Abstract
At our center, relapsed mantle cell lymphoma (MCL) can be treated with maintenance therapy composed of consecutive low-dose lenalidomide and short-term, high-dose dexamethasone (LD regimen), which achieves good responses (longer overall survival and progression-free survival) and low toxicity. Cereblon is probably targeted by both lenalidomide and dexamethasone, which leads to synergistic cytotoxicity in MCL by inhibiting the interleukin-6/signal transducer and activator of transcription 3 (IL-6/STAT3), phosphatidylinositol 3-kinase (PI3K)/AKT and AKT2/Forkhead box O3 (FOXO3A)/BCL2-like 11 (BIM) pathways. The two drugs synergistically inhibit the same pathways, but through different sites. Cereblon was found expressed in most of the MCL tissues (91.3% positivity). Moreover, cereblon expression is positively correlated with LD regimen sensitivity: long-term lenalidomide exposure downregulates cereblon and induces multi-drug resistance against lenalidomide, dexamethasone, cytarabine, cisplatin, and methotrexate in vitro. Removal of lenalidomide resensitizes lenalidomide-resistant MCL cells to lenalidomide and dexamethasone. Our work suggests that rotating the LD regimen with other regimens would improve MCL maintenance therapy.
Abbreviations: MCL, mantle cell lymphoma; CRBN, cereblon; Lenalidomide, Lena; Dexamethasone, Dex; LD, lenalidomide and dexamethasone; OS, overall survival; Ara-C, cytarabine; MTX, methotrexate; DDP, cisplatin; CTX, cyclophosphamide; THP, therarubicin
Keywords: Lenalidomide, Dexamethasone, Cereblon, MCL, IL-6/STAT3
Highlights
-
•
Synergistic inhibition of IL-6/STAT3, PI3K/AKT, and AKT2/FOXO3A/BIM pathways by lenalidomide and dexamethasone
-
•
CRBN expression in MCL may account for the effectiveness of the LD regimen
-
•
Long-term lenalidomide exposure downregulates CRBN expression and induces drug resistance; Lena removal resensitized MCL cells
-
•
Intermittent use of the LD regimen would avoid drug resistance
Consecutive low-dose lenalidomide and short-term, high-dose dexamethasone (LD) maintenance therapy showed good response in relapsed mantle cell lymphoma. The synergistic effect of the two drugs might involve cereblon and such signaling pathways as IL-6/STAT3, PI3K/Akt and Akt2/FoxO3a/Bim. Cereblon expression positively correlated with LD regimen sensitivity. Expression of cereblon in mantle cell lymphoma probably accounts for the effectiveness of the LD regimen.
1. Introduction
Mantle cell lymphoma (MCL) is a distinct subtype of B cell lymphoma composed of small to medium lymphoid cells originating from CD5-positive follicular mantle B cells (Pérez-Galán et al., 2011, McKay et al., 2012). Due to an aggressive clinical disease course and incurability with standard chemotherapy, especially in the relapsed/refractory setting in which median overall survival (OS) is approximately 1–2 years with current therapies, MCL has the worst prognosis of B cell lymphomas (McKay et al., 2012, Goy and Kahl, 2011, Vose, 2012). MCL therapy has progressed in the past decade due to clinical experimentation with novel agents and drug combinations. Most novel drugs were tested in relapsed/refractory patients. Lenalidomide showed particularly promising antitumor activities in MCL patients (Habermann et al., 2009, Zinzani et al., 2013, Goy et al., 2013). Several clinical trials on lenalidomide for treating MCL are underway (NCT01035463, NCT01865110, NCT01996865) (Leonard et al., 2012, Martin et al., 2013, Zaja et al., 2012, Morrison et al., 2014). To date, there is no consensus on the best treatment approach for MCL.
At our center, a retrospective study revealed that a small cohort of patients with relapsed MCL who received lenalidomide and dexamethasone (LD regimen: for every 28 days, 10 mg lenalidomide was administered daily from d1 to d21, together with 20 mg dexamethasone at d1, d8, d15, and d22) as maintenance therapy had prolonged OS and progression-free survival (PFS), and showed low toxicity. Lenalidomide and dexamethasone had an obvious synergistic effect on the induction of apoptosis and inhibition of the cell growth of MCL cell lines in vitro that was better than that in myeloma lymphoma. However, the mechanism of the lenalidomide and dexamethasone synergy was unclear.
