C, membranes were probed with corresponding HRP-conjugated secondary antibodies (Amersham). Protein bands were visualized using an enhanced chemiluminescence system (ECL Plus, Amersham) and film paper. Western blotting was replicated independently three times (n = 3).
2.5. Immunofluorescence
Human biopsies from Achilles tendon (grant No.: 166-08) as well as mouse whole foot were embedded in paraffin and cut in 6 μm thick longitudinal sections. Prior to staining, slides were deparaffinated with xylol and rehydrated via ethanol (100–50% EtOH). For antigen retrieval, sections were treated with 0,2% hyaluronidase for 30 min. Following blocking with 1% BSA/PBS for 2 h sections were incubated overnight at 4 °C with primary antibodies against asporin, fibromodulin, lysyl oxidase (All Abcam), lumican (Santa Cruz, Dallas, USA) and Tnmd (Docheva et al., 2005). Corresponding Alexa Fluor 488-labeled secondary antibodies and nuclear dye 4′, 6-diamidino-2-phenylindole (DAPI) (both Life technology, Carlsbad, USA) were applied at room temperature for 1 h and 5 min, respectively. Photomicrographs were taken with an Axiocam MRm camera on Observer Z1 microscope (Carl Zeiss, Oberkochen, Germany). Confocal photomicrographs of Tnmd staining in human and mouse Achilles tendon were taken using a confocal Leica TSC SP2 microscope (Leica, Wetzlar, Germany). Immunofluorescence experiments were repeated thrice independently.
2.6. Mechanical Stimulation
TSPC underwent axial cyclic strain in a six-station stimulation apparatus driven by an eccentric motor as described previously (Popov et al., 2015). For this, 1 × 105 TSPC were cultured for 4 days on FBS-coated flexible silicone dishes (60 mm × 30 mm). Triplicates were then stretched 60 min/day in the long axis at a frequency of 1 Hz and a magnitude of 5% for 3 consecutive days. In parallel, non-stimulated cells were used as controls on day 3. Directly after stimulation, cells were lysed for mRNA isolation as described above. Mechanical stimulation was performed with 2 different TSPC donors (n = 2) (each in triplicates) and repeated twice independently.
2.7. Luciferase Assay
The assay was performed as previously described by us (Alberton et al., 2012). In brief, human TSPC (3 × 104 cells/cm2) were transfected with 2,5 μg control promoter-less and Tnmd promoter-luciferase plasmids using OptiMEM media and Lipofectamine 2000 kit (Invitrogen). After 6 h, transfection media was replaced with complete growth media, and TSPC were cultured for 2 days. Luciferase activity was then measured with the Luciferase Assay E4030 kit (Promega, Madison, USA) according to the manufacturer's instructions. Cells were lysed in 1 × lysis buffer, 20 μl of the cell lysates were mixed with 100 μl luciferase reagent and immediately measured on Safire II Luminometer (TECAN, Männedorf, Germany). Two transfected TSPC donors were used for two independent mechanical stimulations (n = 4).
2.8. Forced Endurance Training
Forced endurance exercise training with adult Tnmd KO and WT male mice (6 animals per genotype, n = 6) was carried out. Training and testing was performed according to the guidelines of the animal authority of North Rhine Westphalia. Mice were allowed to accommodate 1 week to the new environment and to the treadmill. Tests started once the animals were able to run without any external stimulus. The treadmill (Exer3/6; Columbus Instruments, Columbus, USA) endurance training protocol consisted of a 60 min treadmill exercise, 5 days a week, at a constant speed of 10 m/min and angle of − 10°, which was selected in order to increase the tendon loading during the training. The speed for the training session was sustained by the mice for the daily hourly training session. The training lasted 4 consecutive weeks. A pre- and post-training exhaustive test took place, one day before the first day of training and one day after the last day of training, respectively. In these exhaustive tests, mice were placed to run from 12 m/min with an increasing velocity (ΔV) of 2 m/min every 3 min, until volitional exhaustion. Running time and velocity were recorded for each mouse until they were not able to run anymore and fell off the treadmill. Littermates of 6 animals per genotype, not subjected to training, were used as a sedentary control group. Mice were randomly allocated to either trained or sedentary conditions. For the complete experimental period, mice were fed ad libitum. Lastly, animals were authanized and legs excised and processed for further analysis.
2.9. Pulmonary Capacity Measurements
Each animal from both genotypes was analyzed, prior and post training, for maximal lung volume in an Oxymax Economy System chamber (Columbus Instruments, Columbus, USA). Experiments were performed in the Department of Prof. W. Bloch.
2.10. Atomic Force Microscopy (AFM)
AFM was performed on Achilles tendon sections from sedentary and trained Tnmd KO and WT mice. Measurements were taken with NanoWizard AFM instrument (JPK Instruments, Berlin, Germany) mounted on an inverted optical microscope (Axiovert 200, Zeiss) according to (Kamper et al., 2016, Prein et al., 2016) with slight modifications. To identify and ensure comparable regions between the groups, every 10th slide was analyzed by H&E staining and consecutive slides were taken for AFM. The optical microscope with a 40 × magnification was used to guarantee the correct positioning of the cantilever tip onto the tendon collagen fibers. The AFM had a maximum horizontal scanning range of 100 × 100 μm2 and a vertical range of 15 μm. For AFM imaging and indentation measurements, silicon nitride cantilevers (MLCT, Microcantilever, Bruker, Mannheim) with a nominal spring constant of 0.01 N/m and integrated pyramidal tips with a nominal radius < 20 nm were used. Prior to each measurement, the force constants of cantilevers were determined individually using the thermal noise method described in Butt and Jaschke (1995). The collected data were evaluated with the JPK Data processing software 4.0.23 (JPK instruments). For AFM imaging and collagen diameter analysis the following algorithm was used: 1) polynomial fit for each line of the scan, to correct for a flat beveled underlying surface; 2) low pass filter (Gaussian); 3) high pass filter, to enhance the geometrical outline; and 4) edge detection, to determine the diameter of the individual fibrils.
Elasticity recording and calculation of Young's Modulus was based on AFM-collected force indentation curves. Prior to each fit the following operations were applied: 1) conversion of the detector deflection signal into a force; 2) baseline subtraction: the last 10% of the force-distance curves were used to calculate the baseline and was subtracted from the force signal; 3) contact point determination: the point in which the force curve crosses zero is set as the x-axis zero; 4) calculation of tip sample separation: the height signal is corrected for the deflection of the cantilever.
AFM imaging and force mapping was performed with two tendons per group. Intact fibrils were randomly chosen from three different sections/animal. A total of 60 fibrils per animal were measured for diameter size and Gaussian distribution analysis (75–100 data points are sufficient), whilst 110 force curves were analyzed for Young's Modulus analysis.
2.11. Immunogold Labelling (Post Embedding Technique) and Electron Microscopy
Tendon tissue biopsies and TSPC were fixed in 3% paraformaldehyde for 1 h, washed overnight in PBS/BSA, dehydrated in ascending series with ethanol and embedded in LR-white (London Resin, Plano, Marburg, Germany). Ultrathin sections were prepared on nickel grids, pre-treated with hyaluronidase (1 mg/ml) for 10 min, washed and incubated with 1% BSA in 0,01 M PBS, pH 7,9 and 0,5% Tween for 10 min. Subsequently, sections were incubated with primary antibodies (Tnmd (Docheva et al., 2005), collagen I and decorin, 1:50 in BSA/PBS) overnight at 4 °C and washed with 1% BSA in PBS for 15 min. Secondary antibodies conjugated with 10 nm gold particles (diluted 1:30 in PBS/BSA) were applied for 1 h. After rinsing for 2 × 5 min and fixation with 1% glutaraldehyde, sections were contrasted with tannic acid 1%/20 min, osmium 1%/10 min and uranyl acetate 2%/30 min. Finally, sections were rinsed and examined under transmission electron microscope (Zeiss EM10, Germany).
2.12. Statistical Analysis
Quantitative data and statistical significance was analyzed with GraphPad Prism5 software (GraphPad). Unless otherwise specified, all quantitative data were acquired from at least three independent experiments. Bar charts show mean values and standard deviations. Statistical difference was tested with one-way ANOVA (multigroup) or with unpaired Student's t-test (two groups). In multiple comparisons, one-way ANOVA was followed by Bonferroni post-hoc correction. p-Value of 0.05 was considered statistically significant and indicated in figures as *p < 0.05, **p < 0.005 and ***p < 0.0005.
