Abstract
Dengue is the most prevalent arthropod-borne viral disease worldwide and is caused by the four dengue virus serotypes (DENV-1-4). Sequential heterologous DENV infections can be associated with severe disease manifestations. Here, we present an immunocompetent mouse model of secondary DENV infection using non mouse-adapted DENV strains to investigate the pathogenesis of severe dengue disease. C57BL/6 mice infected sequentially with DENV-1 (strain Puerto Rico/94) and DENV-2 (strain Tonga/74) developed low platelet counts, internal hemorrhages, and increase of liver enzymes. Cross-reactive CD8+ T lymphocytes were found to be necessary and sufficient for signs of severe disease by adoptively transferring of DENV-1-immune CD8+ T lymphocytes before DENV-2 challenge. Disease signs were associated with production of tumor necrosis factor (TNF)-α and elevated cytotoxicity displayed by heterotypic anti-DENV-1 CD8+ T lymphocytes. These findings highlight the critical role of heterotypic anti-DENV CD8+ T lymphocytes in manifestations of severe dengue disease.
Keywords: Dengue virus, Immunocompetent mouse model, Secondary infection, Immune response, CD8+ T lymphocytes
Highlights
-
•
Heterotypic anti-DENV-1 CD8+ T cells are critical for signs of severe dengue disease in sequentially DENV-2 infected mice
-
•
Production of TNF-α by heterotypic anti-DENV-1 CD8+ T lymphocytes is critical for signs of severe dengue disease
-
•
Anti-DENV-1 CD4+ T lymphocytes also contribute to severe dengue disease
Talarico et al. present an immunocompetent mouse model of dengue virus secondary infection which shows signs of severe dengue disease upon heterotypic infection with DENV-1 (PR/94) followed by DENV-2 (Tonga/74). In this model cross-reactive CD4+ and CD8+ T cells contribute to disease, while anti-DENV heterotypic antibodies do not play a critical role in pathogenesis.
1. Introduction
The four serotypes of dengue virus (DENV) cause the most prevalent arthropod-borne viral disease in humans (Guzman and Harris, 2015). An estimated 390 million people are infected by one of the DENV serotypes every year; up to 96 million have apparent DENV infections, with different levels of disease severity (Bhatt et al., 2013). Symptoms in humans range from mild febrile illness with myalgia and rash to severe disease characterized by thrombocytopenia, capillary leakage, bleeding, and increase of liver enzymes (dengue hemorrhagic fever [DHF]) (Gibbons and Vaughn, 2002). The latter may progress to hypovolemic shock (dengue shock syndrome [DSS]) (Martina et al., 2009). Severe dengue disease affects over 2 million people each year and is estimated to cause 21,000 deaths annually (Gubler, 2012). Severe manifestations occur late in illness during viral clearance and are more common in secondary heterologous DENV infections (Yacoub et al., 2013). Infants born to DENV-immune mothers are at greater risk of severe disease during primary infection (Halstead et al., 2002). The global incidence of severe dengue disease is increasing (Guzman et al., 2010).
Several hypotheses have been advanced to explain the pathogenesis of DHF/DSS (Martina et al., 2009). The most widely accepted theory ascribes DHF/DSS to an antibody-dependent enhancement (ADE) of heterologous viral replication in cells of the reticuloendothelial system (Halstead, 2003). A second hypothesis attributes DHF/DSS to a heterotypic T lymphocyte response during secondary DENV infection that is predominantly directed against the first infecting DENV serotype (DENV serotypes exhibit approximately 70% sequence homology) (Pierson and Diamond, 2013, Rothman, 2011). This secondary T cell response is thought to be suboptimal, differing from primary responses in cytokine production and cytotoxic ability (Rothman, 2010, Rothman, 2011). These theories are based on observations derived from human studies (Halstead, 1988, Mongkolsapaya et al., 2003), and the mechanism of illness remains unclear.
While various human studies have suggested a role for T cells in pathogenesis of DENV infections (Bashyam et al., 2006, Mongkolsapaya et al., 2006, Zivna et al., 2002), several recent reports –mainly in immunocompromised mouse models– have described a protective role of T lymphocytes against DENV disease (Prestwood et al., 2012, Yauch et al., 2010, Yauch et al., 2009, Zellweger et al., 2014, Zellweger et al., 2015, Zompi et al., 2012). Therefore, T cell responses seem to play a dual role in DENV infections, in both protection against and pathogenesis of DENV disease.
While imbalances in response to individual serotypes could lead to DHF/DSS in seropositive populations (Halstead, 1982, Sangkawibha et al., 1984), the component/s of the immune response that cause severe dengue disease are still not well understood. A better understanding of DENV pathogenesis would be important for vaccine development in a disease without specific treatment.
The development of animal models for severe dengue disease has been challenging (Plummer and Shresta, 2014, Zellweger and Shresta, 2014, Zompi and Harris, 2012). In this study, we describe an immunocompetent mouse model of secondary DENV infection using non mouse-adapted DENV strains. C57BL/6 mice infected sequentially with DENV-1 (strain Puerto Rico/94) and DENV-2 (strain Tonga/74) developed low platelet counts, internal hemorrhages, and increase of liver enzymes. We subsequently used this model to characterize the mechanisms involved in the pathogenesis of severe dengue disease.
2. Materials and Methods
2.1. Ethics Statement
The animal protocols used in this study were reviewed and approved by the University of Vanderbilt Institutional Animal Care and Use Committee (IACUC) (protocol# M/09/139), Instituto de Ciencia y Tecnología Dr. César Milstein IACUC (protocol#02-2016), Facultad de Ciencias Exactas y Naturales-UBA IACUC (protocol#82) and INFANT Committee. Experiments were performed according to the guidelines of the above-mentioned IACUCs.
2.2. Cell Lines and Virus Preparation
Vero (African green monkey kidney) cells (ATCC Cat# CCL-81, RRID: CVCL_0059) were grown in MEM (GIBCO) supplemented with 5% fetal bovine serum (FBS). The C6/36 mosquito cell line from Aedes albopictus, adapted to grow at 33 °C, was cultured in L-15 Medium (Leibovitz) (GIBCO) supplemented with 0.3% tryptose phosphate broth, 0.02% glutamine, 1% MEM non-essential amino acids solution and 5% FBS. DENV-1 strain Puerto Rico/94 (PR/94) (Blaney et al., 2007) and DENV-2 strain Tonga/74 (Blaney et al., 2004, Gubler et al., 1978) were kindly supplied by Dr. Steve Whitehead (Laboratory of Infectious Diseases, NIAD, NIH, DHHS). DENV-2 strain NGC was generously provided by Dr. Elsa Damonte (Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina), DENV-2 strain 16681 was kindly provided by Dr. Andrea Gamarnik (Fundación Instituto Leloir, Buenos Aires, Argentina) and DENV-2 strain D2S10 was generously supplied by Dr. Eva Harris (University of California, Berkeley, USA). DENV strains were grown in C6/36 cells for 4–6 days. Virus stocks were titrated by plaque formation in Vero cells.
Respiratory syncytial virus (RSV) strain L19 was grown in Vero cells for 3–4 days and virus stock was titrated by plaque formation in Vero cells.
2.3. Mouse Strains and Viral Infection
Five to six week old C57BL/6 J (RRID:IMSR_JAX:000664), B6.129S2-Cd8atm1Mak/J (CD8α −/−, deficient in functional cytotoxic T cells, RRID:IMSR_JAX:002665) and B6;129S–Tnftm1Gkl/J (TNF-α −/−, deficient in the production of tumor necrosis factor (TNF)-α, RRID:IMSR_JAX:003008) mice were purchased from The Jackson Laboratory and housed in the Animal Facilities from University of Vanderbilt, Instituto de Ciencia y Tecnología Dr. César Milstein, Facultad de Ciencias Exactas y Naturales-UBA or Fundación INFANT. In all institutions animals were housed under specific pathogen-free conditions in individual ventilated cages. Both male and female mice were used in all experiments. All experimental procedures were approved and were performed according to the guidelines of the University of Vanderbilt IACUC, Instituto de Ciencia y Tecnología Dr. César Milstein IACUC, Facultad de Ciencias Exactas y Naturales-UBA IACUC and INFANT Committee. In all experiments four to six mice were used per treatment group.
C57BL/6 mice were inoculated intraperitoneally (i.p.) with 5 × 105 PFU of DENV-1, DENV-2, or C6/36 cell supernatant (placebo). After 60 days mice were challenged i.p. with 1 × 106 PFU of DENV-2.
2.4. Measurement of Hematological and Biochemical Parameters
Blood samples were obtained from the facial vein of mice, collected in tubes containing K2-EDTA (Wiener lab), and after centrifugation at 2000 × g for 10 min at room temperature, plasma samples were separated. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatine kinase (CK), and lactate dehydrogenase (LDH) were measured by UV- or -kinetics method at different days post-secondary infection with DENV-2 (0, 4, and 7 days) using an Architect 8100 chemistry analyzer (Abbott Laboratories). Platelet counts were measured by microscopy on a CELLDYN 3700 (Abbott Laboratories) according to the manufacturer's instructions.
Increase of liver enzymes was defined as ALT and/or AST plasma values significantly above the ones obtained for control mice. Platelet counts significantly lower than the value obtained for control mice were also considered a primary outcome of disease. Plasma values of CK, LDH and ALP significantly higher than the ones obtained for control mice were considered secondary outcomes of disease. All normal laboratory values for C57BL/6 mice were obtained from the Comparative Pathology Laboratory, Vanderbilt University Medical Center, and the Mouse Phenome Database at The Jackson Laboratory.
2.5. Histology
Mice were euthanized by cervical dislocation 7 days post-secondary DENV-2 infection and tissues (liver, kidney, and spleen) were harvested and immediately fixed in 10% formalin in PBS. Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
2.6. Measurement of Bleeding Time
Measurement of bleeding time in mice was performed as previously described (Weiss et al., 2002). Seven days post-secondary DENV-2 infection, mice were anesthetized with an intraperitoneal injection of a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg of body weight, respectively), and 5 mm of the distal tip of the tail was amputated. The tail was blotted with filter paper every 5 s, and the bleeding time was recorded as the time at which the tail no longer stained the paper. Bleeding time ranges vary considerably in the literature. Therefore, we defined prolonged bleeding time in the study group as values significantly exceeding the values obtained in control mice.
2.7. Vascular Permeability Assay
Vascular leakage was assessed using Evans blue dye as a marker for albumin extravasation as previously described (Shresta et al., 2006), with some modifications. Briefly, 7 days post-secondary DENV-2 infection C57BL/6 mice were anesthetized with an intraperitoneal injection of a mixture of ketamine and xylazine and 0.2 ml of Evans blue (0.5% [w/v] in PBS) (Sigma Aldrich) was injected intravenously into the anesthetized mice. Two hours after injection, the animals were euthanized and extensively perfused with sterile PBS (4 ml), the spleen, kidney and liver were then harvested and placed into pre-weighed tubes containing formamide (Sigma-Aldrich, 2 ml/g tissue). Samples were incubated at 55 °C for 2.5 h. Evans blue concentrations in formamide extracts were subsequently quantitated by measuring absorbance at 610 nm. A calibration curve with 2-fold serial dilutions of Evans blue standards in formamide was performed in parallel. Data were expressed as ng Evans blue per g of tissue.