Based on the excellent synergistic effect of lenalidomide and dexamethasone, their mechanisms of action might share some common targets. Cereblon (CRBN) is a direct and therapeutically important molecular target of lenalidomide (Broyl et al., 2013, Lopez-Girona et al., 2012), while the target of dexamethasone in MCL is unknown. In addition, the signaling pathways involved in regulating apoptosis and cell cycle that are responsive to lenalidomide and dexamethasone are unclear. Several signaling pathways have been implicated in MCL cell growth including Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3), phosphatidylinositol 3-kinase (PI3K)/AKT, and AKT2/FOXO3A/BIM. A major driver of STAT3 activation is the cytokine interleukin-6 (IL-6), which signals through a heterodimeric IL-6 receptor (IL-6Rα/IL-6Rβ) to activate JAKs and induce STAT3 tyrosine phosphorylation. STAT3 activation in turn promotes IL-6 production and IL-6R expression, completing the positive feedback loop of the IL-6/STAT3 axis in MCL cells (Sansone and Bromberg, 2012, Snyder et al., 2014, Wang et al., 2009, Carbone et al., 2015, Zhang et al., 2012). AKT activation decreases cells in G0/G1 by phosphorylating the cell cycle inhibitory proteins p21WAF1/CIP1 and p27KIP1 (Zhang et al., 2012). Activation of the AKT isoform AKT2 phosphorylates Forkhead box O3 (FOXO3A), inducing FOXO3A inactivation and reducing apoptosis.
In this study, we used CRBN short interfering RNA (siRNA) to show that CRBN was likely involved in the synergy between lenalidomide and dexamethasone. We detected CRBN expression in most of the MCL patients we examined, which together with low toxicity of the drugs probably underlied the effectiveness of the LD regimen as maintenance therapy. We explored how lenalidomide and dexamethasone might affect the IL-6/STAT3, PI3K/AKT and AKT2/FOXO3A pathways. We found that inhibition of IL-6/STAT3, PI3K/AKT and AKT2/FOXO3A/BIM activities, which are crucial for lenalidomide's inhibition of cell growth and promotion of apoptosis were also involved in dexamethasone-induced cell cycle arrest. We also found that CRBN expression correlated positively with LD regimen sensitivity, whereas long-term lenalidomide and dexamethasone exposure downregulated CRBN and induced multi-drug resistance. Removing lenalidomide re-upregulated CRBN and restored the LD regimen sensitivity, which provides a rationale for the intermittent use of the LD regimen to avoid drug resistance in MCL treatment.
2. Materials and Methods
2.1. Cell Lines and Antibodies
The JeKo-1 cell line was obtained from the Cell Bank of the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The Z138 and REC-1 cell lines were obtained from the Biology Corporation of Meiyan. JeKo-1 cells were cultured in RPMI 1640 medium (Gibco) containing 20% fetal bovine serum (FBS; HyClone), 1% antibiotics/antimycotics in a humidified 5% CO2 incubator at 37 °C. Z138 and REC-1 cells were similarly cultured except 10% FBS was added in medium. The CRBN antibody was purchased from Sigma-Aldrich. Other antibodies used for western blot analysis were purchased from Cell Signaling Technology. The apoptosis and cell cycle detection kits were purchased from Sigma-Aldrich. The antibodies for flow cytometry, including those against CD126 and CD130, were purchased from eBioscience. The IL-6 enzyme-linked immunoassay (ELISA) kit was purchased from R&D Systems.
2.2. Lenalidomide and Dexamethasone Treatment
Dexamethasone (Sigma-Aldrich) was dissolved as previously described (Zhang et al., 2012). Lenalidomide (Selleckchem) was dissolved in dimethyl sulfoxide. JeKo-1, Z138, and REC-1 cells were treated with either control reagents or with lenalidomide for 72 h and/or dexamethasone for 24 h. Following incubation, the cells were harvested as previously described to assess apoptosis, cell cycle status, and for western blot analysis (Wang et al., 2009). For the treatment of CRBN knockdown cells, lenalidomide was added to the cell suspension 16 h after siRNA transfection, and dexamethasone was added 20 h before cell cycle and apoptosis detection. The cell cycle and apoptosis were detected 72 h after siRNA transfection.