3. Results
3.1. Tnmd is a Mechanosensitive Gene
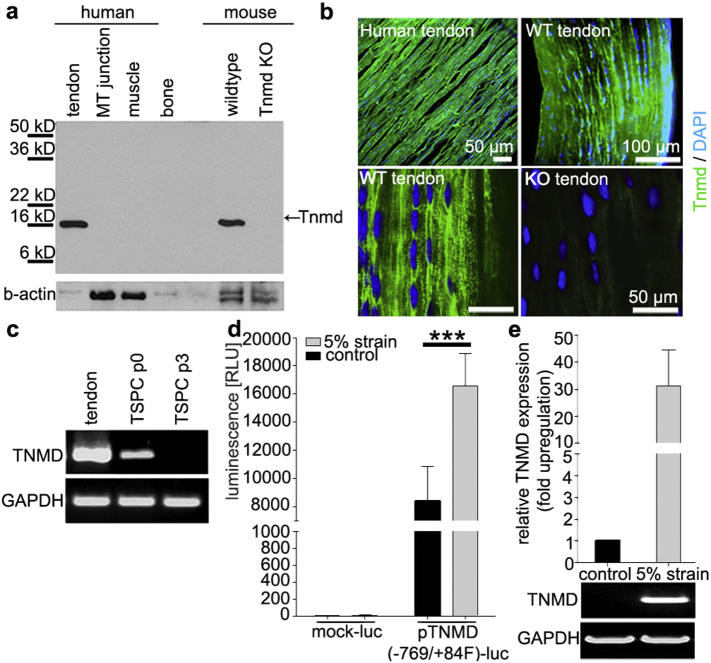
Tnmd is a gene abundantly expressed in tendons and ligaments (Brandau et al., 2001, Shukunami et al., 2001, Docheva et al., 2005). The protein has a highly conserved cleavable C-terminal cysteine-rich domain (Brandau et al., 2001, Shukunami et al., 2001) identified as an important regulator for TSPC proliferation and senescence as well as for tendon maturation (Alberton et al., 2015 and reviewed in Dex et al., 2016). Western blot analysis of human musculoskeletal elements identified the expression of Tnmd C-terminal domain predominantly in tendons at approximately 16 kD (Fig. 1a), as shown previously by us and others (Docheva et al., 2005, Kimura et al., 2008). Immunofluorescent stainings localized the Tnmd protein in the ECM of human and WT mouse Achilles tendon sections (Fig. 1b) as well as in human fibrocartilage, but not in hyaline cartilage (Supplementary Fig. 1). Tnmd transcripts were abundant in human tendon total mRNA extracts, but in vitro culturing of previously characterized human TSPC (Kohler et al., 2013, Popov et al., 2015) led to a rapid loss of Tnmd mRNA (Fig. 1c). Interestingly, upon 5% axial cyclic strain, the Tnmd promoter was strongly activated and expression significantly upregulated as validated by luciferase assay and semi-quantitative PCR (Fig. 1d and e).
Fig. 1.
Tnmd expression in human musculoskeletal elements and mechano-regulation. (a) Representative western blot with anti-C-terminal Tnmd antibody on human (tendon, MTJ myotendinous junction, muscle and bone) and mouse (tendon) tissues. Detection of beta actin served as loading control. (b) Representative immunofluorescent detection of Tnmd in human and mouse Achilles tendons. Tnmd expression in green and nuclear DAPI in blue colour. Upper panel: low magnification epi-fluorescence images; lower panel: confocal microscopy. (c) Semi-quantitative PCR for Tnmd expression in cultured human TSPC (passage 0 and 3). The house-keeping gene GAPDH was used for normalization. Western blotting, staining and PCR were reproduced three times (n = 3). (d) Luciferase activity assays (expressed in RLU, relative light units) with human TSPC transfected with a promoter-less (mock-luc) or Tnmd promoter (− 769/+84F Tnmd genomic fragment)-driven luciferase (pTnmd-luc) plasmids without or undergoing mechanical stimulation of 5% axial strain. Two independent stimulations with two different donors were carried out (n = 4). (e) Semi-quantitative PCR bands and densitometric evaluation of Tnmd expression against GAPDH in human TSPC (passage 3) prior and after mechanical stimulation (presented in fold change). Statistical significance: ***p < 0.0005.
In conclusion, the C-terminal domain of Tnmd protein is deposited in the ECM of human and mouse Achilles tendons. Tnmd expression in human TSPC rapidly diminishes in two- dimensional static culture, but is restored upon axial stretching, indicating the mechanosensitivity of the TNMD gene.
3.2. Tnmd is Required for Optimal Running Performance
Mice naturally cover large distances of over 5 km daily (Koteja et al., 1999). To compare their running ability, WT and Tnmd KO mice were subjected to a forced endurance running protocol depicted in Fig. 2a. Even in the initial exhaustive tests, mutant mice ran for significantly less time. With increasing speed of 12–18 m/min more mutant mice dropped out earlier and none run at 18 m/min (Fig. 2b left and c left). The training regime of 10 m/min was based on 70% of the average maximum velocity of both genotypes (Fig. 2b left). Moreover, in the post-exhaustive tests, mutant mice ran for significantly less time and were unable to run over 12 m/min with 70% (4 out of 6 Tnmd KO mice) failing at that speed (Fig. 2b left and c). This suggested that Tnmd KO tendons may experience fatigue and if that is the case, training further worsens their performance. In contrast, the beneficial effects of training were clear in the control animals where more mice were able to sustain speeds between 12 and 14 m/min. The observed drop at 18 m/min may be related to muscle fiber composition shift during endurance running. Supplementary Fig. 2 depicts the stacked areas of the data point in Fig. 2c and stack area calculation in percentage to the WT sedentary animals. Maximum speed reached was not different between genotypes nor affected by training (Fig. 2b right).
Fig. 2.
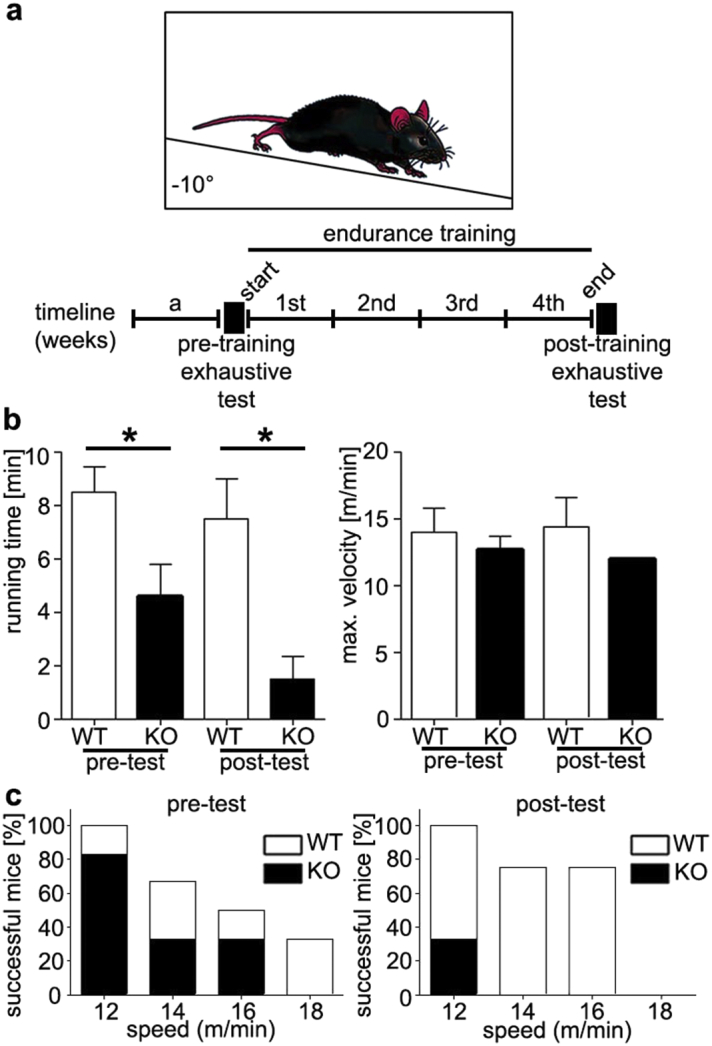
Tnmd KO and WT performance in endurance running tests. (a) Experimental setup of the forced endurance running experiments. Mice ran in a treadmill at an angle of − 10°. Beneath is the timeline: a, 1 week of acclimatization, start exhaustive test, followed by 5 days training for 4 consecutive weeks and end exhaustive test. (b) Average running time (left graph) and average of the maximum velocity (right graph) achieved by each group in the exhaustive tests. (c) Bar graphs present the success rate of both groups at each speed of 12–18 m/min in the start (left) and end (right) exhaustive tests. Six animals per genotype (n = 6) were tested. Statistical significance: *p < 0.05.