2.8. Quantitation of Virus in Blood and Tissues by Real Time RT-PCR
Blood was extracted at different times (0, 6, 18, 24, 48, 72, 96 and 168 h) after DENV infection and collected in tubes containing K2-EDTA (Wiener lab.). RNA was extracted from plasma samples using QIAamp Viral RNA Minikit (QIAGEN) according to the manufacturer's instructions.
After blood extraction at the corresponding post-infection times, mice were euthanized by cervical dislocation. The lymph nodes (mesenteric, periportal and celiac) as well as a portion of the tissues (spleen, liver, kidney) were immediately snap-frozen in tubes containing a mixture of dry ice/ethanol and later stored at − 80 °C. Total RNA was extracted from tissues using TRIzol (Invitrogen) according to the manufacturer's instructions.
The amount of viral RNA in blood and tissues was quantified by using StepOnePlus Real-Time PCR System (Applied Biosystems) employing TaqMan technology. The primers and probe used are targeted to amplify nucleotides 10,589 to 10,699 within the viral 3′UTR (Callahan et al., 2001). Beta-actin was used as endogenous control. Standard curves were generated using 10-fold serial dilutions of viral RNA obtained from purified DENV-1 or DENV-2 suspensions.
2.9. Characterization of Antibody Response
Antibodies against DENV were measured in serum on days 0, 30, 45 and 60 after primary DENV infection and on days 0 (60 days post-initial inoculation), 10, 18 and 25 after the DENV-2 challenge. Initially, sera were tested for IgG antibodies to DENV-1 and DENV-2 E proteins using E protein-specific immunoassays, and for neutralizing antibodies to DENV-1 and DENV-2 by 50% plaque reduction neutralization titer (PRNT50) assay.
2.10. DENV E Protein-specific IgG Immunoassays
The purification of DENV envelope (E) protein ectodomains was performed as previously described (Talarico et al., 2013). Protein-specific immunoassays were conducted similarly to those described in previous publications (Delgado et al., 2009, Polack et al., 2003). Briefly, ninety-six well plates were coated with 400 ng/ml of DENV-1 or DENV-2 E proteins and incubated at 37 °C for 2 h followed by an overnight incubation at 4 °C. After washing with PBS containing 0.05% Tween-20, wells were blocked with 5% skim milk in PBS for 1 h at 37 °C. Subsequently, 2-fold serial dilutions of serum samples were added to wells and incubated for 1 h at 37 °C. After washing, the secondary antibody (goat anti-mouse HRP-conjugated IgG, KPL) was added. After 1 h incubation at 37 °C, TMB substrate (BD) was added to wells. The reaction was stopped with the addition of H2SO4 1 M and optical density (O.D.) was measured at 450 nm.
2.11. Neutralization Assay
To determine anti-DENV neutralizing antibodies in sera from DENV-1 and DENV-2 immunized mice, a plaque reduction neutralization assay against DENV serotypes was performed as previously described, with some modifications (Talarico et al., 2013). Briefly, mice sera were heat-inactivated at 56 °C for 30 min and four-fold serial dilutions of the serum samples (1/20 to 1/5120 in MEM) were mixed with an equal volume containing 100 PFU of DENV-2 in MEM containing 2% FBS. The mixtures were incubated at 37 °C for 1 h and then transferred to Vero cells plated in 24-well plates. After 1 h incubation at 37 °C, cells were overlaid with MEM 2% FBS containing 1% methylcellulose (Sigma) and further incubated at 37 °C for 7 days. Cells were fixed with formaldehyde 10%, stained with crystal violet 10% and viral plaques were counted. Neutralization titers were determined as the reciprocal of the highest serum dilution yielding a 50% reduction in viral plaques (PRNT50).
2.12. B Cell Depletion in Mice
C57BL/6 mice were infected with 5 × 105 PFU of DENV-1 or DENV-2 via f.p. injection. On day four the ipsilateral popliteal lymph nodes in the hind legs corresponding to the inoculation site were removed. Control mice were infected with DENV-1 via f.p. injection and underwent mock surgery or were inoculated with C6/36 cell supernatant (placebo) f.p. Briefly, C57BL/6 mice were anesthetized using an intraperitoneal injection of a mixture of ketamine and xylazine. After depilation and skin antisepsis, the ipsilateral popliteal lymph nodes were removed. After surgery enrofloxacin (30 mg/kg) was administered every 24 h for 3 days as prophylaxis and tramadol (30 mg/kg i.p.) was administered every 24 h for 4 days as an analgesic. Post-surgical wound healing and behavior was monitored. Sixty days after primary infection all mice were challenged i.p. with 1 × 106 PFU of DENV-2. The efficacy of B cell depletion was tested by DENV-specific IgG immunoassay.
2.13. T Cell Depletion in Mice
C57BL/6 mice were infected i.p. with 5 × 105 PFU of DENV-1 and were administered anti-CD8 mAb (Clone 2.43 (anti-Lyt2.2), BioXCell Cat# BE0061 RRID:AB_1125541) and/or anti-CD4 mAb (Clone Gk1.5 (anti-L3T4), BioXCell Cat# BE0003–1 RRID:AB_1107636) i.p. three days before DENV-2 challenge (daily doses of 100 μg of mAb on days 57, 58, and 59 post-primary infection). C57BL/6 control mice were inoculated i.p. with an isotype control mAb, using the same inoculation scheme. CD8+ and CD4+ T cell depletion was confirmed by flow cytometry using anti-CD8 FITC and anti-CD4 FITC antibodies (BD Biosciences). Sixty days after primary infection with DENV-1, all mice were challenged i.p. with 1 × 106 PFU of DENV-2.
2.14. Adoptive Transfer of CD4+ or CD8+ T Cells From DENV Infected C57BL/6 Mice and Subsequent DENV Infection
For the T cell adoptive transfer experiments, C57BL/6 mice were infected i.p. with DENV-1 or DENV-2. On day sixty post-infection, spleens were removed following euthanasia, splenocytes were separated using a Ficoll gradient (GE Healthcare) and then CD4+ or CD8+ T lymphocytes were isolated using magnetic microbeads (Miltenyi Biotec). The purity of cells was assessed by flow cytometry. CD4+ or CD8+ T cells were transferred to naïve C57BL/6 mice via tail vein injection (107 cells/mouse), and 24 h later all mice were challenged i.p. with 1 × 106 PFU of DENV-2. Control C57BL/6 mice were transferred i.v. with sterile saline solution (placebo) and challenged i.p. with DENV-2.
2.15. Adoptive Transfer of CD8+ T Cells From DENV Infected C57BL/6 or TNF-α −/− Mice and Subsequent DENV Infection
C57BL/6 and TNF-α −/− mice were infected i.p. with DENV-1. Sixty days later, spleens were removed following euthanasia, splenocytes were separated using a Ficoll gradient (GE Healthcare) and CD8+ T lymphocytes were isolated using magnetic microbeads (Miltenyi Biotec). CD8+ T cells from C57BL/6 and TNF-α −/− mice were transferred to CD8α −/− mice via tail vein injection, and 24 h later all mice were infected i.p. with DENV-2.
2.16. Granzyme B Production in Supernatants From Stimulated Splenocytes
C57BL/6 mice were infected i.p. with DENV-1, DENV-2, RSV or C6/36 cell supernatant (placebo) and after 60 days mice were challenged i.p. with DENV-2. On day seven post-secondary infection with DENV-2, spleens were removed following euthanasia and splenocytes were separated using a Ficoll gradient (GE Healthcare). Spleen mononuclear cells (1 × 106 cells) were incubated per well in 200 μl of RPMI (GIBCO) with 10% FBS in the presence of UV-irradiated DENV-2 (MOI equivalent of 0.5), or RPMI 10% FBS alone. After 72 h of incubation at 37 °C supernatant fluids were removed and assayed for granzyme B release by using an immunoassay (eBioscience).
2.17. Statistical Analysis
Bar, XY graphs and statistical analyses (mean, standard error, and statistical tests) were generated with GraphPad Prism. The one-way ANOVA and Bonferroni's multiple comparison post-test correction was used to determine statistical significance among multiple groups. The Student's un-paired t-test was used to evaluate differences between two treatment groups. Statistical significance is depicted in figures: *p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. A Mouse Model of Secondary DENV Infection
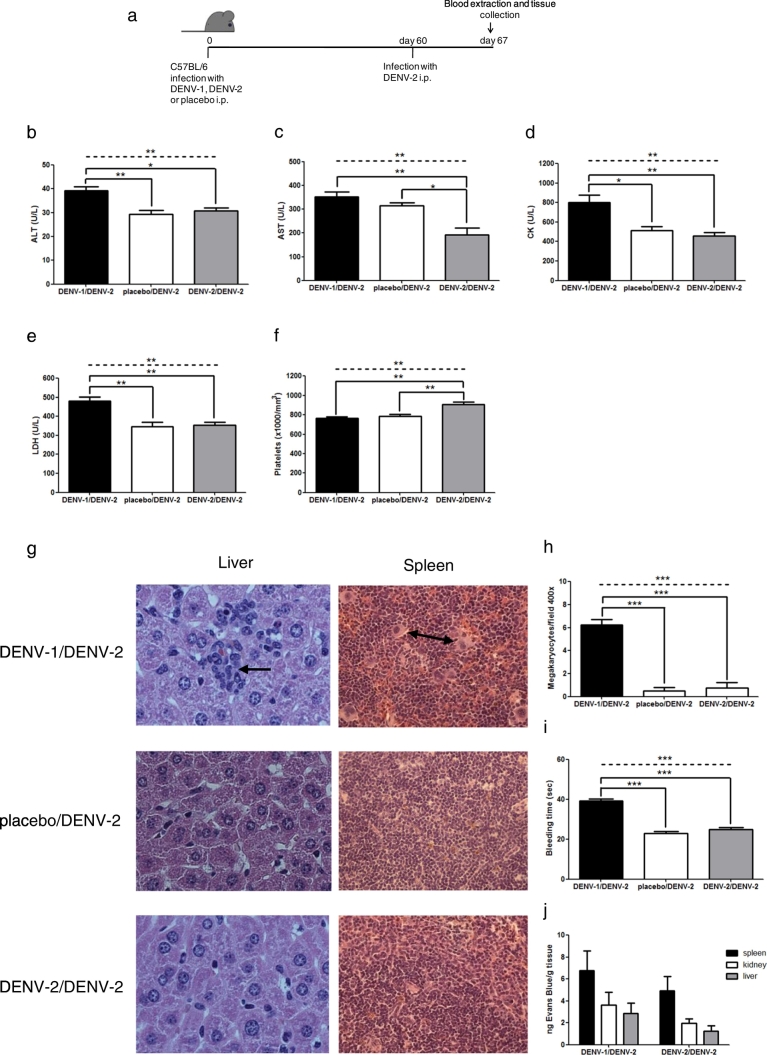
First, C57BL/6 mice were infected i.p. with 5 × 105 PFU of DENV-1 (a human strain isolated from Puerto Rico in 1994; DENV-1 PR/94). Control mice were infected with 5 × 105 PFU of DENV-2 (a human strain isolated in Tonga in 1974; DENV-2 Tonga/74) or inoculated with C6/36 cell supernatant as placebo. Sixty days after primary inoculation, all mice were challenged i.p. with 1 × 106 PFU of DENV-2 (strain Tonga/74) (Fig. 1a). Hematological and biochemical parameters as well as histopathology and vascular permeability in heterotypic DENV-infected mice were compared to control mice at 7 days post-secondary infection. This time-point was selected because secondary antibody responses and cytotoxic T lymphocyte (CTL) responses against DENV-2 were high seven days post-infection, and CTLs decreased thereafter (Suppl. Fig. S1 and Fig. 4d). Mice infected with DENV-1 followed by DENV-2 developed greater increase of liver enzymes (Fig. 1b, c), elevated CK and LDH levels in plasma (Fig. 1d, e), lower platelet counts (Fig. 1f) with a marked increase in megakaryocytes in the spleen (Fig. 1g, h) and hematopoietic centers in the liver (Fig. 1g), prolonged bleeding time (Fig. 1i) and internal hemorrhages evidenced by hemosiderin-laden macrophages in the spleen (Suppl. Fig. S2a) compared to both control groups (except in AST and platelet count, where significant differences were observed only with homotypic infection). A trend towards increased vascular permeability in spleen, kidney and liver was observed in DENV-1/DENV-2 sequentially infected mice, compared to those that received homotypic infection (Fig. 1j); however, no rise in hematocrit was detected in DENV heterotypic infected mice compared to control groups (Suppl. Fig. S2b).