2.3. Drug Combination Analysis
The effect of drug combination was analyzed using the CI method, as defined by the following equation: CI = (OD490)AB / [(OD490)A + (OD490)B], where (OD490)AB was the absorbance of the LD regimen treatment group, and (OD490)A and (OD490)B were the absorbance of the groups treated with lenalidomide and dexamethasone alone, respectively. CI > 1 indicated antagonism, CI = 1 indicated additivity, CI < 1 indicated synergy, and CI < 0.7 indicated significant synergy. Each CI ratio was the mean value calculated from at least three independent experiments.
2.4. IL-6 and IL-6R Detection
JeKo-1 cells were treated with 200 ng/mL lenalidomide for 1 week before the IL-6 concentration was measured using ELISA. IL-6R (CD130/CD126) was detected by flow cytometry after one-week treatment of the cells with 200 ng/mL lenalidomide and 2-day treatment with 20 μM dexamethasone.
2.5. SiRNA Constructs and Transfection
Three CRBN siRNAs were synthesized by GenePharma.; the siRNA information is available in the Supplemental materials. The CRBN siRNAs were transfected into JeKo-1 cells using the transfection reagent INTERFERin (Dakewe Biotech) according to the manufacturer's protocol. Transfection efficiency was determined by flow cytometry (BD Biosciences) 8 h post-transfection using 5-carboxyfluorescein (FAM)-labeled siRNA (GenePharma). 12 h and 24 h post-transfection, proteins were analyzed by western blotting.
2.6. Development of Lenalidomide-resistant Cell Lines
Lenalidomide-resistant JeKo-1 cells were selected by 2-week treatment with 0.5 μM lenalidomide. Then, lenalidomide treatment was discontinued for 1 week in one group of cells. For viability assays, cultures were initiated with 2 × 105 cells/mL and treated with 200 ng/mL lenalidomide for 3 days. Apoptosis and cell cycle status were then detected using apoptosis and cell cycle kits, respectively (Sigma-Aldrich). The cells were harvested at 3, 7, 10, and 14 days to detect CRBN expression by western blotting. Following the 1-week discontinuation of lenalidomide treatment, the cells were again incubated with lenalidomide for 3 days and subjected to apoptosis, cell cycle status, and CRBN expression analyses.
2.7. Treatment of Cells With Chemotherapeutic Agents
JeKo-1 cells were separated into lenalidomide-susceptible, lenalidomide-resistant, and lenalidomide-resensitized groups. The cells were treated for 24 h with ara-C (20 μM), CTX (50 μM), DDP (20 μM), MTX (10 μM), or THP (1 μM), and apoptosis was measured following the treatment.
2.8. Retrospective Study of Patient Outcomes and Immunohistochemistry
Our study involved the examination of the data of patients with MCL collected at Huadong Hospital affiliated with Fudan University, Shanghai, China, from 2009 to 2016, which received the approval of the Huadong Hospital ethical committee. We obtained forms of written informed consent from 23 participants prior to their inclusion in the study. CRBN expression in the lymph nodes was detected by immunohistochemistry and bone marrow pathology. In the LD maintenance therapy group, patients received 10 mg lenalidomide orally on days 1–21 of a 28-day cycle. Dexamethasone (20 mg) was given orally on day 1, 8, 15, and 22 every 28 days after complete or partial remission following chemotherapy. The first-line therapy was induction chemotherapy consisting of ara-C and autologous hematopoietic stem cell transplantation. The LD regimen was administered in relapsed or refractory patients until they achieved complete response or partial response. The patient follow-up methods are available in the Supplemental materials.
2.9. Statistical Methods
A two-tailed Student's t-test was used to evaluate differences between in vitro test groups. p-Values < 0.05 were considered statistically significant. Comparisons of the clinical features of the Lena + Dex maintenance and non-maintenance therapy groups were performed using the Student's t-test and chi-squared test. Survival was assessed using the Kaplan-Meier curve method, and survival between groups was compared using the log-rank test.