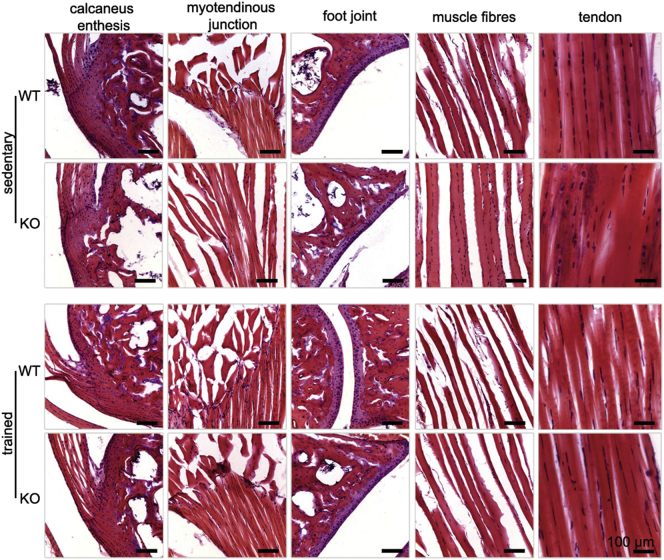
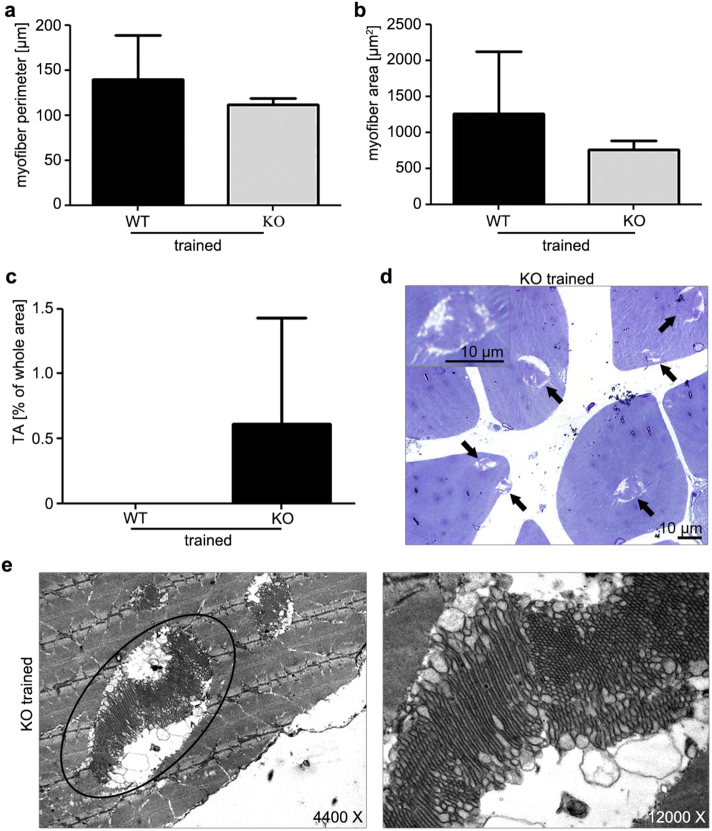
Endurance performance is multifactorial and can reflect the status of the musculoskeletal and pulmonary system. For this, musculoskeletal elements and foot joints were histologically examined (Supplementary Fig. 3) and pulmonary capacity, which are considered the “gold standard” when studying the cardiovascular, pulmonary, and metabolic adaptations to exercise, (data not shown) were measured revealing no differences. Morphometric evaluation of gastrocnemius myofibre perimeter and area showed reduced values in trained Tnmd KO mice (Supplementary Fig. 4a and b). Furthermore, we noticed a specific kind of myopathy only in trained Tnmd KO animals, namely tubular aggregates (TA) (Engel et al., 1970), which are atypical membranous structures originating from the terminal cisterns of the sarcoplasmic reticulum (Morgan-Hughes, 1998, Pavlovicova et al., 2003). The screening and TA counting of ultrathin muscle sections demonstrated approximately 1% of TA per area in mutant myofibers (Supplementary Fig. 4c, d and e). Though their incidence is rare, they represent an important indicator of skeletal muscle disease associated with aging (Boncompagni et al., 2012).
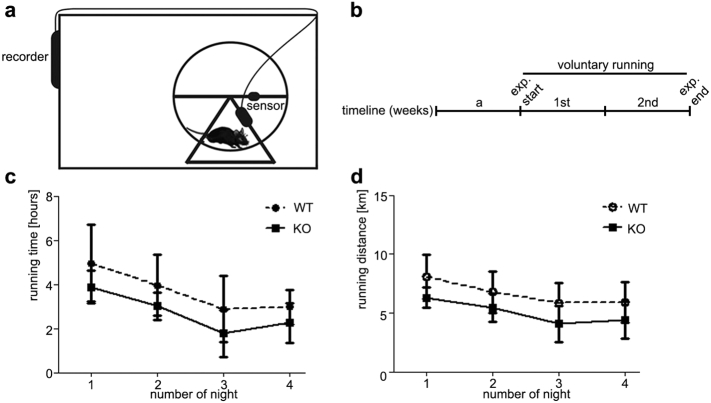
The response to forced exercise also depends on the adaptive capability and neural system of the mice (Lerman et al., 2002) because physical activity levels synergize environmental factors with genetic and biological components (Lightfoot, 2011). Therefore, we also examined the mice by voluntary running tests (Supplementary Fig. 5a and b). This investigation confirmed that even voluntarily Tnmd KO mice performed inferiorly as reflected by their running time and distance covered (Supplementary Fig. 5d, c and d). The registered tendency was not significant due to lower technical measurement accuracy of the running wheel set-up.
In sum, our results revealed that Tnmd KO mice are inferior runners compared to WT littermates in endurance tests, hence demonstrating that Tnmd is essential for the intrinsic properties of tendons allowing optimal running performance.
3.3. Tnmd Regulates the Structural and Biomechanical Properties of Tendon Collagen I Fibers and Their Response to Training
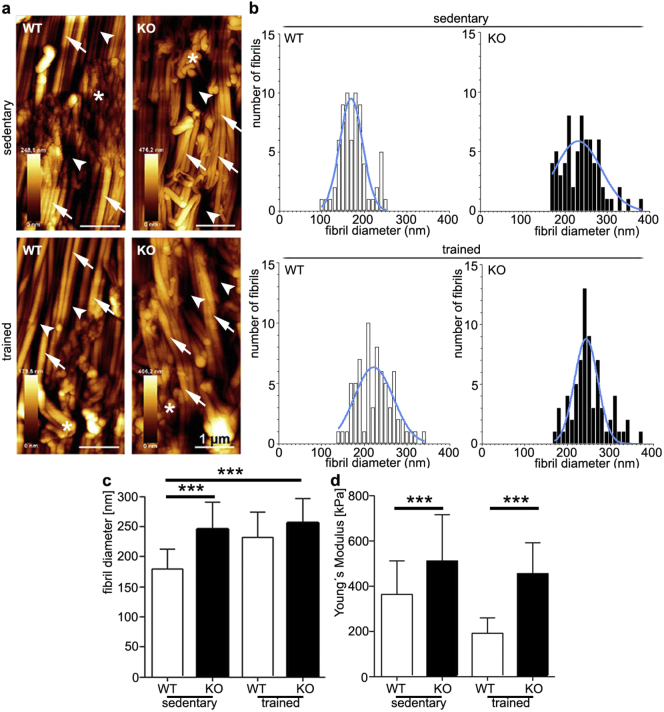
We utilized AFM technology to analyze collagen fiber diameter distribution and stiffness because it is the only tool that enables measurements of both parameters on the same tissue section. We examined the collagen topography, average fiber diameter and diameter distribution in WT and KO Achilles tendons from sedentary and trained mice by AFM. We found that collagen fibrils of Tnmd KO tendons were less compact, more frayed and interrupted by gaps (Fig. 3a). Comparison of fibril diameters revealed significantly larger mean values in Tnmd KO tendons (245,1 nm ± 44,8 nm) and a wider Gaussian distribution compared to controls (mean diameter of 179,3 nm ± 32,9 nm) with a narrower Gaussian distribution (Fig. 3b and c). Thicker collagen fibril phenotypes were previously described in thrombospondin-2 (Kyriakides et al., 1998), lumican (Chakravarti et al., 1998) and decorin KO mice (Zhang et al., 2006). WT mice fibril diameter ranged from 100 to 260 nm, whereas small fibrils were entirely missing in the Tnmd KO tendons and the range shifted to 190–390 nm (Fig. 3b). Interestingly, training led to widening of the Gaussian distribution and to an increase in fibril thickness in WT tendon, but this latter response was absent in the mutants, and the Gaussian distribution became narrower (Fig. 3b and c). Taking advantage of the AFM-based nano-indentation, we measured the elasticity of single collagen fibers and calculated average Young's Modulus (YM) (Fig. 3d). Here, Tnmd KO mice had significantly stiffer collagen I fibrils with YM of 511,5 ± 148.5 kPa in the sedentary group, which was slightly reduced to 453,9 ± 136,7 kPa in the trained group. In contrast, sedentary WT mice had softer collagen fibrils and responded more efficiently to endurance exercise by further increasing their elasticity by 47% (trained group YM 192,3 ± 66,2 kPa vs sedentary group YM 362,6 ± 148,5 kPa). Fig. 3d furthermore reveals that training led to a more homogenous tissue ultrastructure in WT tendons as reflected by a lower standard deviation.
Fig. 3.