Fig. 1.
A mouse model of DENV secondary infection. (a) C57BL/6 mice were infected i.p. with DENV-1, cell supernatant (placebo) or DENV-2 and 60 days later, challeged i.p. with DENV-2. (b–f) Hematological and biochemical parameters at 7 days post-secondary infection in mice infected sequentially with DENV-1/DENV-2, placebo/DENV-2, and DENV-2/DENV-2. (g) Histopathology showing hematopoietic centers in liver (arrow) and megakaryocytes in spleen (arrow) of mice infected with DENV-1/DENV-2 compared to mice infected with placebo/DENV-2, and those receiving two doses of DENV-2. Magnification: 400 ×. H&E staining. (h, i) Scores for megakaryocytes and bleeding time. (j) Mice immune against DENV-1 or DENV-2 and later infected with DENV-2 received Evans Blue 7 days post-secondary infection, and 2 h later the dye was extracted from tissues with formamide and absorbance was measured at 610 nm. ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; LDH: lactate dehydrogenase. Values represent means ± SEM (n = 4–6/group). Two independent experiments were performed that showed similar results. Statistical significance between groups using one-way ANOVA is depicted with a dashed line at the top of the graph, and Bonferroni's multiple comparison post-test is indicated with solid lines connecting two bars (*p < 0.05, **p < 0.01, ***p < 0.001).
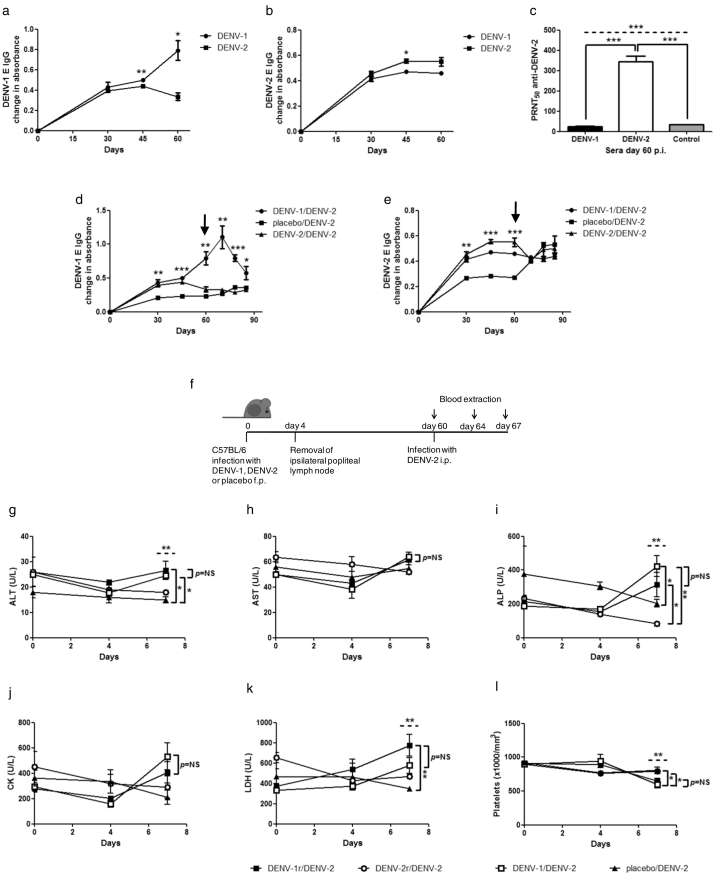
Fig. 4.
Characterization of DENV antibodies elicited after DENV primary or secondary infection and role of B lymphocytes in DENV secondary infection. (a) IgG anti-DENV-1 E, (b) IgG anti-DENV-2 E and (c) neutralization (PRNT50) against DENV-2 in sera from DENV-1 and DENV-2 i.p. infected C57BL/6 mice. (d) IgG anti-DENV-1 E and (e) IgG anti-DENV-2 E in sera from DENV-1, cell supernatant (placebo) or DENV-2 i.p. infected mice, challenged i.p. with DENV-2 at day 60 p.i. Arrows indicate DENV-2 challenge at day 60 p.i. (f–l) C57BL/6 mice were infected f.p. with DENV-1, DENV-2 or cell supernatant (placebo), resected of the ipsilateral popliteal lymph nodes 4 days later (DENV-1r, DENV-2r) and challenged with DENV-2 i.p. 60 days post-primary infection. (g–l) Hematological and biochemical parameters at different times post-secondary infection with DENV-2 (0, 4, and 7 days). ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; CK: creatine kinase; LDH: lactate dehydrogenase. Values represent means ± SEM (n = 4–6/group). Two independent experiments were performed that showed similar results. NS, non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, determined by (a, b) Student's un-paired t-test or (c–e, g–l) one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line). (g–l) p = NS for all DENV-1/DENV-2 vs. DENV-1r/DENV-2 groups.
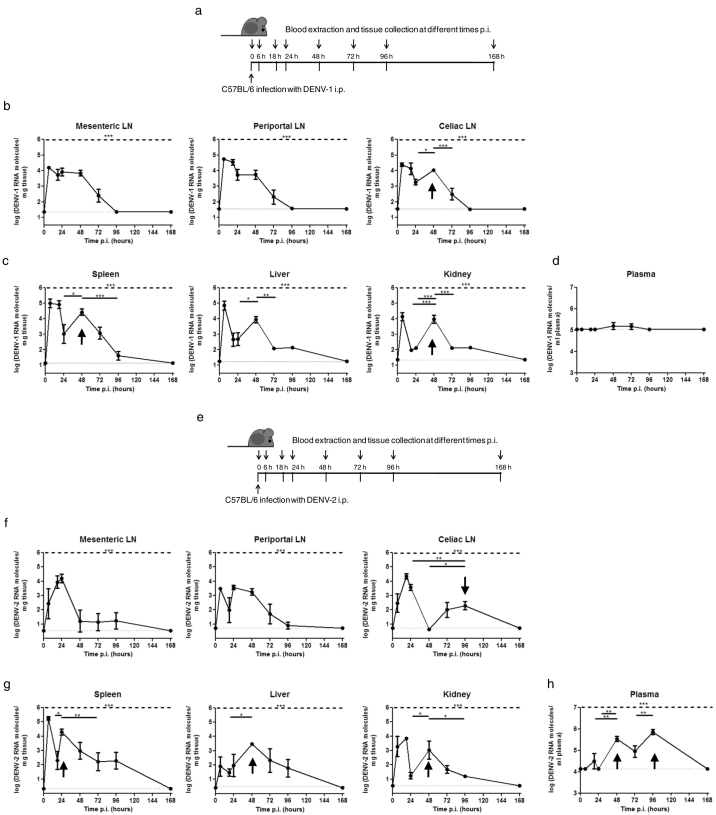
After primary infection with DENV-1 i.p. (Fig. 2a-d), viral RNA was detected in the mesenteric, periportal and celiac lymph nodes from 6 to 72 h p.i. Viral RNA levels in mesenteric and periportal lymph nodes were high during the first 48 h, subsequently decreasing over time (Fig. 2b). Viral RNA was also detected in the spleen, liver and kidney for 96 h (Fig. 2c). In these tissues and celiac lymph nodes, DENV RNA levels peaked around 48 h p.i., suggesting transient replication (see arrow). Viremia levels were low to undetectable up to 168 h p.i. with DENV-1 i.p. (Fig. 2d). These results suggest that DENV-1 reached the lymph nodes by i.p. inoculation and spread quickly to solid organs, where it was able to sustain transient replication. When mice were infected with DENV-2 i.p. (Fig. 2e–h), viral RNA was found in lymph nodes up to 96 h p.i. (Fig. 2f). As with DENV-1 primary infection, transient replication was found in tissues and celiac lymph nodes with peak viral RNA levels around 24 to 96 h p.i. (Fig. 2f, g; see arrow). Unlike DENV-1, DENV-2 plasma levels were higher and peaked between 48 and 96 h p.i., decreasing thereafter (Fig. 2h).
Fig. 2.
Virus detection by qRT-PCR in tissues and plasma after primary DENV-1 and DENV-2 infection. C57BL/6 mice were infected with (a, b, c, d) DENV-1 or (e, f, g, h) DENV-2 i.p., and at different times p.i. (0, 6, 18, 24, 48, 72, 96, and 168 h), mice were euthanized and (b, f) draining lymph nodes (LN) (mesenteric, periportal and celiac), (c, g) organs (spleen, liver and kidney) and (d, h) plasma were collected. Total RNA was extracted from tissues and DENV RNA was detected by qRT-PCR. Arrows indicate transient viral replication. LN: lymph node. Values represent means ± SEM (n = 6–8/group). Two independent experiments were performed that showed similar results. *p < 0.05, **p < 0.01, ***p < 0.001 determined by one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line). The statistical significance of post-test comparisons is only depicted for the DENV RNA peaks that suggest transient replication. Dotted lines show limits of detection.