3. Results
3.1. Low-dose Lenalidomide and Short-term High-dose Dexamethasone Improved Survival in a Small Cohort of Patients With MCL With Good Tolerance
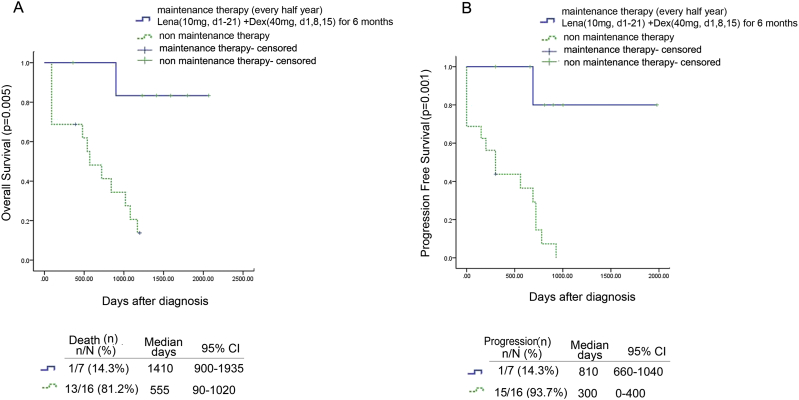
Twenty-three patients were included in the retrospective study of the administration of the LD regimen as maintenance therapy of MCL. MCL diagnosis was confirmed by lymph node biopsy or bone marrow biopsy. Table 1 shows the clinical features and survival of patients treated with or without Lena + Dex (LD) regimen maintenance therapy group and non-maintenance therapy group. There was no significant difference in the clinical features that could affect survival. Fig. 1A and B show the OS and PFS of patients in both groups. In the LD maintenance therapy group, the median OS was 1410 days (range, 900–1935 days) and the median PFS was 810 days (range, 660–1040 days). In the non-maintenance therapy group, the median OS was 555 days (range, 90–1020 days) and the median PFS was 300 days (range, 0–400 days). The LD maintenance therapy group displayed significantly improved OS and PFS compared with the non-maintenance therapy group (p = 0.005 and p = 0.001, respectively). All patients tolerated the maintenance therapy. No patient discontinued the therapy due to adverse effects. Of the seven patients treated with the LD regimen, one had mild thrombocytopenia and another had symptoms of fatigue. No patient had infections related to lenalidomide treatment (Supplemental Table 1).
Table 1.
Clinical features and survival of patients in the Lena and non-Lena treatment groups.
| Parameter | Patients n/N (%) |
Median (95% CI) | p |
|---|---|---|---|
| Age at diagnosis | ≫ 50 y | 0.868 | |
| Lena | 7/7 | 59.4 (51–68) | |
| Non-Lena | 15/16 | 62.3 (56.2–68.1) | |
| Sex | Male | 0.061 | |
| Lena | 6/7 | ||
| Non-Lena | 12/16 | ||
| Ki67 | ≫ 50% | 0.819 | |
| Lena | 3/7 | 40 (10–70)% | |
| Non-Lena | 6/16 | 30 (15–70)% | |
| MIPI | ≫ 5 | 0.096 | |
| Lena | 5/6 | 6 (5–8) | |
| Non-Lena | 8/17 | 4.5 (3–7) | |
| Treatment response after first relapse | 1 (CR)/2 (PR)/3 (SD)/4 (PD) | 0.083 | |
| Lena | 5/2 | ||
| Non-Lena | 6/5/4/1 | ||
| Auto-HSCT | Yes (0)/No (1) | 0.639 | |
| Lena | 5/2 | ||
| Non-Lena | 10/6 | ||
| Fever, sweating, weight loss | Yes | 0.144 | |
| Lena | 3/7 | ||
| Non-Lena | 4/16 | ||
| CRBN(+) | (+) | 0.350 | |
| Lena | 7/7 | ||
| Non-Lena | 14/16 | ||
| Disease-free survival | Relapse | 0.001 | |
| Lena | 1/7 (14.3%) | 300 (0–1259.7) | |
| Non-Lena | 14/16 (87.5%) | 0 | |
| Overall survival | Death | 0.005 | |
| Lena | 1/7 (14.3%) | 900 (463.6–1336.4) | |
| Non-Lena | 13/16 (81.2%) | 570 (240.4–899.6) |
95% CI, 95% confidence interval; Lena, lenalidomide; MIPI, MCL International Prognostic Index; auto-HSCT, autologous hematopoietic stem cell transplant; CRBN, cereblon.