Atomic force microscopy analysis on nano-topography, distribution of diameter size and elastic modulus of collagen I fibrils in Achilles tendons of sedentary and trained WT and Tnmd KO mice. (a) Representative AFM topographical images. Arrows: indicate collagen I fibers, arrowheads: interfibrular matrix, asterisk: section artifacts. (b) Distribution of collagen I fibril diameter size; blue curves present Gaussian distribution. (c) Average collagen fibril diameter (n = 60). (d) Calculation of mean Young's Moduli denoting the elastic properties of collagen I fibrils (n = 110). Bars represent mean ± standard deviation. Statistical significance: ***p < 0.005.
Thus, our data presents a striking collagen I phenotype of the Tnmd-deficient tendons; hereupon, we conclude that this gene is important for regulating the lateral thickness and stiffness of single collagen I fibers and for proper fiber adaptation to exercise by modifying their width and elasticity.
3.4. Tnmd is Required for Proper Collagen I Cross-linking
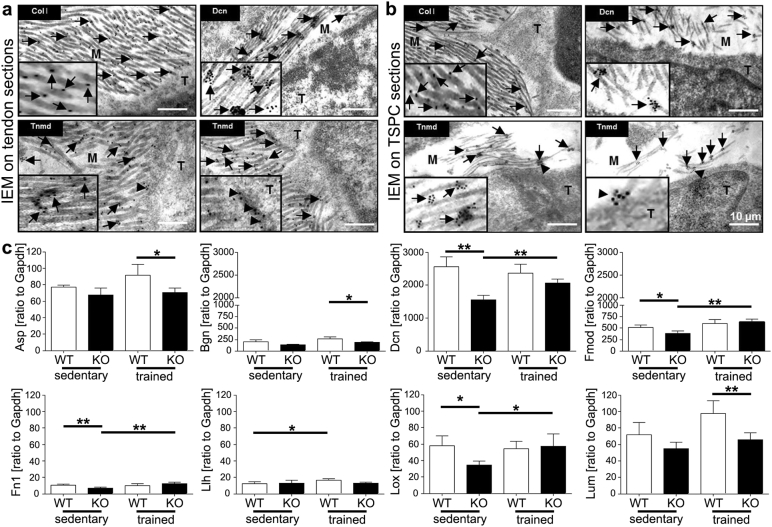
To explain this collagen-related phenomenon, we next investigated the exact localization of TNMD by immuno-gold labelling with anti-Tnmd C-terminal, collagen I and decorin antibodies together with electron microscopy imaging on Achilles tendon tissue (Fig. 4a) and TSPC sections (4b).
Fig. 4.
Tnmd localization in extracellular matrix and investigation of collagen I regulatory and cross-linking genes. Immunogold electron microscopy imaging of Achilles tendon (a) and TSPC sections (b) with immunogold labelling for collagen I (Col I), decorin (Dcn) and C-terminal Tnmd antibodies. Black arrows point to sites of epitope expression in the extracellular matrix (M) and arrowheads point to sites of epitope expression on tenocytes (T). (c) Quantitative PCR analysis for asporin (Asp), biglycan (Bgn), decorin (Dcn), fibromodulin (Fmod), fibronectin (Fn1), lysyl hydroxylase (Llh), lysyl oxidase (Lox) and lumican (Lum) on total cDNA from sedentary and trained Tnmd KO and WT tendons. N = 4. Statistical significance: *p < 0.05 and **p < 0.005.
High collagen I signals were observed directly in the bundles of thick ECM fibrils in tendon and TSPC sections. Further, gold-particles attached to decorin antibodies were grouped in between the mesh of collagen fibrils in a manner distinctive for proteoglycan distribution. Fig. 4a and b also revealed strong labelling for Tnmd in the ECM in both tendon sections and secreted by TSPC; its epitope was detected in small clusters or singularly localized between or along collagen fibers, differing from the larger clusters seen for decorin. TNMD was also found on the cell membrane in both tendon cells and TSPC in regions where collagen fibers were attached or protruding. Importantly, no expression along the naked cell membrane was visible, suggestive of intracellular cleavage of TNMD, which when secreted, deposits in the ECM in association with collagen I fibrils.
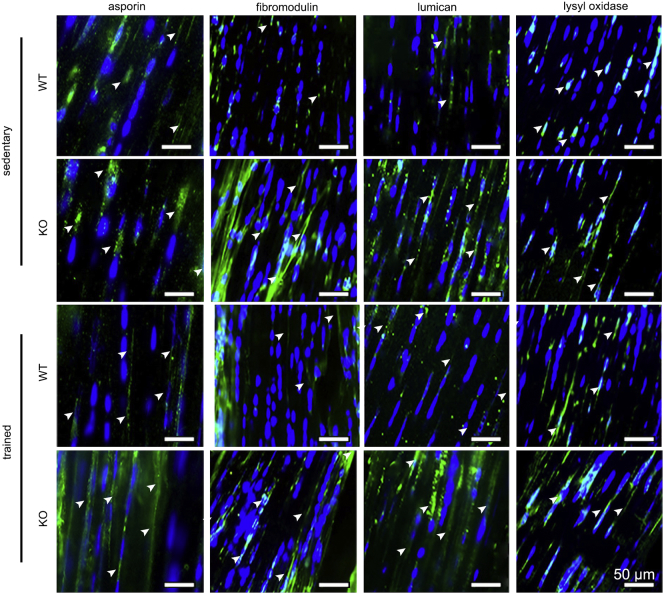
Lastly, we evaluated whether Tnmd affects collagen I fibril regulatory proteoglycans and cross-linkers, by investigating the expression of asporin, biglycan, fibromodulin, decorin, lysyl hydroxylase and lysyl oxidase genes in sedentary and endurance-trained tendons. Quantitative PCR identified high expression for decorin; moderate for fibromodulin and biglycan; and low for lumican, asporin, lysyl oxidase, lysyl hydroxylase and fibronectin (Fig. 4c). Loss of Tnmd resulted in significant downregulation of decorin, fibromodulin, fibronectin and lysyl oxidase mRNA levels. Regarding adaptation to exercise, only genes that were significantly downregulated in resting conditions were significantly upregulated in the mutants after training. This was markedly different to WT mice, which responded by significantly upregulating only lysyl hydroxylase, a pro-collagen I cross-linker (Yeowell and Walker, 2000). Intriguingly, at the protein level we detected higher levels of fibromodulin, lumican and lysyl oxidase in Tnmd KO prior to exercise; however, their protein deposition was unchanged after training (Supplementary Fig. 6). Only asporin responded to exercise by exhibiting a more fibrillary pattern. In contrast, WT animals had more pronounced lysyl oxidase signals in the ECM after running.
Taken together, our results suggest that TNMD/Tnmd is a mechanosensitive gene important for tendon running performance, proper adaptation to exercise and maintenance of structurally and functionally integral collagen fibers.
4. Discussion
Mechanical stimuli are important for development, homeostasis and regeneration of musculoskeletal tissues such as bone (Palomares et al., 2009), cartilage (Khoshgoftar et al., 2011) and muscle (Candiani et al., 2010). Muscle-restricted deletion of integrin-linked kinase, the downstream partner of the mechano-receptor integrin β1, leads to muscular dystrophy at the site of myotendinous junctions, a phenotype that is further enhanced by endurance exercise training (Wang et al., 2008). Conversely, mice lacking decorin and undergoing exercise are protected from the onset of osteoarthritis (Gronau et al., 2017). Mechanical stimulation was recognized as indispensable for tendon development and homeostasis (Yang et al., 2005, Galloway et al., 2013). One recent study by Maeda et al., 2011 indicated that in tendons physical forces are translated into biochemical signals via a signaling loop between TGF-β3 growth factor-Smad 2/3 mediators and Scleraxis (Scx) transcriptional activity. In another study, it was discovered that Scx and Osterix antagonistically regulate tensile force-responsive remodeling of periodontal ligaments and alveolar bone (Takimoto et al., 2015). However, the exact involvement of other mechano-sensitive genes and their signaling cascades in tendon tissues remains largely unknown and further efforts in understanding such pathways are fundamental for improving therapeutic strategies for tendinopathies.
We have previously described that Tnmd KO mice have a very mild developmental phenotype, consisting of reduced tenocyte density and increased collagen fiber diameter (Docheva et al., 2005). These data suggested that Tnmd is not a master-switch gene, but rather a fine-tuner. For this reason, we decided to examine whether TNMD is a mechanosensitive gene and to challenge control and KO animals by endurance running.