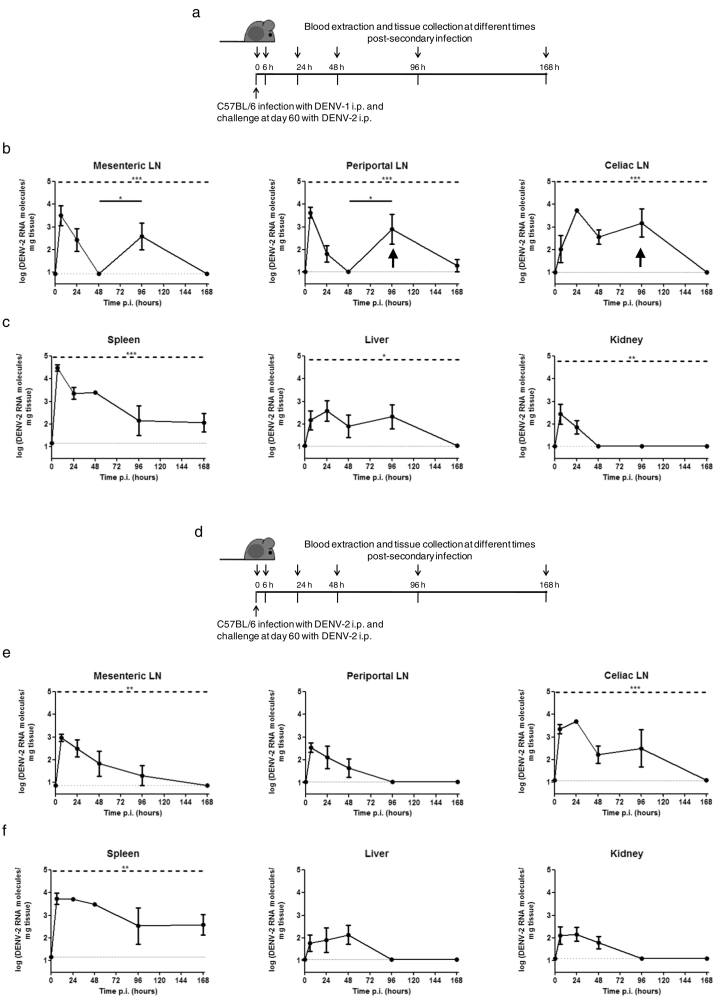
When mice were challenged with DENV-2 60 days after primary infection, viral RNA was detected in mesenteric, periportal and celiac lymph nodes from 6 to 96–168 h post homotypic and heterotypic infections (Fig. 3b, e). DENV-2 RNA initially peaked at 6–24 h in both groups. In heterotypic infections, viral RNA levels peaked again in mesenteric and periportal lymph nodes, suggesting transient replication (Fig. 3b; see arrow). Conversely, RNA levels decreased steadily in homotypic infections (Fig. 3e).
Fig. 3.
Virus detection by qRT-PCR in tissues after secondary homotypic and heterotypic DENV infection. C57BL/6 mice were infected i.p. with (a, b, c) DENV-1 or (d, e, f) DENV-2 and 60 days later, challeged i.p. with DENV-2. At different times post secondary infection (0, 6, 24, 48, 96, and 168 h), mice were euthanized and (b, e) draining lymph nodes (LN) (mesenteric, periportal and celiac) and (c, f) organs (spleen, liver and kidney) were collected. Total RNA was extracted from tissues and DENV-2 RNA was detected by qRT-PCR. Arrows indicate transient viral replication. LN: lymph node. Values represent means ± SEM (n = 6–8/group). Two independent experiments were performed that showed similar results. *p < 0.05, **p < 0.01, ***p < 0.001 determined by one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line). The statistical significance of post-test comparisons is only depicted for the DENV RNA peaks that suggest transient replication. Dotted lines show limits of detection.
DENV-2 RNA was also found in spleen, liver and kidney up to 48–168 h after homotypic and heterotypic infections (Fig. 3c, f), exhibiting lower RNA levels in liver and kidney compared to spleen. After initial increase in viral RNA due to input virus, viral RNA in spleen subsequently decreased and maintained low to moderate levels up to 168 h in both homotypic and heterotypic infections. Interestingly, moderate DENV-2 RNA levels in liver were maintained up to 96 h in heterotypically infected mice, while a steady decrease in viral RNA level was observed in homotypically challenged mice. Unlike primary infection, DENV-2 plasma levels were undetectable after secondary homotypic and heterotypic infections (data not shown).
These results indicate that DENV-1 strain PR/94 and DENV-2 strain Tonga/74 are able to sustain short-lived replication in C57BL/6 mice.
In order to characterize which cells were infected upon sequential DENV-1/DENV-2 infection, splenocytes were subjected to magnetic cell sorting using microbeads. DENV-2 RNA was found in CD11b+ and Ly-6G+ cells but not in T cells 48–96 h post-secondary infection, suggesting that macrophages and neutrophils are targets of DENV replication in C57BL/6 mice (Suppl. Fig. S3).
3.2. Other Heterologous DENV Challenge and Candidate DENV Strains for a Model of Severe Disease
The sequence of DENV serotype infections has been reported to influence disease severity (Fried et al., 2010). In fact, certain DENV serotypes, but not others, associate with severe manifestations in secondary infections (Anantapreecha et al., 2005). Therefore, we investigated if DENV-1 infection was altered by prior DENV-2 infection in C57BL/6 mice. Hematological and biochemical parameters in mice infected sequentially with DENV-2 followed by DENV-1 were not significantly different from those of homotypic DENV-1/DENV-1 and placebo/DENV-1 infected mice (Suppl Fig. S4). Furthermore, no histological alterations were detected in the three groups of mice (data not shown). These results suggest that DENV-2 priming does not alter the disease phenotype produced by secondary DENV-1 infection in our model.
Beyond secondary heterologous DENV infection, viral strains may also affect DENV pathogenesis (Kouri et al., 1989, Rico-Hesse, 2003). We therefore compared in our mouse model the effect of different DENV-2 strains. Interestingly, minimal to no signs of severe dengue disease were observed in wt mice sequentially infected with DENV-1 PR/94 followed by DENV-2 strains NGC, mouse-adapted and neurovirulent (Gruenberg et al., 1988), 16681, initially isolated from an hemorrhagic fever patient and subsequently passaged in cell cultures (Kinney et al., 1997), and D2S10, peripherally mouse-adapted by alternately passaged in mice and mosquito cells (Shresta et al., 2006) (data not shown).
Furthermore, in order to determine whether the disease phenotype observed in heterotypic DENV-1/DENV-2 infected mice was DENV-specific, we immunized mice with an unrelated virus before DENV-2 infection. We observed that mice sequentially infected with RSV followed by DENV-2 did not present significant differences in hematological and biochemical parameters compared to both homotypic DENV-2/DENV-2 and placebo/DENV-2 infected mice (Suppl. Fig. S5). Additionally, no histopathological alterations were found in these three groups of mice (data not shown), suggesting that the disease signs observed in DENV-1/DENV-2 infected mice are DENV-specific.
Overall, these results suggest that DENV-1 PR/94 followed by DENV-2 Tonga/74 may share cross-reactive determinants -absent in other combinations- that are important in eliciting signs of severe dengue disease in C57BL/6 mice, as certain sequential infection are more severe in humans.
3.3. Cross-reactivity and Neutralization of Antibodies Elicited After DENV Infection
We then sought to determine the specificity and biological activity of homotypic and heterotypic antibodies against DENV after primary infection in C57BL/6 mice. We examined the serotype-specific and cross-reactive antibody responses elicited by DENV-1 and DENV-2 by immunoassay using purified E proteins from DENV-1 and DENV-2. DENV-1 infection led to a long-lived IgG response against DENV-1 E, while the heterotypic response elicited by DENV-1 against DENV-2 E plateaued at 45 days (Fig. 4a, b). Conversely, primary infection of mice with DENV-2 elicited a low response against DENV-1 E that decreased at day 60 and a slightly higher response against DENV-2 E, which contained neutralizing antibodies (PRNT50 343 ± 27 against DENV-2) (Fig. 4a-c). Heterologous DENV-2 infection of DENV-1-immune mice was associated with an early secondary rise in anti-DENV-1 E IgG (à la original antigenic sin), which peaked on day 70 (day 10 post-challenge) and subsequently decreased (Fig. 4d). The DENV-2 E IgG response in DENV-2/DENV-2 sequentially infected mice did not increase after challenge with DENV-2 on day 60 (Fig. 4e).
3.4. Antibodies in the Pathogenesis of Severe Dengue Disease in Mice
To investigate whether antibodies were critical for the pathogenesis of severe dengue disease, C57BL/6 mice received DENV-1 and DENV-2 via footpad (f.p.) and were depleted of primed B cells by resection of ipsilateral popliteal lymph nodes 4 days post-primary infection (Fig. 4f). This strategy abrogates the homotypic and heterotypic B cell responses (Suppl. Fig. S6), while maintaining a primed T cell response that is generated before lymph node resection (Delgado et al., 2009, Finke and Acha-Orbea, 2001) (Suppl. Fig. S1). Two additional groups of mice that initially received DENV-1 f.p. and mock surgery, or C6/36 cell supernatant via f.p. also served as controls. Sixty days after primary infection, all groups were challenged with DENV-2 i.p. Hematological and biochemical parameters were analyzed at 0, 4 and 7 days post-challenge with DENV-2 and compared between groups (Fig. 4g–l). Outcomes in DENV-1-primed B cell-depleted mice were not significantly different from those of DENV-1/DENV-2-infected, non-depleted mice. Similar to non-depleted mice with sequential DENV-1/DENV-2 infections, these depleted animals had significantly higher plasma levels of ALT, ALP and LDH (Fig. 4g, i, k) and a trend towards increased levels of CK (Fig. 4j) and lower platelets (Fig. 4l) than control mice inoculated with placebo followed by DENV-2 infection or DENV-2-primed B cell-depleted mice infected with DENV-2. No differences were found in AST levels between the four groups of mice (Fig. 4h). These results suggest that signs of severe disease in mice can occur in the absence of primed B cells.
3.5. Heterotypic Anti-DENV-1 CD8+ T Lymphocytes are Critical for Signs of Severe Dengue Disease in Mice
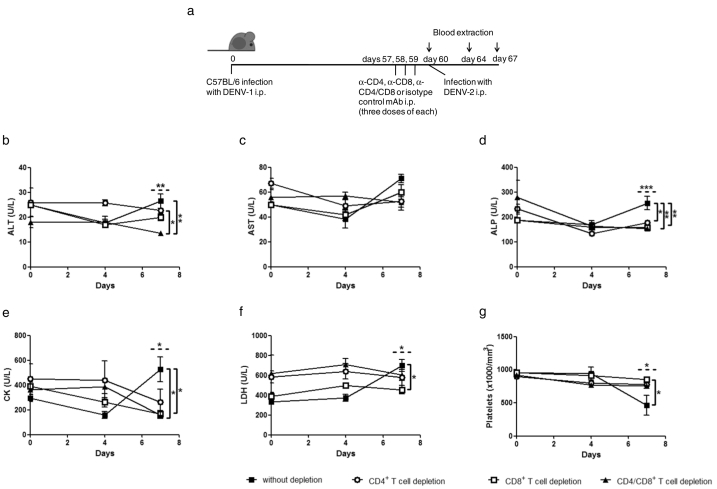
A role for heterotypic T cells in the pathogenesis of dengue has been suggested by several investigators (Friberg et al., 2011b, Mongkolsapaya et al., 2006, Yacoub et al., 2013), and CD4+ and CD8+ T lymphocytes that are cross-reactive among DENV serotypes have been described in both humans and mice (Beaumier and Rothman, 2009, Friberg et al., 2011a, Rivino et al., 2013, Zompi et al., 2012). Therefore, we next explored the role of CD8+ and CD4+ T cells in severe dengue disease. We compared outcomes in DENV-1-immune C57BL/6 mice depleted of CD8+ and/or CD4+ T lymphocytes vs. DENV-1-immune C57BL/6 mice inoculated with a control irrelevant mAb. After depletion, all mice were challenged i.p. with DENV-2 (Fig. 5a). Interestingly, seven days post-challenge, non-depleted mice had significantly higher levels of ALT, ALP, CK and LDH in plasma than mice depleted of CD8+ and/or CD4+ T lymphocytes (Fig. 5b, d-f). Differences in all tested biochemical parameters appeared to be greater between control mice and the CD8+ T cell-depleted group than between control and CD4+ T cell-depleted mice. Furthermore, control, but not CD8+ and/or CD4+ T cell-depleted mice, exhibited significantly lower platelet counts at seven days post-challenge with DENV-2 (Fig. 5g). These findings suggest that CD8+ T lymphocytes play a critical role in dengue pathogenesis in mice with a secondary role for CD4+ T cells.