Fig. 1.
Survival of patients with MCL receiving LD maintenance Therapy.
(A) Kaplan–Meier OS curves of patients in the Lena + Dex(LD) maintenance therapy group (n = 7) and the non-maintenance therapy group (n = 16); (B) Kaplan–Meier PFS curves of patients in the Lena + Dex(LD) maintenance therapy group (n = 7) and the non-maintenance therapy group (n = 16).
3.2. Low-dose Lenalidomide and High-dose Dexamethasone Caused Higher Apoptosis Rate, Cell Cycle Arrest, and IL-6 Signaling Inhibition in MCL Cell Lines
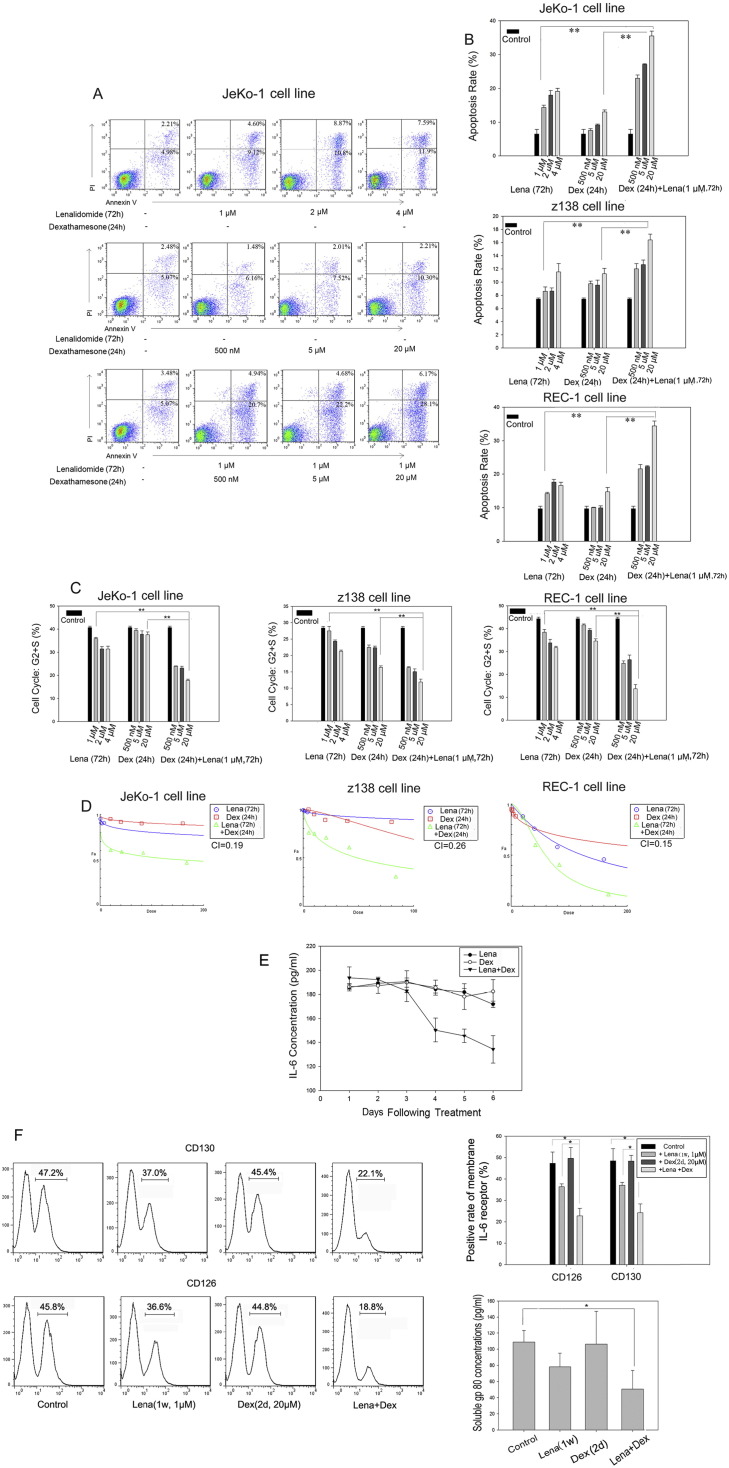
We assessed the sensitivity of the JeKo-1, Z138, and REC-1 MCL cell lines to lenalidomide. After 3 days of treatment, 1 μM lenalidomide induced significant levels of apoptosis in each cell line (Fig. 2A, B, p < 0.05), but not in myeloma cell lines (data not shown). Dexamethasone was added to the culture medium after 48-hour lenalidomide pretreatment and cells were further incubated with both drugs for 24 h. Addition of 20 μM dexamethasone greatly increased lenalidomide-induced apoptosis as compared with either drug alone (Fig. 2B, p < 0.01). We also assessed the inhibition of JeKo-1, Z138, and REC-1 cell growth by either drug alone or in combination. We found that 20 μM dexamethasone combined with 1 μM lenalidomide greatly increased MCL cell growth arrest as compared with either drug alone in these cell lines (Fig. 2C, p < 0.01). Moreover, we concluded that lenalidomide and dexamethasone had a significant synergistic inhibitory effect on MCL cell growth, based on a combination index (CI) of 0.17 in JeKo-1 cells, 0.26 in Z138 cells, and 0.15 in REC-1 cells as calculated by CompuSyn software (Fig. 2D).
Fig. 2.
Apoptosis, cell cycle arrest, and IL-6/IL-6R levels in MCL cell lines treated with lenalidomide and dexamethasone.