Tendons are comprised of abundant ECM, mainly collagen I, and low number of cells (Heinemeier and Kjaer, 2011). A small percentage of these cells are TSPC, a distinctive cell population with common adult stem cell characteristics suggested to modulate tendon homeostasis (Popov et al., 2015, Bi et al., 2007, Kohler et al., 2013). Mechanical loading of TSPC at 4% cyclic uniaxial stretch stimulated their proliferation and differentiation into tenocytes (Zhang and Wang, 2010), promoted the production of collagen I, whilst suppressing inflammatory responses (Yang et al., 2005). We have previously shown that 8% biaxial-loaded human TSPC respond by upregulation of mechano-sensitive genes and production of the proteoglycans fibromodulin and lumican (Popov et al., 2015). Loss of Tnmd transcripts in cultivated human periodontal ligament cells, tenocytes (Itaya et al., 2009, Mazzocca et al., 2011) and in rat tendon fibroblasts has been shown (Jelinsky et al., 2010). We suggest this downregulation results from lack of mechanical stimuli in vitro. Furthermore, a study on the effect of release of tensile strain on engineered human tendon-constructs seeded with tendon fibroblasts reported downregulation of TNMD expression (Bayer et al., 2014). In our study, the PCR and luciferase data on control and mechanically stretched human TSPC showed clearly that TNMD upregulates with mechanical stretching. Interestingly, TSPC transduced with Tnmd promoter, but not undergoing stretching had increased luciferase activity, which we suggest is due to endogenous expression of upstream Tnmd regulators in TSPC such as Scx (Alberton et al., 2012).
When subjected to exhaustive tests and endurance running exercise Tnmd-deficient mice exhibited a significantly different performance phenotype, comprised of lower running time, earlier running test failure and worsening of running performance after training regime. These results suggest that Tnmd is important for optimal running performance. In order to exclude side-effects of Tnmd gene KO on mice running performance, we carried out gross morphological assessment of musculoskeletal elements and foot joints revealing no obvious differences between genotypes. Interestingly, we detected a TA myopathy only in the trained KO animals, therefore at present we considered the onset of this pathology secondary to the tendon functional maladaptation to running. The initial study of tenomodulin by Brandau et al., 2001, suggested a low messenger expression of Tnmd in the brain, which so far has not been validated on the protein level. Still, to examine any potential neural side-effects, we performed voluntarily running tests where the animals were not forced to run, and in these tests Tnmd KO animals showed willingness to run, suggesting no profound behavioral differences, but still a tendency for inferior running. Follow up investigations will be undertaken to further study the Tnmd-related TA muscle phenotype as well as possible effects of Tnmd in the neural system.
Collagen I is the main determinant of tendon mechanical strength and function. Our immunogold labelling imaging co-localized Tnmd to collagen I fibers in human Achilles tendon and TSPC sections. Strong labelling for TNMD was observed in the ECM in both tendon sections and secreted by TSPC. Additionally, it was also found on the cell membrane in both tendon cells and TSPC in regions where collagen fibers were attached or protruding. Importantly, no expression along the naked cell membrane was visible, suggestive of intracellular cleavage of Tnmd, with subsequent export and deposition in the ECM in close association with collagen I fibers.
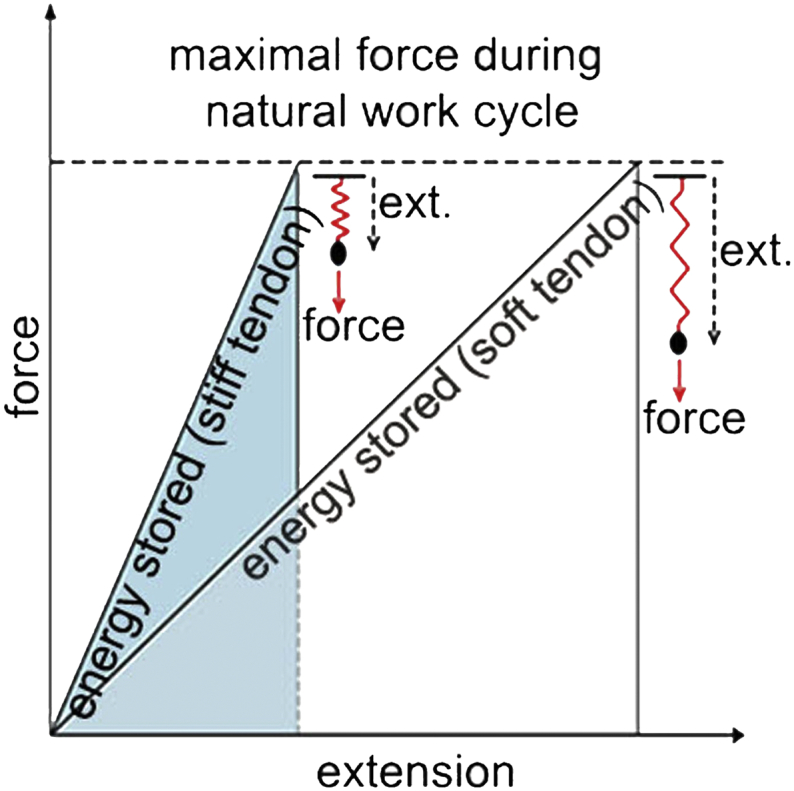
Since Tnmd KO mice harbor very mild developmental changes, including interesting ultrastructural phenotype characterized by irregular and thicker collagen I fibrils when examined by electron microscopy (Docheva et al., 2005), we hypothesized possible structural and biomechanical alterations of the tendon tissue on nano-level. To pinpoint such we implicated AFM topography and force indentation analyses, a technology that has been largely used in cartilage research, demonstrating solid data on nano-structural and -biomechanical assessment of cartilage pathologies (Gronau et al., 2017, Prein et al., 2016, Stolz et al., 2009). AFM of collagen I fibers in sedentary and trained Achilles tendons confirmed these previous observations and additionally revealed that the fibers are significantly stiffer in the KO than in WT tendons. Moreover, while fiber size increased and stiffness decreased with training in control mice, the KO tendons were non-responsive. The ability of tendons to stretch and recoil is important to save energy during locomotion for economic force generation (Alexander, 1991). However, at the same time they must not undergo irreversible deformation. Physiological stretching of tendons in vivo decreases crimp numbers close to 50% in order to reduce the degree of fibril undulation (Franchi et al., 2007). This is more important for tendons than muscle, since muscle can elongate by almost 10% while in contrast the whole tendon unit can only lengthen by 3–4% (Elsalanty et al., 2007). Vilarta et al., observed more aligned and intensely packed fibrils in Achilles tendons of rats after exercise (Vilarta and Vidal Bde, 1989). Stiffening of human tendons was observed in aging or diabetic individuals due to increased pathological cross-linking of collagen fibrils (Shadwick, 1990, Couppe et al., 2016). Stiffness of tendons varies with age, sex, physical activity and fatigue (Kubo et al., 2001b, Kubo et al., 2001a, Kubo et al., 2001c, Reeves, 2006). Interestingly, different types of tendons modulate their stiffness to each other, for example in endurance running, tendons in the knee joints become stiffer and in planter joints softer to guarantee optimal running performance (Kubo et al., 2015). Supplementary Fig. 7 depicts the explanation of this phenomenon. When subjected to the same amount of mechanical load, stiffer tendons extend less and in turn store less energy. In contrast, tendons that are more elastic extend further, store more energy and can therefore prevent premature rupturing. In endurance training the tendon spring softens; the softer the spring, the more elastic potential energy can be stored and less restoring force is needed resulting in more economic running.
A rise in blood-flow (Langberg et al., 1998), collagen-turnover (Langberg et al., 2000), glucose uptake (Hannukainen et al., 2005) and altered regulation of matrix metalloproteases (Koskinen et al., 2004) are detected in tendons during exercise. Tendons also adapt differently depending on the nature of the exercise. For example, Svensson et al. (2016) used resistance training protocol (building up strength, anaerobic endurance and size of skeletal muscles; involving fast-twitch muscle fibers) that resulted in tendon stiffening. In contrast, Wood and Brooks (2016) reported endurance running (involving slow-twitch fibers) led to more elastic tendon tissues in tendons of old mice, which is in line with our results. Endurance running in humans leads to softening of Achilles tendons (Ooi et al., 2015) and tendons of young mice adjusted to treadmill running by alterations in collagen fiber diameter, distribution, cross-sectional area and number (Michna, 1984, Michna and Hartmann, 1989, Majima et al., 2003). It will be of great importance in subsequent studies to investigate more precisely the structural-functional relationship between tendon tissue and both muscle fiber types by applying different exercise protocols as well as to couple AFM data with whole tendon biomechanical tests.