Fig. 5.
Role of heterotypic DENV-1-specific CD4+ and CD8+ T lymphocytes in DENV-2 secondary infection. (a) C57BL/6 mice were infected with DENV-1 and 57 days later were passively transferred with anti-CD4+ and/or anti-CD8+ or isotype control mAb i.p. (three daily doses of mAb) in order to deplete T cell populations. Twenty four hours later, all mice were challenged with DENV-2 i.p. (b–g) Hematological and biochemical parameters were measured at different days post-secondary infection with DENV-2 (0, 4, and 7 days). ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; CK: creatine kinase; LDH: lactate dehydrogenase. Values represent means ± SEM (n = 4–6/group). Two independent experiments were performed that showed similar results. *p < 0.05, **p < 0.01, ***p < 0.001, determined by one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line).
3.6. Heterotypic Anti-DENV-1 CD8+ T Lymphocytes are Sufficient for Manifestations of Severe Dengue Disease
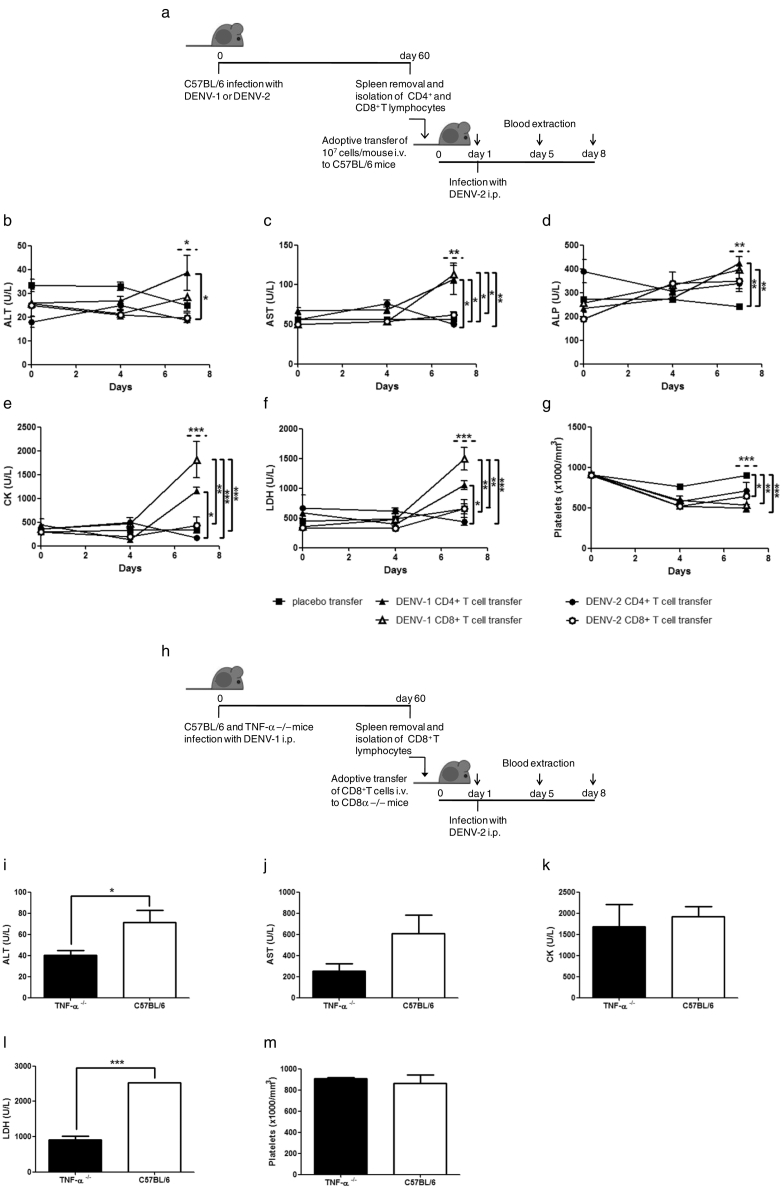
We then explored whether T cells are sufficient to elicit signs of severe dengue disease by adoptively transferring different subsets of primed T lymphocytes to naïve mice. DENV-1-immune or DENV-2-immune CD4+ or CD8+ T lymphocytes were transferred to naive C57BL/6 mice, and mice were then infected with DENV-2 (Fig. 6a). Mice receiving DENV-1-immune CD4+ or CD8+ T lymphocytes exhibited higher plasma levels of ALT, AST, ALP, CK and LDH at seven days post-secondary infection compared to control naïve mice and recipients of adoptively transferred DENV-2-immune CD4+ or CD8+ T lymphocytes (Fig. 6b-f). Furthermore, a significant decrease in platelets was observed in mice receiving DENV-1-immune CD4+ or CD8+ T compared to control mice (Fig. 6g). These experiments suggest that both CD8+ and CD4+ T lymphocytes are sufficient to elicit certain signs of severe dengue disease. Moreover, a trend towards a greater effect on measured outcomes appeared to be associated with heterotypic CD8+ T lymphocytes than with CD4+ T lymphocytes.
Fig. 6.
Heterotypic DENV-1-specific CD8+ T lymphocytes are sufficient to elicit signs of severe dengue disease in DENV-2 infected mice. (a–g) C57BL/6 mice were transferred i.v. with CD4+ or CD8+ T cells from DENV-1 or DENV-2 infected mice, and 1 day later were infected with DENV-2 i.p. (b–g) Hematological and biochemical parameters were measured at different days post-secondary infection with DENV-2 (0, 4, and 7 days). (h–m) CD8α −/− mice were transferred with CD8+ T cells from DENV-1 infected TNF-α −/− or C57BL/6 mice, and 1 day later were challenged with DENV-2 i.p. (i–m) Hematological and biochemical parameters were measured at 7 days post-secondary infection with DENV-2. ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; LDH: lactate dehydrogenase. Values represent means ± SEM (n = 4–6/group). Two independent experiments were performed that showed similar results. *p < 0.05, **p < 0.01, ***p < 0.001, determined by (b–g) one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line) or (i–m) Student's un-paired t-test.
We further characterized the CD4+ T lymphocyte population by exploring interferon (IFN)-γ and interleukin (IL)-4 production, cell surface expression of activation markers (CD71, CD40L and inducible T cell costimulator (ICOS)) and lymphoproliferation. Mice infected with DENV-1 followed by DENV-2 exhibited a type 1 bias of the CD4+ T helper (Th) response due to absence of detection of IL-4 in plasma (Suppl. Fig. S7a, b), augmented expression of the activation markers CD71, CD40L and ICOS and increased T lymphocyte proliferation compared to control mice (Suppl. Fig. S7c, d).
3.7. The Production of TNF-α by Heterotypic Anti-DENV-1 CD8+ T Lymphocytes is Critical for Signs of Severe Dengue Disease
Several studies suggest a role for TNF-α in dengue pathogenesis, activating vascular endothelial cells and leading to vascular leakage (Carvalho et al., 2014, Kurane, 2007, Sierra et al., 2010). It is, however, unclear which cells are the source of TNF-α associated with disease progression. Activated monocytes/macrophages, dendritic cells and T cells are known to produce TNF-α in DENV infections (Chu et al., 2015, Chunhakan et al., 2015, Watanabe et al., 2015). Therefore, we investigated whether TNF-α production by heterotypic CD8+ T lymphocytes was critical for severe dengue disease. For this purpose, we conducted an adoptive transfer experiment using DENV-1-immune CD8+ T lymphocytes from TNF-α −/− mice and naïve CD8α −/− recipients. These mice were subsequently challenged with DENV-2. Control CD8α −/− mice received DENV-1-immune CD8+ T lymphocytes from C57BL/6 mice and were also challenged with DENV-2 (Fig. 6h). At seven days post-challenge, control CD8α −/− mice exhibited increase of liver enzymes and high levels of LDH in plasma compared to CD8α −/− mice that received DENV-1-immune CD8+ T lymphocytes from TNF-α −/− mice (Fig. 6i, j, l), suggesting that production of TNF-α by heterotypic CD8+ T lymphocytes is critical for these signs of severe dengue disease. No significant differences in CK plasma levels or platelet counts were detected between the two groups of mice (Fig. 6k, m).
3.8. Increased CTL Activity in Sequentially DENV-1/DENV-2 Infected Mice
The major mechanism of CTL-mediated destruction of target cells involves the delivery of cytolytic effector molecules, including perforin and granzymes. These proteins are stored in the cytoplasmic granules of CTLs and are released during CTL-mediated target cell lysis. Granzyme B is the main CTL granule component that is implicated in apoptosis of target cells (Lord et al., 2003). In order to gain some mechanistic insight into why heterotypic anti-DENV-1 CD8+ T lymphocytes were responsible for signs of severe dengue in DENV-2 infected mice, we measured CTL activity in mice splenocytes by detecting the release of granzyme B. Splenocytes were isolated from mice infected with DENV-1 followed by DENV-2 at 7 days post-secondary infection and were then exposed to DENV-2 antigen during 72 h. Control groups included mice sequentially infected with DENV-2/DENV-2, RSV/DENV-2 and placebo/DENV-2. Interestingly, splenocytes from mice infected sequentially with DENV-1/DENV-2 exhibited a significantly increased release of granzyme B upon stimulation with DENV-2 antigen compared to control groups (Fig. 7).
Fig. 7.
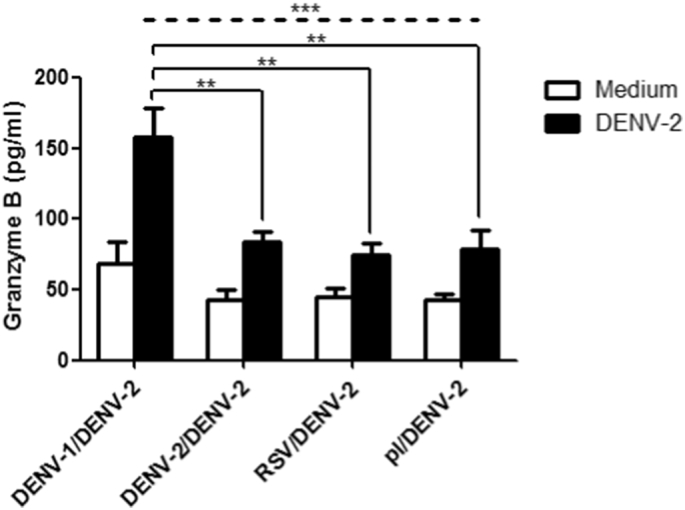
Increased granzyme B release from stimulated splenocytes from heterotypic DENV-2 infected mice. C57BL/6 mice were infected i.p. with DENV-1, DENV-2, RSV or C6/36 cell supernatant (placebo) and after 60 days mice were challenged i.p. with DENV-2. Seven days post-secondary infection, splenocytes were isolated, stimulated with UV-irradiated DENV-2 or medium and assayed for granzyme B release by immunoassay. Values represent means ± SEM (n = 5/group). Two independent experiments were performed that showed similar results. **p < 0.01, ***p < 0.001, determined by one-way ANOVA (dashed line) and Bonferroni's multiple comparison post-test (solid line).