(A) Flow cytometry analysis of JeKo-1 cell apoptosis in the presence of lenalidomide and/or dexamethasone. (B) Apoptosis of JeKo-1, Z138, and REC-1 cells in the presence of lenalidomide and/or dexamethasone. All assays were performed in triplicate. Data are the mean ± SD. **p < 0.01. (C) Cell cycle status in JeKo-1, Z138, and REC-1 cells in the presence of lenalidomide and/or dexamethasone. All assays were performed in triplicate. Data are the mean ± SD. **p < 0.01. (D) CI in MCL cell lines treated with lenalidomide and dexamethasone at a proportion of 1:20. (E) IL-6 expression in JeKo-1 culture medium in the presence of 1 μM lenalidomide for 6 days and/or 20 μM dexamethasone for day 1, 3, and 5; (F, left) Histogram of IL-6R (CD126 and CD130) expression in JeKo-1 cells by flow cytometry in the presence of lenalidomide (1 μM) and/or dexamethasone (20 μM); (right) expression of IL-6R(CD126 and CD130) and soluble GP80 (IL-6R) detected by ELISA in the presence of lenalidomide (1 μM) and/or dexamethasone (20 μM) in Jeko-1 cells. Data are the mean ± SD. (*p < 0.05).
IL-6 exerts its biological action by binding to a membrane receptor complex consisting of IL-6Rβ (CD130) and IL-6Rα (CD126) (Zhang et al., 2012). Human MCL cells express membrane CD130/CD126 as well as the soluble CD130, GP80. We examined the effects of lenalidomide and dexamethasone alone or in combination on IL-6 secretion and IL-6R expression in MCL cells. JeKo-1 cells were incubated with 1 μM lenalidomide and/or 20 μM dexamethasone for 6 days. IL-6 levels in the culture medium and IL-6R expression (membrane CD130/CD126 and soluble GP80) were assessed. Lenalidomide alone inhibited IL-6 secretion in a time-dependent manner (Fig. 2E, p = 0.02, d1 vs d6) and inhibited IL-6R (CD130/CD126) expression (p < 0.01) (Fig. 2F). Dexamethasone alone did not inhibit IL-6 secretion (Fig. 2E, p = 0.53, d1 vs d6) and IL-6R (CD130/CD126) expression (Fig. 2F, p = 0.61, and p = 0.90, respectively) but enhanced lenalidomide-induced inhibition of IL-6 secretion (Fig. 2E, p = 0.002, d1 vs d6) and IL-6R (CD130/CD126 and GP80) expression (Fig. 2F, p < 0.01, p < 0.01, and p = 0.02, respectively).