Collagen I and various proteoglycans contribute to the tensile strength and viscoelastic properties of the tendon tissue, respectively (Puxkandl et al., 2002, Robinson et al., 2004). Furthermore, proteoglycans and cross-linking enzymes modulate collagen I fibrillogenesis during rest and training (Kalamajski and Oldberg, 2010, Kwansa et al., 2014). Therefore, we last examined what effect Tnmd exerts on cross-linker genes in resting and exercise conditions. Comparison of mRNA levels of cross-linkers in tendons of WT mice revealed only one significant exercise-responder gene - lysyl hydroxylase. In contrast, the tendons of the mutant mice exhibited a very different molecular response to exercise: namely all genes that were significantly downregulated in sedentary animals responded by upregulation in the trained. The expression of the extracellular collagen cross-linker gene Lox was augmented in the KO mice as a result of exercise. Heinemeier et al. (2007) have shown Lox upregulation in rat tendon with increased loading, which is suggested to potentiate the tendon collagen cross-linking process. At the protein level, we observed discrepancies in the expression profile, a phenomenon similar to that seen in the development of early osteoarthritis in articular cartilage: comparison between osteoarthritic versus healthy patients revealed that link protein, among several other genes, increased 50-fold at the mRNA level compared with only a three-fold increase at the protein level in the cartilage of diseased donors (Cs-Szabo et al., 1997). At present, how Tnmd exactly regulates collagen I fibrillogenesis remains difficult to decipher due to our lack of understanding of Tnmd signaling pathways. Our data greatly emphasize the need for future studies to accurately explain the signaling pathways of this gene protein.
Taken together, our study demonstrates that TNMD/Tnmd, a mechanosensitive gene, is required for proper tendon tissue adaptation to endurance running. The data advance our understanding of the structural-functional relationships of tendon tissues.
Funding Sources
German Research Foundation, D.D. grant Nr. DO1414/3-1.
Conflict of Interest
Authors have no conflict of interest.
Author Contributions
S.D. performed experimental design, voluntary running experiments, collagen cross-linking experiments and wrote the manuscript; P.A. contributed to experimental design, prepared specimens and performed H&E stainings of mouse limbs and arranged sections for AFM experiments; L.W. and W.B. did forced endurance training and tests and pulmonary examinations and muscle analyses; T.S., S.B. and H. C-S. provided AFM data; S.M. made available cadaver human tissue biopsies and contributed with histological interpretation; M.S. carried out immunogold labelling and EM; A.I. contributed to mechanical stimulation of TSPC; C.S. provided plasmids for luciferase assay; M.S. approved manuscript; D.D. made project conception and experimental design, wrote and approved manuscript.
Acknowledgments
D.D. acknowledges the support of the German Research Foundation (DO1414/3-1). TS, SB and HCS acknowledge the support through the CANTER research focal point of the Bavarian State Ministry for Science and Education. The funders were not involved in study design, data collection, data analysis, interpretation and writing of the report. We thank Martina Burggraf for technical assistance in generating data for Fig. 1.
Appendix A. Supplementary materials and methods
A.1. Semiquantitative PCR
Total RNA from human TSPC was isolated with Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany). For cDNA synthesis, 1μg total RNA and AMV First-Strand cDNA Synthesis Kit (Life technologies) were used. PCR was performed with Taq DNA Polymerase (Life technologies) in MGResearch instrument (BioRad, Germany). The conditions for TNMD and GAPDH PCR are given in Supp. Table 1. PCR bands were quantified densitometrically using BioCapt software (Vilber Lourmat, Germany). Values were normalized to GAPDH and results reported as fold change to control, none-mechanically stimulated cells. For enabling fold-upregulation calculation, the PCR 0 value from the measurement in the control group was set to 1.
A.2. Immunofluorescence
Immunofluorescence on human patella, meniscus, tendon and fibrocartilage was performed as described in the main text. Biopsies from cadaveric human cartilage (patella), meniscus and enthesis fibrocartilage were donor donated to the Anatomy Institute, LMU, Munich. Cryo-sections (12 μm thick) were rehydrated in PBS and immunofluorescence was carried with antibodies for collagen II and Tnmd with final nuclear, DAPI counterstain. Photomicrographs were taken with Axiocam MRm camera on Observer Z1 microscope (Carl Zeiss).
A.3. Hematoxilin/Eosin staining
H&E staining was performed on paraffin sections of mouse lower foot as follows: after deparafinization and xylol treatment, re-hydration in descending alcohol row (100/H2O-50/PBS%) and dH2O wash were carried out. Hematoxylin solution of 0.1% was applied for 5 min, followed by intense washing with tap water. Next, slides were rinsed with dH2O and incubated in 0.1% eosin solution for 5 min. Slides were dehydrated in ascending ethanol row to 100%, cleared by two xylol steps and finally mounted with DPX mounting media (Sigma-Aldrich). Pictures were taken on an Axiovert 100 microscope using AxioCam ICc3 color camera (Carl Zeiss).
A.4. Processing of tissue samples for electron microscopy (EM)
Muscle specimens for EM were processed in the Department of Prof. W. Bloch as described by Wang et al., 2008. Briefly, muscle tissue was fixed in pre-cooled 4% PFA ON at 4°C. After fixation, specimens were rinsed 3x10 min in 1 M cacodylate buffer and then treated with 1% uranyl acetate in 70% ethanol for 8 h at 4°C. Biopsies were subsequently dehydrated in a graded series of ethanol and finally embedded in Araldite (Serva, Heidelberg, Germany). Ultrathin sections of 30-60 nm were cut with an ultramicrotome (Reichert-Leica) with a diamond knife and placed on copper grids. Transmission electron microscopy was performed using an electron microscope 902A, Carl Zeiss (Zeiss, Jena, Germany).
A.5. Voluntary running
Voluntary running behavior was compared between adult Tnmd KO and WT male mice (six per genotype, n = 6). Study design was approved by the central animal facility of the Medical faculty of the LMU. First week mice were acclimatized to the experimental cage containing a standard free-spinning mouse running wheel (∅ 12 cm) equipped with speedometer (bike computer 8000 Speedmaster, Sigma-Electro, Neustadt, Germany). The second and third week each mouse was placed in the experimental cage, left overnight to voluntarily use the wheel. This was repeated for 4 consecutive days each week. Running time and distance were measured, however the technical accuracy of the wheel/speedometer system was lower compared to the treadmill apparatuses.
Supp. Table 1.
Semiquantitative PCR conditions.
| Forward primer | Reverse primer | Size (bp) | Annealing Temp (°C) | |
|---|---|---|---|---|
| TNMD | 5´-aagacccgtcacgccagacaag-3´ | 5´-ttcacagacgcggcggcaatag-3´ | 153 | 52 |
| GAPDH | 5´-caactacatggtttacatgttc-3´ | 5´-gccagtggactccacgac-3´ | 181 | 50 |
Fig. S1.
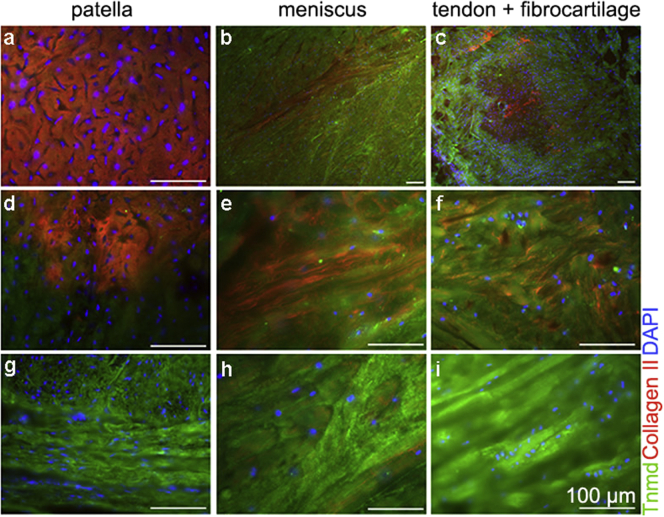
Tnmd expression in human patella, meniscus and enthesis fibrocartilage. Immunofluorescense with anti-collagen II (in red), Tnmd (in green) antibodies and nuclear DAPI (in blue) counterstain. (a) Central cartilage region of patella, (b) meniscus (low magnification), (c) enthesis (tendon to fibrocartilage area) (low magnification), (d) transition zone to patella tendon, (e) meniscus double-labelled area, (f) fibrocartilage double-labelled area, (g) patella tendon, (h) meniscus area positive only for Tnmd, and (i) tendon area positive only for Tnmd.
Fig. S2.
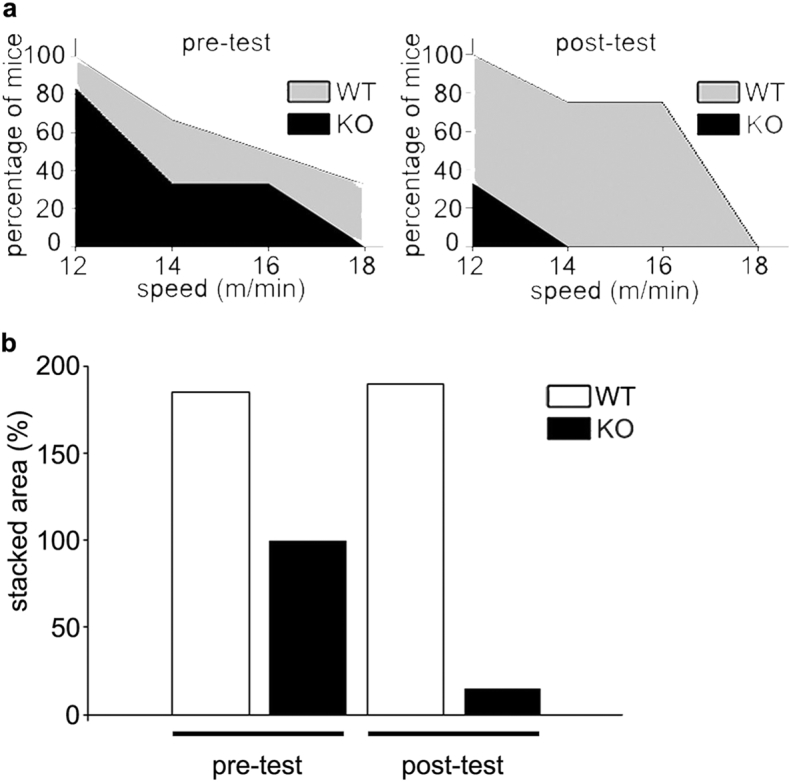
Stacked areas presenting the success rate of both genetypes in pre- and post-exhaustive tests. (a) Stacked area plot and (b) cumulative stacked area in percentage.