4. Discussion
The mechanism of illness in severe dengue disease has been elusive and numerous hypotheses have been proposed in the DENV literature for decades. The challenge of establishing immunocompetent animal models reproducing disease signs has been a major hurdle to better understand DENV pathogenesis. In this study, we describe an immunocompetent mouse model of secondary DENV infection, in which DENV-1/DENV-2 sequentially infected mice develop increase of liver enzymes, bleeding and low platelet counts, key signs of severe dengue disease in patients (Kalayanarooj et al., 1997, Phuong et al., 2004, Sam et al., 2013).
Given that the vast majority of DHF/DSS cases result from secondary infections, theories on DENV pathogenesis are based on the presence of heterotypic antibody (Halstead, 2003) or T lymphocyte cross-reactivity (Rothman, 2010). In the first group, the most widely accepted theory attributes disease severity to ADE of heterologous viral replication in cells of the reticuloendothelial system. The potential pathogenic link between ADE and DHF/DSS remains an unsolved issue in DENV pathogenesis. In humans, some studies found an association between DENV titers and severity of illness (Vaughn et al., 2000), while others did not (Laoprasopwattana et al., 2005). Interestingly, DHF/DSS ensues after acute symptomatology subsides, suggesting an aberrant anamnestic immune response rather than direct effects from viral replication (Rothman, 2009). In vivo ADE has been shown in immunocompromised mouse models using mice deficient in both IFN-α/β receptor (Ifnar) and IFN-γ receptor (Ifngr) (Balsitis et al., 2010, Milligan et al., 2015, Zellweger et al., 2010) and in Ifnar −/− mice (Orozco et al., 2012, Zellweger et al., 2010). In our model, high levels of viral replication were not detected in tissues and plasma during disease, signs of severe dengue disease followed viral clearance, and B cell depletion did not alter disease phenotypes, suggesting that there are mechanisms different from enhanced viremia that contribute to disease.
Signs of exacerbated disease in our mice model were milder than those observed in humans during severe disease, due to the use of immunocompetent C57BL/6 mice that are semipermissive to DENV infection (Zellweger and Shresta, 2014, Zompi and Harris, 2012). DENV's ability to subvert human but not murine IFN-α/β (Ifna) signaling may be the major reason behind DENV's efficient replication in human but not in mouse cells (Ashour et al., 2010, Morrison and Garcia-Sastre, 2014). However, we found that DENV permissive cells in our model were CD11b+ cells but not T cells, similarly to what is observed in human target cells (Kou et al., 2008, Noisakran et al., 2010). Interestingly, DENV-2 RNA was also found in Ly-6G+ cells after sequential DENV-1/DENV-2 infection indicating that neutrophils are additional targets for DENV infection in C57BL/6 mice.
Other murine models of severe dengue disease use immunocompromised mice deficient in Ifnar and Ifngr (Balsitis et al., 2010, Milligan et al., 2015, Zellweger et al., 2010) or only Ifnar (Beatty et al., 2015, Orozco et al., 2012, Prestwood et al., 2012, Schmid and Harris, 2014), key components of the immune system present in humans during illness (De La Cruz Hernandez et al., 2014, Libraty et al., 2002, Nguyen et al., 2004), and a single DENV infection in the absence or presence of ADE. These models support robust DENV replication, and exhibit many signs of severe dengue disease.
Different DENV serotypes, and even strains of the same serotype, differ in their ability to cause severe disease (Fried et al., 2010, Nisalak et al., 2003, Rico-Hesse, 2003). In particular, DENV-2 has been associated with severe disease manifestations in reports from Thailand and Taiwan (Chen et al., 2007, Fried et al., 2010, Vaughn et al., 2000). Additionally, DENV-2 and DENV-4 in Thailand were more likely to produce DHF cases in secondary infections, while DENV-1 and DENV-3 appeared to elicit DHF in both primary and secondary infections (Anantapreecha et al., 2005). Furthermore, in Cuba and Thailand DENV-2 infections in DENV-1 exposed individuals associated with DHF manifestations (Guzman et al., 1991, Sangkawibha et al., 1984). In line with these reports, we observed that DENV-1/DENV-2 sequential infection in C57BL/6 mice led to exacerbated disease compared to homotypic DENV-2/DENV-2 and primary DENV-2 infections. Conversely, the reverse heterologous challenge DENV-2/DENV-1 did not alter disease phenotypes in comparison to controls.
In our mouse model of secondary DENV infection, we used non-mouse adapted DENV strains. DENV-1 PR/94 was isolated in 1994 in Puerto Rico and was reported to replicate efficiently in rhesus monkeys similarly to other DENV-1 strains (Blaney et al., 2007, Whitehead et al., 2003). DENV-2 Tonga/74 was isolated from a 1974 epidemic in the Pacific island of Tonga and was characterized by producing mild illness (Gubler et al., 1978). Interestingly, despite a lower level of virulence in comparison to other DENV-2 strains (Blaney et al., 2004), DENV-2 Tonga/74 infection in DENV-1 PR/94 immune C57BL/6 mice elicited signs of severe disease. These signs were absent with other combinations of more virulent mouse-adapted and non mouse-adapted DENV-2 strains. Our results suggest that factors different from viral virulence, such us sharing cross-reactive T cell determinants, are important in eliciting signs of severe disease.
Hypotheses postulating a role for heterotypic T lymphocyte cross-reactivity in the DENV pathogenesis are based on the “original antigenic sin” phenomenon (Midgley et al., 2011, Mongkolsapaya et al., 2003, Rothman, 2011). In the context of DHF/DSS, the expression “original antigenic sin” postulates that cross-reactive T cells raised against the first infecting DENV serotype predominate during a secondary DENV infection and show suboptimal degranulation capacity and high cytokine production which may contribute to the development of DHF/DSS signs (Duangchinda et al., 2010, Mongkolsapaya et al., 2006, Rothman, 2009).
Interestingly, a protective role for T cells in dengue disease, including heterotypic T cells, has also been reported in several studies, mainly in immunocompromised mouse models (Elong Ngono et al., 2016, Prestwood et al., 2012, Yauch et al., 2010, Yauch et al., 2009, Zellweger et al., 2014, Zellweger et al., 2015, Zompi et al., 2012). CD8+ T cell depletion prior to infection has been shown to increase viral load in various organs upon infection with DENV-2 in Ifnar−/− mice (Yauch et al., 2009). Similarly, depletion of CD4+ and CD8+ T lymphocytes reduced survival of DENV-2-primed BALB/c mice challenged with a lethal DENV-2 strain (Amorim et al., 2016). In line with these studies, a recent report described that cross-reactive T cells were able to enhance viral clearance in tissues from Ifnar−/− HLA*B0702 transgenic mice despite having lower magnitude and avidity than serotype-specific T cells (Elong Ngono et al., 2016). Furthermore, recent studies analyzing DENV-specific T cell responses in humans in a hyperendemic setting suggested an HLA-linked protective role for both CD4+ T and CD8+ T cells against DENV infection (Weiskopf et al., 2013, Weiskopf et al., 2015).
Therefore, the comprehension of the specific role of T cells in either protection against or pathogenesis of dengue disease is important for the safe development of preventive and therapeutic strategies (Ghosh and Dar, 2015). In our study, CD8+ and CD4+ T cell-depleted C57BL/6 mice did not develop signs of severe dengue disease, suggesting that heterotypic cross-reactive CD8+ and/or CD4+ T lymphocytes played a critical role in pathogenesis. Adoptive transfer experiments further suggested that CD8+ T lymphocytes are sufficient to elicit certain signs of severe dengue disease in mice and, to a lesser extent, CD4+ T lymphocytes also contribute to disease. Patients with DHF/DSS present increased levels of IFN-γ and TNF-α, compared to those with non-DHF/DSS (Pang et al., 2007). Our mice sequentially infected with DENV-1/DENV-2 also showed a Th1 bias. TNF-α is one of the major mediators of inflammation, induced by a wide range of pathogenic stimuli (Carvalho et al., 2014). Our results suggest that production of TNF-α by low affinity heterotypic anti-DENV CTL is a critical component of DENV pathogenesis, mainly affecting the liver manifestations of disease.
Despite the protective role for T cells in immunocompromised mouse models, various reports using immunocompetent mice showed that intrahepatic infiltration of activated immune cells positively correlated with DENV-induced liver damage, characterized by elevated liver enzymes (Chen et al., 2004, Franca et al., 2010, Paes et al., 2005). Sung et al. demonstrated that both splenic and intrahepatic CD8+ T cells in DENV-2 infected C57BL/6 mice recognized DENV epitopes, and that intrahepatic infiltrating CD8+ T cells were cytotoxic and caused liver cell death (Sung et al., 2012). In our mouse model, we observed that DENV-1/DENV-2 sequentially infected mice showed infiltration of immune cells in liver and that splenocytes from these mice exhibited increased cytotoxicity, characterized by augmented release of granzyme B, compared to control groups. We hypothesize that transient DENV infection of lymphoid tissues and extended maintenance of moderate viral RNA levels in solid organs may trigger the recruitment of immune cells to solid organs, mainly the liver. In this regard, the elevated cytotoxicity displayed by heterotypic anti-DENV-1 CD8+ T lymphocytes against DENV-2 infected hepatocytes may be responsible for the distinct disease phenotype detected in heterotypic DENV-1/DENV-2 infected mice, characterized by increased liver enzymes, LDH and CK. In fact, anti-DENV-1 CD8+ T cell increased cytotoxicity against DENV-2 antigen may be associated with cross-reactive determinants and warrants further investigation.
In our mouse model, DENV-1/DENV-2 sequentially infected C57BL/6 mice developed exacerbated disease characterized by increased plasma levels of liver enzymes, CK and LDH, decreased platelet counts and bleeding, accompanied by immune cell infiltration in liver and increased megakaryocytes in spleen. Plasma leakage, which is characterized by rising hematocrit, hypoalbuminemia, ascites or pleural effusion, has been originally reported as the pathophysiological hallmark that determines disease severity and that differentiates DHF and DSS from dengue fever (Nimmannitya, 1987). Even though a trend towards augmented vascular permeability was observed in spleen, kidney and liver in sequentially DENV-1/DENV-2 infected C57BL/6 mice compared to homotypic DENV-2/DENV-2 infected mice, no increase in hematocrit was detected. This aspect of severe dengue disease that is partially reproduced in our immunocompetent mouse model may reflect the semipermissive nature of mice to DENV, probably aligned with the inability of DENV to subvert the IFN pathway (Ashour et al., 2010, Morrison and Garcia-Sastre, 2014, Zellweger and Shresta, 2014, Zompi and Harris, 2012). Sequentially DENV-1/DENV-2 infected mice were more permissive for liver damage than for vascular leakage, important pathogenic outcome for severe dengue disease in humans, revealing a caveat of our mouse model compared to human disease. Thrombocytopenia and bleeding have also been observed in severe dengue patients and the mechanisms involved have been hypothesized to be related to DENV direct or indirect affection of bone marrow progenitor cells by inhibiting their function (de Azeredo et al., 2015). These signs of severe disease were observed in sequentially DENV-1/DENV-2 infected mice and appeared to be mediated by heterotypic anti-DENV-1 CD8+ T cells, by either cytotoxicity or TNF-α production.
In summary, we present an immunocompetent mouse model of DENV secondary infection that exhibits certain signs of severe dengue disease upon heterotypic infection with DENV-1 PR/94 followed by DENV-2 Tonga/74. Disease signs in this model were not dependent on viral burden or anti-DENV B lymphocytes. Conversely, cross-reactive CD8+ T lymphocytes and CD4+ T lymphocytes critically contributed to disease.
Our findings highlight the importance of CD4+ and CD8+ T lymphocyte responses in dengue pathogenesis and the need to further study these responses in human subjects.
Funding Sources
This work was funded by grants from UBS Optimus Foundation (ID 2007-00206), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 0118), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2008-1073) and Fundación Bunge y Born (Year 2010), Argentina. LBT, PLA, LII and EBD are members of Research Career from CONICET and ABB and YPR are fellows from the same institution.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
LBT and FPP conceived and designed experiments. LBT, JPB, ABB, JMB, AF, AGG, AM, CS, DRH, AL, PLA, MTC and GAM performed experiments. LBT, JPB, ABB, JMB, DRH, AL and FPP analyzed the data. LBT, YPR, LII, FAR, EBD, EH and FPP contributed with materials. LBT and ABB performed graphics and analyzed bibliography. LBT, EBD, EH and FPP wrote the manuscript.
Acknowledgments
We thank Dr. Steve Whitehead (NIAD, NIH, DHHS, USA) and Dr. Andrea Gamarnik (Fundación Instituto Leloir, Buenos Aires, Argentina) for the kind provision of viral strains.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.033.
Appendix A. Supplementary data
Supplementary material
References
- Amorim J.H., dos Santos Alves R.P., Bizerra R., Araujo Pereira S., Ramos Pereira L., Nascimento Fabris D.L., Santos R.A., Romano C.M., de Souza Ferreira L.C. Antibodies are not required to a protective immune response against dengue virus elicited in a mouse encephalitis model. Virology. 2016;487:41–49. doi: 10.1016/j.virol.2015.10.006. (pii) [DOI] [PubMed] [Google Scholar]
- Anantapreecha S., Chanama S., A-n A., Naemkhunthot S., Sa-Ngasang A., Sawanpanyalert P., Kurane I. Serological and virological features of dengue fever and dengue haemorrhagic fever in Thailand from 1999 to 2002. Epidemiol. Infect. 2005;133:503–507. doi: 10.1017/s0950268804003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J., Morrison J., Laurent-Rolle M., Belicha-Villanueva A., Plumlee C.R., Bernal-Rubio D., Williams K.L., Harris E., Fernandez-Sesma A., Schindler C. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8:410–421. doi: 10.1016/j.chom.2010.10.007. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azeredo E.L., Monteiro R.Q., de-Oliveira Pinto, L.M. Thrombocytopenia in dengue: interrelationship between virus and the imbalance between coagulation and fibrinolysis and inflammatory mediators. Mediat. Inflamm. 2015;2015:313842. doi: 10.1155/2015/313842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis S.J., Williams K.L., Lachica R., Flores D., Kyle J.L., Mehlhop E., Johnson S., Diamond M.S., Beatty P.R., Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyam H.S., Green S., Rothman A.L. Dengue virus-reactive CD8 + T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 2006;176:2817–2824. doi: 10.4049/jimmunol.176.5.2817. (pii) [DOI] [PubMed] [Google Scholar]
- Beatty P.R., Puerta-Guardo H., Killingbeck S.S., Glasner D.R., Hopkins K., Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa3787. (pii) [DOI] [PubMed] [Google Scholar]
- Beaumier C.M., Rothman A.L. Cross-reactive memory CD4 + T cells alter the CD8 + T-cell response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. Viral Immunol. 2009;22:215–219. doi: 10.1089/vim.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney J.E., Jr., Hanson C.T., Hanley K.A., Murphy B.R., Whitehead S.S. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 2004;4(39) doi: 10.1186/1471-2334-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney J.E., Jr., Sathe N.S., Hanson C.T., Firestone C.Y., Murphy B.R., Whitehead S.S. Vaccine candidates for dengue virus type 1 (DEN1) generated by replacement of the structural genes of rDEN4 and rDEN4Delta30 with those of DEN1. Virol. J. 2007;4:23. doi: 10.1186/1743-422X-4-23. (1743-422X-4-23 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan J.D., Wu S.J., Dion-Schultz A., Mangold B.E., Peruski L.F., Watts D.M., Porter K.R., Murphy G.R., Suharyono W., King C.C. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 2001;39:4119–4124. doi: 10.1128/JCM.39.11.4119-4124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho D.M., Garcia F.G., Terra A.P., Lopes Tosta A.C., Silva Lde A., Castellano L.R., Silva Teixeira D.N. Elevated dengue virus nonstructural protein 1 serum levels and altered toll-like receptor 4 expression, nitric oxide, and tumor necrosis factor alpha production in dengue hemorrhagic fever patients. J. Trop. Med. 2014;2014:901276. doi: 10.1155/2014/901276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.C., Lai S.Y., Sung J.M., Lee S.H., Lin Y.C., Wang W.K., Chen Y.C., Kao C.L., King C.C., Wu-Hsieh B.A. Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J. Med. Virol. 2004;73:419–431. doi: 10.1002/jmv.20108. [DOI] [PubMed] [Google Scholar]
- Chen R.F., Yang K.D., Wang L., Liu J.W., Chiu C.C., Cheng J.T. Different clinical and laboratory manifestations between dengue haemorrhagic fever and dengue fever with bleeding tendency. Trans. R Soc. Trop. Med. Hyg. 2007;101:1106–1113. doi: 10.1016/j.trstmh.2007.06.019. (S0035-9203(07)00232-5 [pii]) [DOI] [PubMed] [Google Scholar]
- Chu H., George S.L., Stinchcomb D.T., Osorio J.E., Partidos C.D. CD8 + T cell responses in Flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J. Infect. Dis. 2015 doi: 10.1093/infdis/jiv258. (jiv258 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunhakan S., Butthep P., Yoksan S., Tangnararatchakit K., Chuansumrit A. Vascular leakage in dengue hemorrhagic fever is associated with dengue infected monocytes, monocyte activation/exhaustion, and cytokines production. Int. J. Vasc. Med. 2015;2015:917143. doi: 10.1155/2015/917143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz Hernandez S.I., Puerta-Guardo H., Flores-Aguilar H., Gonzalez-Mateos S., Lopez-Martinez I., Ortiz-Navarrete V., Ludert J.E., Del Angel R.M. A strong interferon response correlates with a milder dengue clinical condition. J. Clin. Virol. 2014;60:196–199. doi: 10.1016/j.jcv.2014.04.002. (pii) [DOI] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P., Diaz L., Trento A., Chang H.Y., Mitzner W. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangchinda T., Dejnirattisai W., Vasanawathana S., Limpitikul W., Tangthawornchaikul N., Malasit P., Mongkolsapaya J., Screaton G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16922–16927. doi: 10.1073/pnas.1010867107. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A., Chen H.W., Tang W.W., Joo Y., King K., Weiskopf D., Sidney J., Sette A., Shresta S. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine. 2016;13:284–293. doi: 10.1016/j.ebiom.2016.10.006. (S2352-3964(16)30461-3 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke D., Acha-Orbea H. Differential migration of in vivo primed B and T lymphocytes to lymphoid and non-lymphoid organs. Eur. J. Immunol. 2001;31:2603–2611. doi: 10.1002/1521-4141(200109)31:9<2603::aid-immu2603>3.0.co;2-8. ([pii] 10.1002/1521-4141(200109)31:9<2603::AID-IMMU2603>3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- Franca R.F., Zucoloto S., da Fonseca B.A. A BALB/c mouse model shows that liver involvement in dengue disease is immune-mediated. Exp. Mol. Pathol. 2010;89:321–326. doi: 10.1016/j.yexmp.2010.07.007. (pii) [DOI] [PubMed] [Google Scholar]
- Friberg H., Bashyam H., Toyosaki-Maeda T., Potts J.A., Greenough T., Kalayanarooj S., Gibbons R.V., Nisalak A., Srikiatkhachorn A., Green S. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci. Report. 2011;1:51. doi: 10.1038/srep00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H., Burns L., Woda M., Kalayanarooj S., Endy T.P., Stephens H.A., Green S., Rothman A.L., Mathew A. Memory CD8 + T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol. Cell Biol. 2011;89:122–129. doi: 10.1038/icb.2010.61. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried J.R., Gibbons R.V., Kalayanarooj S., Thomas S.J., Srikiatkhachorn A., Yoon I.K., Jarman R.G., Green S., Rothman A.L., Cummings D.A. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Dar L. Dengue vaccines: challenges, development, current status and prospects. Indian J. Med. Microbiol. 2015;33:3–15. doi: 10.4103/0255-0857.148369. (doi: IndianJMedMicrobiol_2015_33_1_3_148369 [pii]) [DOI] [PubMed] [Google Scholar]
- Gibbons R.V., Vaughn D.W. Dengue: an escalating problem. BMJ. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg A., Woo W.S., Biedrzycka A., Wright P.J. Partial nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue virus type 2, New Guinea C and PUO-218 strains. J. Gen. Virol. 1988;69(Pt 6):1391–1398. doi: 10.1099/0022-1317-69-6-1391. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. The economic burden of dengue. Am.J.Trop. Med. Hyg. 2012;86:743–744. doi: 10.4269/ajtmh.2012.12-0157. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J., Reed D., Rosen L., Hitchcock J.R., Jr. Epidemiologic, clinical, and virologic observations on dengue in the Kingdom of Tonga. Am.J.Trop. Med. Hyg. 1978;27:581–589. doi: 10.4269/ajtmh.1978.27.581. [DOI] [PubMed] [Google Scholar]
- Guzman M.G., Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. (pii) [DOI] [PubMed] [Google Scholar]
- Guzman M.G., Kouri G., Bravo J., Soler M., Martinez E. Sequential infection as risk factor for dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) during the 1981 dengue hemorrhagic Cuban epidemic. Mem. Inst. Oswaldo Cruz. 1991;86:367. doi: 10.1590/s0074-02761991000300011. (doi: S0074-02761991000300011 [pii]) [DOI] [PubMed] [Google Scholar]
- Guzman M.G., Halstead S.B., Artsob H., Buchy P., Farrar J., Gubler D.J., Hunsperger E., Kroeger A., Margolis H.S., Martinez E. Dengue: a continuing global threat. Nat. Rev. Microbiol. 2010;8:S7–16. doi: 10.1038/nrmicro2460. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S.B. Immune enhancement of viral infection. Prog. Allergy. 1982;31:301–364. [PubMed] [Google Scholar]
- Halstead S.B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- Halstead S.B., Lan N.T., Myint T.T., Shwe T.N., Nisalak A., Kalyanarooj S., Nimmannitya S., Soegijanto S., Vaughn D.W., Endy T.P. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg. Infect. Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayanarooj S., Vaughn D.W., Nimmannitya S., Green S., Suntayakorn S., Kunentrasai N., Viramitrachai W., Ratanachu-eke S., Kiatpolpoj S., Innis B.L. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- Kinney R.M., Butrapet S., Chang G.J., Tsuchiya K.R., Roehrig J.T., Bhamarapravati N., Gubler D.J. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. doi: 10.1006/viro.1997.8500. (S0042-6822(97)98500-7 [pii]) [DOI] [PubMed] [Google Scholar]
- Kou Z., Quinn M., Chen H., Rodrigo W.W., Rose R.C., Schlesinger J.J., Jin X. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 2008;80:134–146. doi: 10.1002/jmv.21051. [DOI] [PubMed] [Google Scholar]
- Kouri G.P., Guzman M.G., Bravo J.R., Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull. World Health Organ. 1989;67:375–380. [PMC free article] [PubMed] [Google Scholar]
- Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 2007;30:329–340. doi: 10.1016/j.cimid.2007.05.010. (S0147-9571(07)00053-7 [pii]) [DOI] [PubMed] [Google Scholar]