Fig. S3.
Representative H&E staining of musculoskeletal elements in Tnmd KO and WT sedentary and trained animals.
Fig. S4.
Gastrocnemius muscle analyses in Tnmd KO and WT trained animals. (a) Perimeter and (b) area of myofibers measured on toluidine blue-stained muscle sections. (c) Tubular aggregate (TA) count per total (expressed in percentage) myofiber area. (d) Light microscopy (arrows depict TA) and (e) electron microscopy (two different magnifications) images of muscle ultrathin sections of trained Tnmd KO animals.
Fig. S5.
Tnmd KO and WT performance in voluntary running tests. (a) Experimental setup of the voluntary running experiment. (b) Timeline: a, 1 week of acclimatization followed by two weeks of voluntary running, as in each mouse performance was recorded for 4 consecutive nights. (c) Running time and (d) distance covered per night. Bars represent mean ± standard deviation. Six animal per genotype were tested (n = 6).
Fig. S6.
Comparison of protein expression of collagen I cross-linking genes between genotypes and in response to training. Immunofluorescence with asporin, fibromodulin, lumican and lysyl oxidase antibodies on Achilles tendon sections.
Fig. S7.
Cartoon model explaining the physical paradigm of energy stored in stiff versus soft tendons (ext., extension). The softer the spring, more elastic potential energy can be stored in the tendon and less restoring force is needed, hence resulting in more economic running.
References
- Alberton P., Popov C., Pragert M., Kohler J., Shukunami C., Schieker M., Docheva D. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberton P., Dex S., Popov C., Shukunami C., Schieker M., Docheva D. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 2015;24(5):597–609. doi: 10.1089/scd.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R.M. Elastic mechanisms in primate locomotion. Z. Morphol. Anthropol. 1991;78(3):315–320. [PubMed] [Google Scholar]
- Bayer M.L., Schjerling P., Herchenhan A., Zeltz C., Heinemeier K.M., Christensen L., Krogsgaard M., Gullberg D., Kjaer M. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One. 2014;9(1):e86078. doi: 10.1371/journal.pone.0086078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Ralphs J.R. Tendons and ligaments—an overview. Histol. Histopathol. 1997;12(4):1135–1144. [PubMed] [Google Scholar]
- Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W., Li L., Leet A.I., Seo B.M., Zhang L., Shi S., Young M.F. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Boncompagni S., Protasi F., Franzini-Armstrong C. Sequential stages in the age-dependent gradual formation and accumulation of tubular aggregates in fast twitch muscle fibers: SERCA and calsequestrin involvement. Age (Dordr.) 2012;34(1):27–41. doi: 10.1007/s11357-011-9211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandau O., Meindl A., Fassler R., Aszodi A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev. Dyn. 2001;221(1):72–80. doi: 10.1002/dvdy.1126. [DOI] [PubMed] [Google Scholar]
- Butt H.J., Jaschke M. Calculation of thermal noise in atomic force microscopy. Nanotechnology. 1995;6:1–7. [Google Scholar]
- Candiani G., Riboldi S.A., Sadr N., Lorenzoni S., Neuenschwander P., Montevecchi F.M., Mantero S. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J. Appl. Biomater. Biomech. 2010;8(2):68–75. [PubMed] [Google Scholar]
- Chakravarti S., Magnuson T., Lass J.H., Jepsen K.J., LaMantia C., Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 1998;141(5):1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppe C., Svensson R.B., Kongsgaard M., Kovanen V., Grosset J.F., Snorgaard O., Bencke J., Larsen J.O., Bandholm T., Christensen T.M., Boesen A., Helmark I.C., Aagaard P., Kjaer M., Magnusson S.P. Human Achilles tendon glycation and function in diabetes. J. Appl. Physiol. (1985) 2016;120(2):130–137. doi: 10.1152/japplphysiol.00547.2015. [DOI] [PubMed] [Google Scholar]
- Cs-Szabo G., Melching L.I., Roughley P.J., Glant T.T. Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum. 1997;40(6):1037–1045. doi: 10.1002/art.1780400607. [DOI] [PubMed] [Google Scholar]
- Dex S., Lin D., Shukunami C., Docheva D. Tenogenic modulating insider factor: systematic assessment on the functions of tenomodulin gene. Gene. 2016;587(1):1–17. doi: 10.1016/j.gene.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D., Hunziker E.B., Fassler R., Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsalanty M., Makarov M., Cherkashin A., Birch J., Samchukov M. Changes in pennate muscle architecture after gradual tibial lengthening in goats. Anat. Rec. 2007;290(5):461–467. doi: 10.1002/ar.20513. [DOI] [PubMed] [Google Scholar]
- Engel W.K., Bishop D.W., Cunningham G.G. Tubular aggregates in type II muscle fibers: ultrastructural and histochemical correlation. J. Ultrastruct. Res. 1970;31(5–6):507–525. doi: 10.1016/s0022-5320(70)90166-8. [DOI] [PubMed] [Google Scholar]
- Franchi M., Fini M., Quaranta M., De Pasquale V., Raspanti M., Giavaresi G., Ottani V., Ruggeri A. Crimp morphology in relaxed and stretched rat Achilles tendon. J. Anat. 2007;210(1):1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway M.T., Lalley A.L., Shearn J.T. The role of mechanical loading in tendon development, maintenance, injury, and repair. J. Bone Joint Surg. Am. 2013;95(17):1620–1628. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronau T., Kruger K., Prein C., Aszodi A., Gronau I., Iozzo R.V., Mooren F.C., Clausen-Schaumann H., Bertrand J., Pap T., Bruckner P., Dreier R. Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin. Ann. Rheum. Dis. 2017;76(2) doi: 10.1136/annrheumdis-2016-209319. 442–229. [DOI] [PubMed] [Google Scholar]
- Hannukainen J., Kalliokoski K.K., Nuutila P., Fujimoto T., Kemppainen J., Viljanen T., Laaksonen M.S., Parkkola R., Knuuti J., Kjaer M. In vivo measurements of glucose uptake in human Achilles tendon during different exercise intensities. Int. J. Sports Med. 2005;26(9):727–731. doi: 10.1055/s-2005-837458. [DOI] [PubMed] [Google Scholar]
- Heinemeier K.M., Kjaer M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011;11(2):115–123. [PubMed] [Google Scholar]
- Heinemeier K.M., Olesen J.L., Haddad F., Langberg H., Kjaer M., Baldwin K.M., Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007;582(Pt 3):1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya T., Kagami H., Okada K., Yamawaki A., Narita Y., Inoue M., Sumita Y., Ueda M. Characteristic changes of periodontal ligament-derived cells during passage. J. Periodontal Res. 2009;44(4):425–433. doi: 10.1111/j.1600-0765.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Jelinsky S.A., Archambault J., Li L., Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J. Orthop. Res. 2010;28(3):289–297. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- Kalamajski S., Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29(4):248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kamper M., Hamann N., Prein C., Clausen-Schaumann H., Farkas Z., Aszodi A., Niehoff A., Paulsson M., Zaucke F. Early changes in morphology, bone mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX −/− mice. Matrix Biol. 2016;49:132–143. doi: 10.1016/j.matbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Khoshgoftar M., van Donkelaar C.C., Ito K. Mechanical stimulation to stimulate formation of a physiological collagen architecture in tissue-engineered cartilage: a numerical study. Comput. Methods Biomech. Biomed. Eng. 2011;14(2):135–144. doi: 10.1080/10255842.2010.519335. [DOI] [PubMed] [Google Scholar]
- Kimura N., Shukunami C., Hakuno D., Yoshioka M., Miura S., Docheva D., Kimura T., Okada Y., Matsumura G., Shin'oka T., Yozu R., Kobayashi J., Ishibashi-Ueda H., Hiraki Y., Fukuda K. Local tenomodulin absence, angiogenesis, and matrix metalloproteinase activation are associated with the rupture of the chordae tendineae cordis. Circulation. 2008;118(17):1737–1747. doi: 10.1161/CIRCULATIONAHA.108.780031. [DOI] [PubMed] [Google Scholar]
- Kohler J., Popov C., Klotz B., Alberton P., Prall W.C., Haasters F., Muller-Deubert S., Ebert R., Klein-Hitpass L., Jakob F., Schieker M., Docheva D. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013;12(6):988–999. doi: 10.1111/acel.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen S.O., Heinemeier K.M., Olesen J.L., Langberg H., Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J. Appl. Physiol. (1985) 2004;96(3):861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- Koteja P., Swallow J.G., Carter P.A., Garland T., Jr. Energy cost of wheel running in house mice: implications for coadaptation of locomotion and energy budgets. Physiol. Biochem. Zool. 1999;72(2):238–249. doi: 10.1086/316653. [DOI] [PubMed] [Google Scholar]