- Laoprasopwattana K., Libraty D.H., Endy T.P., Nisalak A., Chunsuttiwat S., Vaughn D.W., Reed G., Ennis F.A., Rothman A.L., Green S. Dengue Virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J. Infect. Dis. 2005;192:510–519. doi: 10.1086/431520. (JID34061 [pii]) [DOI] [PubMed] [Google Scholar]
- Libraty D.H., Endy T.P., Houng H.S., Green S., Kalayanarooj S., Suntayakorn S., Chansiriwongs W., Vaughn D.W., Nisalak A., Ennis F.A. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 2002;185:1213–1221. doi: 10.1086/340365. (JID011089 [pii]) [DOI] [PubMed] [Google Scholar]
- Lord S.J., Rajotte R.V., Korbutt G.S., Bleackley R.C. Granzyme B: a natural born killer. Immunol. Rev. 2003;193:31–38. doi: 10.1034/j.1600-065x.2003.00044.x. (doi: 044 [pii]) [DOI] [PubMed] [Google Scholar]
- Martina B.E., Koraka P., Osterhaus A.D. Dengue virus pathogenesis: an integrated view. Clin. Microbiol. Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C.M., Bajwa-Joseph M., Vasanawathana S., Limpitikul W., Wills B., Flanagan A., Waiyaiya E., Tran H.B., Cowper A.E., Chotiyarnwong P. An in-depth analysis of original antigenic sin in dengue virus infection. J. Virol. 2011;85:410–421. doi: 10.1128/JVI.01826-10. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G.N., Sarathy V.V., Infante E., Li L., Campbell G.A., Beatty P.R., Harris E., Barrett A.D., Bourne N. A dengue virus type 4 model of disseminated lethal infection in AG129 mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125476. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsapaya J., Dejnirattisai W., Xu X.N., Vasanawathana S., Tangthawornchaikul N., Chairunsri A., Sawasdivorn S., Duangchinda T., Dong T., Rowland-Jones S. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003;9:921–927. doi: 10.1038/nm887. (pii) [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J., Duangchinda T., Dejnirattisai W., Vasanawathana S., Avirutnan P., Jairungsri A., Khemnu N., Tangthawornchaikul N., Chotiyarnwong P., Sae-Jang K. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J. Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. (pii) [DOI] [PubMed] [Google Scholar]
- Morrison J., Garcia-Sastre A. STAT2 signaling and dengue virus infection. JAKSTAT. 2014;3 doi: 10.4161/jkst.27715. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Lei H.Y., Nguyen T.L., Lin Y.S., Huang K.J., Le B.L., Lin C.F., Yeh T.M., Do Q.H., Vu T.Q. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J. Infect. Dis. 2004;189:221–232. doi: 10.1086/380762. (JID30900 [pii]) [DOI] [PubMed] [Google Scholar]
- Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J. Trop. Med. Public Health. 1987;18:392–397. [PubMed] [Google Scholar]
- Nisalak A., Endy T.P., Nimmannitya S., Kalayanarooj S., Thisayakorn U., Scott R.M., Burke D.S., Hoke C.H., Innis B.L., Vaughn D.W. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am.J.Trop. Med. Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- Noisakran S., Onlamoon N., Songprakhon P., Hsiao H.M., Chokephaibulkit K., Perng G.C. Cells in dengue virus infection in vivo. Adv. Virol. 2010;2010:164878. doi: 10.1155/2010/164878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S., Schmid M.A., Parameswaran P., Lachica R., Henn M.R., Beatty R., Harris E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012;93:2152–2157. doi: 10.1099/vir.0.045088-0. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes M.V., Pinhao A.T., Barreto D.F., Costa S.M., Oliveira M.P., Nogueira A.C., Takiya C.M., Farias-Filho J.C., Schatzmayr H.G., Alves A.M. Liver injury and viremia in mice infected with dengue-2 virus. Virology. 2005;338:236–246. doi: 10.1016/j.virol.2005.04.042. (S0042-6822(05)00270-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Pang T., Cardosa M.J., Guzman M.G. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol. Cell Biol. 2007;85:43–45. doi: 10.1038/sj.icb.7100008. (7100008 [pii]) [DOI] [PubMed] [Google Scholar]
- Phuong C.X., Nhan N.T., Kneen R., Thuy P.T., van Thien C., Nga N.T., Thuy T.T., Solomon T., Stepniewska K., Wills B. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the world health organization classification system helpful? Am. J. Trop. Med. Hyg. 2004;70:172–179. (doi: 70/2/172 pii) [PubMed] [Google Scholar]
- Pierson T.C., Diamond M.S. Flaviviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Philadelphia; Lippincott-Raven: 2013. pp. 747–794. [Google Scholar]
- Plummer E.M., Shresta S. Mouse models for dengue vaccines and antivirals. J. Immunol. Methods. 2014;410:34–38. doi: 10.1016/j.jim.2014.01.001. (pii) [DOI] [PubMed] [Google Scholar]
- Polack F.P., Hoffman S.J., Crujeiras G., Griffin D.E. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat. Med. 2003;9:1209–1213. doi: 10.1038/nm918. (pii) [DOI] [PubMed] [Google Scholar]
- Prestwood T.R., Morar M.M., Zellweger R.M., Miller R., May M.M., Yauch L.E., Lada S.M., Shresta S. Gamma interferon (IFN-gamma) receptor restricts systemic dengue virus replication and prevents paralysis in IFN-alpha/beta receptor-deficient mice. J. Virol. 2012;86:12561–12570. doi: 10.1128/JVI.06743-11. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R. Microevolution and virulence of dengue viruses. Adv. Virus Res. 2003;59:315–341. doi: 10.1016/s0065-3527(03)59009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivino L., Kumaran E.A., Jovanovic V., Nadua K., Teo E.W., Pang S.W., Teo G.H., Gan V.C., Lye D.C., Leo Y.S. Differential targeting of viral components by CD4 + versus CD8 + T lymphocytes in dengue virus infection. J. Virol. 2013;87:2693–2706. doi: 10.1128/JVI.02675-12. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman A.L. T lymphocyte responses to heterologous secondary dengue virus infections. Ann. N. Y. Acad. Sci. 2009;1171(Suppl. 1):E36–E41. doi: 10.1111/j.1749-6632.2009.05055.x. (pii) [DOI] [PubMed] [Google Scholar]
- Rothman A.L. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr. Top. Microbiol. Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- Rothman A.L. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011;11:532–543. doi: 10.1038/nri3014. (pii) [DOI] [PubMed] [Google Scholar]
- Sam S.S., Omar S.F., Teoh B.T., Abd-Jamil J., AbuBakar S. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002194. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S., Jatanasen S., Salitul V., Phanthumachinda B., Halstead S.B. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Schmid M.A., Harris E. Monocyte recruitment to the dermis and differentiation to dendritic cells increases the targets for dengue virus replication. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004541. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S., Sharar K.L., Prigozhin D.M., Beatty P.R., Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. (80/20/10208 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B., Perez A.B., Vogt K., Garcia G., Schmolke K., Aguirre E., Alvarez M., Kern F., Kouri G., Volk H.D. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell. Immunol. 2010;262:134–140. doi: 10.1016/j.cellimm.2010.02.005. (pii) [DOI] [PubMed] [Google Scholar]
- Sung J.M., Lee C.K., Wu-Hsieh B.A. Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046292. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico L.B., Bugna J., Wimmenauer V., Espinoza M.A., Quipildor M.O., Hijano D.R., Beccaria M., Wurster V., Cavagnaro L.E., Martinez D. T helper type 2 bias and type 17 suppression in primary dengue virus infection in infants and young children. Trans. R. Soc. Trop. Med. Hyg. 2013;107:411–419. doi: 10.1093/trstmh/trt044. (pii) [DOI] [PubMed] [Google Scholar]
- Vaughn D.W., Green S., Kalayanarooj S., Innis B.L., Nimmannitya S., Suntayakorn S., Endy T.P., Raengsakulrach B., Rothman A.L., Ennis F.A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. (JID990867 [pii]) [DOI] [PubMed] [Google Scholar]
- Watanabe S., Chan K.W., Wang J., Rivino L., Lok S.M., Vasudevan S.G. Dengue virus infection with highly neutralizing levels of cross-reactive antibodies causes acute lethal small intestinal pathology without a high level of viremia in mice. J. Virol. 2015;89:5847–5861. doi: 10.1128/JVI.00216-15. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Angelo M.A., de Azeredo E.L., Sidney J., Greenbaum J.A., Fernando A.N., Broadwater A., Kolla R.V., De Silva A.D., de Silva A.M. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8 + T cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2046–E2053. doi: 10.1073/pnas.1305227110. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Bangs D.J., Sidney J., Kolla R.V., De Silva A.D., de Silva A.M., Crotty S., Peters B., Sette A. Dengue virus infection elicits highly polarized CX3CR1 + cytotoxic CD4 + T cells associated with protective immunity. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E4256–E4263. doi: 10.1073/pnas.1505956112. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E.J., Hamilton J.R., Lease K.E., Coughlin S.R. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- Whitehead, S.S., Falgout, B., Hanley, K.A., Blaney Jr, J.E., Jr., Markoff, L., and Murphy, B.R. (2003). A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 77, 1653–1657. [DOI] [PMC free article] [PubMed]
- Yacoub S., Mongkolsapaya J., Screaton G. The pathogenesis of dengue. Curr. Opin. Infect. Dis. 2013;26:284–289. doi: 10.1097/QCO.0b013e32835fb938. [DOI] [PubMed] [Google Scholar]
- Yauch L.E., Zellweger R.M., Kotturi M.F., Qutubuddin A., Sidney J., Peters B., Prestwood T.R., Sette A., Shresta S. A protective role for dengue virus-specific CD8 + T cells. J. Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch L.E., Prestwood T.R., May M.M., Morar M.M., Zellweger R.M., Peters B., Sette A., Shresta S. CD4 + T cells are not required for the induction of dengue virus-specific CD8 + T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 2010;185:5405–5416. doi: 10.4049/jimmunol.1001709. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Shresta S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014;5:151. doi: 10.3389/fimmu.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Prestwood T.R., Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Eddy W.E., Tang W.W., Miller R., Shresta S. CD8 + T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014;193:4117–4124. doi: 10.4049/jimmunol.1401597. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Tang W.W., Eddy W.E., King K., Sanchez M.C., Shresta S. CD8 + T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J. Virol. 2015;89:6494–6505. doi: 10.1128/JVI.00036-15. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivna I., Green S., Vaughn D.W., Kalayanarooj S., Stephens H.A., Chandanayingyong D., Nisalak A., Ennis F.A., Rothman A.L. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J. Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]
- Zompi S., Harris E. Animal models of dengue virus infection. Viruses. 2012;4:62–82. doi: 10.3390/v4010062. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zompi S., Santich B.H., Beatty P.R., Harris E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J. Immunol. 2012;188:404–416. doi: 10.4049/jimmunol.1102124. (pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material