- Kubo K., Kanehisa H., Ito M., Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J. Appl. Physiol. (1985) 2001;91(1):26–32. doi: 10.1152/jappl.2001.91.1.26. [DOI] [PubMed] [Google Scholar]
- Kubo K., Kanehisa H., Kawakami Y., Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int. J. Sports Med. 2001;22(2):138–143. doi: 10.1055/s-2001-11337. [DOI] [PubMed] [Google Scholar]
- Kubo K., Kanehisa H., Kawakami Y., Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. Eur. J. Appl. Physiol. 2001;84(1–2):162–166. doi: 10.1007/s004210000337. [DOI] [PubMed] [Google Scholar]
- Kubo K., Miyazaki D., Shimoju S., Tsunoda N. Relationship between elastic properties of tendon structures and performance in long distance runners. Eur. J. Appl. Physiol. 2015;115(8):1725–1733. doi: 10.1007/s00421-015-3156-2. [DOI] [PubMed] [Google Scholar]
- Kwansa A.L., De Vita R., Freeman J.W. Mechanical recruitment of N- and C-crosslinks in collagen type I. Matrix Biol. 2014;34:161–169. doi: 10.1016/j.matbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Kyriakides T.R., Zhu Y.H., Smith L.T., Bain S.D., Yang Z., Lin M.T., Danielson K.G., Iozzo R.V., LaMarca M., McKinney C.E., Ginns E.I., Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998;140(2):419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H., Bulow J., Kjaer M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol. Scand. 1998;163(2):149–153. doi: 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Langberg H., Skovgaard D., Asp S., Kjaer M. Time pattern of exercise-induced changes in type I collagen turnover after prolonged endurance exercise in humans. Calcif. Tissue Int. 2000;67(1):41–44. doi: 10.1007/s00223001094. [DOI] [PubMed] [Google Scholar]
- Lerman I., Harrison B.C., Freeman K., Hewett T.E., Allen D.L., Robbins J., Leinwand L.A. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J. Appl. Physiol. 2002;92(6):2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Lightfoot J.T. Current understanding of the genetic basis for physical activity. J. Nutr. 2011;141(3):526–530. doi: 10.3945/jn.110.127290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Sakabe T., Sunaga A., Sakai K., Rivera A.L., Keene D.R., Sasaki T., Stavnezer E., Iannotti J., Schweitzer R., Ilic D., Baskaran H., Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr. Biol. 2011;21(11):933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majima T., Yasuda K., Tsuchida T., Tanaka K., Miyakawa K., Minami A., Hayashi K. Stress shielding of patellar tendon: effect on small-diameter collagen fibrils in a rabbit model. J. Orthop. Sci. 2003;8(6):836–841. doi: 10.1007/s00776-003-0707-x. [DOI] [PubMed] [Google Scholar]
- Mazzocca A.D., Chowaniec D., McCarthy M.B., Beitzel K., Cote M.P., McKinnon W., Arciero R. In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers. Knee Surg. Sports Traumatol. Arthrosc. 2011 doi: 10.1007/s00167-011-1711-x. [DOI] [PubMed] [Google Scholar]
- Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res. 1984;236(2):465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- Michna H., Hartmann G. Adaptation of tendon collagen to exercise. Int. Orthop. 1989;13(3):161–165. doi: 10.1007/BF00268040. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J.A. Tubular aggregates in skeletal muscle: their functional significance and mechanisms of pathogenesis. Curr. Opin. Neurol. 1998;11(5):439–442. doi: 10.1097/00019052-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Morse D.L., Gillies R.J. Molecular imaging and targeted therapies. Biochem. Pharmacol. 2010;80(5):731–738. doi: 10.1016/j.bcp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C.C., Schneider M.E., Malliaras P., Counsel P., Connell D.A. Prevalence of morphological and mechanical stiffness alterations of mid Achilles tendons in asymptomatic marathon runners before and after a competition. Skelet. Radiol. 2015;44(8):1119–1127. doi: 10.1007/s00256-015-2132-6. [DOI] [PubMed] [Google Scholar]
- Oshima Y., Shukunami C., Honda J., Nishida K., Tashiro F., Miyazaki J., Hiraki Y., Tano Y. Expression and localization of tenomodulin, a transmembrane type chondromodulin-I-related angiogenesis inhibitor, in mouse eyes. Invest. Ophthalmol. Vis. Sci. 2003;44(5):1814–1823. doi: 10.1167/iovs.02-0664. [DOI] [PubMed] [Google Scholar]
- Palomares K.T., Gleason R.E., Mason Z.D., Cullinane D.M., Einhorn T.A., Gerstenfeld L.C., Morgan E.F. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. 2009;27(9):1123–1132. doi: 10.1002/jor.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovicova M., Novotova M., Zahradnik I. Structure and composition of tubular aggregates of skeletal muscle fibres. Gen. Physiol. Biophys. 2003;22(4):425–440. [PubMed] [Google Scholar]
- Pennisi E. Tending tender tendons. Science. 2002;295(5557):1011. doi: 10.1126/science.295.5557.1011. [DOI] [PubMed] [Google Scholar]
- Popov C., Burggraf M., Kreja L., Ignatius A., Schieker M., Docheva D. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol. 2015;16:6. doi: 10.1186/s12867-015-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prein C., Warmbold N., Farkas Z., Schieker M., Aszodi A., Clausen-Schaumann H. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. 2016;50:1–15. doi: 10.1016/j.matbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Puxkandl R., Zizak I., Paris O., Keckes J., Tesch W., Bernstorff S., Purslow P., Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002;357(1418):191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves N.D. Adaptation of the tendon to mechanical usage. J. Musculoskelet. Neuronal Interact. 2006;6(2):174–180. [PubMed] [Google Scholar]
- Robinson P.S., Lin T.W., Reynolds P.R., Derwin K.A., Iozzo R.V., Soslowsky L.J. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J. Biomech. Eng. 2004;126(2):252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Shadwick R.E. Elastic energy storage in tendons: mechanical differences related to function and age. J. Appl. Physiol. (1985) 1990;68(3):1033–1040. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- Shukunami C., Oshima Y., Hiraki Y. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem. Biophys. Res. Commun. 2001;280(5):1323–1327. doi: 10.1006/bbrc.2001.4271. [DOI] [PubMed] [Google Scholar]
- Stolz M., Gottardi R., Raiteri R., Miot S., Martin I., Imer R., Staufer U., Raducanu A., Düggelin M., Baschong W., Daniels A.U., Friederich N.F., Aszodi A., Aebi U. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009;4(3):186–192. doi: 10.1038/nnano.2008.410. [DOI] [PubMed] [Google Scholar]
- Svensson R.B., Heinemeier K.M., Couppe C., Kjaer M., Magnusson S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. (1985) 2016;121(6):1353–1362. doi: 10.1152/japplphysiol.00328.2016. [DOI] [PubMed] [Google Scholar]
- Takimoto A., Kawatsu M., Yoshimoto Y., Kawamoto T., Seiryu M., Takano-Yamamoto T., Hiraki Y., Shukunami C. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development. 2015;142(4):787–796. doi: 10.1242/dev.116228. [DOI] [PubMed] [Google Scholar]
- Vilarta R., Vidal Bde C. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989;9(1):55–61. doi: 10.1016/s0934-8832(89)80019-8. [DOI] [PubMed] [Google Scholar]
- Wang H.V., Chang L.W., Brixius K., Wickstrom S.A., Montanez E., Thievessen I., Schwander M., Muller U., Bloch W., Mayer U., Fassler R. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J. Cell Biol. 2008;180(5):1037–1049. doi: 10.1083/jcb.200707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L.K., Brooks S.V. Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J. Orthop. Res. 2016;43(2):346–353. doi: 10.1002/jor.22824. [DOI] [PubMed] [Google Scholar]
- Yang G., Im H.J., Wang J.H. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeowell H.N., Walker L.C. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol. Genet. Metab. 2000;71(1–2):212–224. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J.H. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J. Orthop. Res. 2010;28(5):639–643. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ezura Y., Chervoneva I., Robinson P.S., Beason D.P., Carine E.T., Soslowsky L.J., Iozzo R.V., Birk D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell Biochem. 2006;98(6):1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]