3.3. CRBN Knockdown Decreased Lenalidomide and Dexamethasone Efficiency in MCL Cell Lines
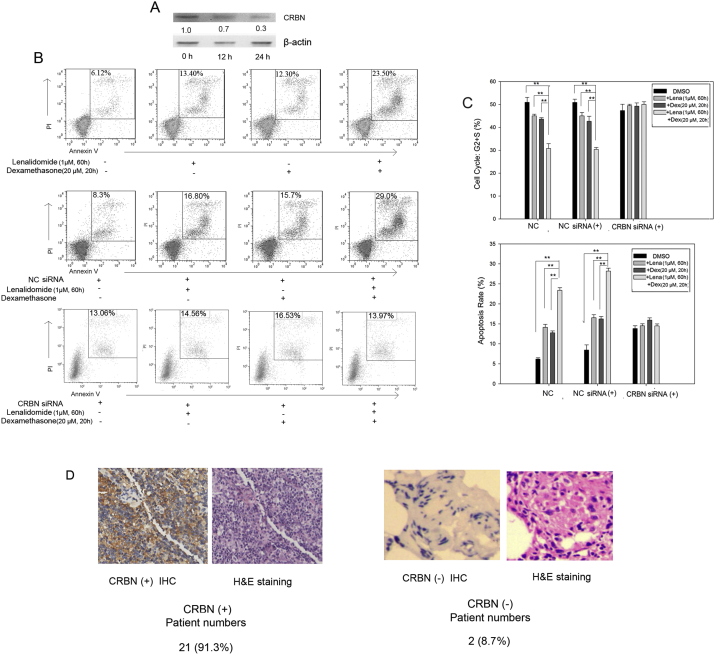
Given the synergistic effect of lenalidomide and dexamethasone, we speculated that the two drugs probably shared a common target. As CRBN is a target of lenalidomide, we performed siRNA knockdown of CRBN to explore its pharmaceutical significance. The transfection efficiency of siRNA in the MCL cells was over 90% (data not shown). CRBN siRNA reduced CRBN expression 24 h post-transfection as shown by western blot (Fig. 3A). Subsequently, we analyzed lenalidomide- and dexamethasone-induced cell cycle arrest and apoptosis. Lenalidomide (1 μM) was added to the culture medium 12 h after transfection with CRBN siRNA or negative control siRNA and the drug was replenished every 24 h. Dexamethasone (20 μM) was added to the culture medium 48 h after transfection. After 72 h following transfection, cell cycle status and apoptosis were analyzed using a cell cycle detection kit and an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit, respectively. Lenalidomide and dexamethasone had synergistic effects on inducing cell cycle arrest (p < 0.01, Fig. 3B). The effects were not observed following CRBN gene silencing (p = 0.25). Lenalidomide- and dexamethasone-induced apoptosis and cell cycle arrest also decreased after CRBN knockdown (p > 0.05; Fig. 3C). These data demonstrate that CRBN is critically involved in the actions of both lenalidomide and dexamethasone in MCL cells. Accordingly, we performed immunohistochemical detection of CRBN expression in tumor biopsy samples. Of the 23 patients with MCL, 21 had CRBN-positive biopsies (Fig. 3D). The percentage of CRBN positivity among patients with MCL was higher than that in multiple myeloma in the study by Annemiek et al., in which among 73 patients with myeloma who received thalidomide, only 30 had detectable CRBN (Broyl et al., 2013).
Fig. 3.
Lenalidomide and dexamethasone targeted CRBN.
(A) CRBN expression at 12 h and 24 h following CRBN gene silencing. (B) Apoptosis rate after CRBN silencing in the presence of lenalidomide (1 μM) and dexamethasone (20 μM). (C) Detection of cell cycle status and apoptosis rate after CRBN silencing in the presence of lenalidomide (1 μM) and dexamethasone (20 μM). Data are the mean ± SD (**p < 0.05). (D) CRBN expression in biopsy samples (40 ×) from patients with MCL. NC siRNA, Negative control siRNA.
3.4. Lenalidomide and Dexamethasone Synergistically Downregulated the IL-6/STAT3, PI3K/AKT and AKT2/Foxo3a/Bim Pathways in MCL Cells
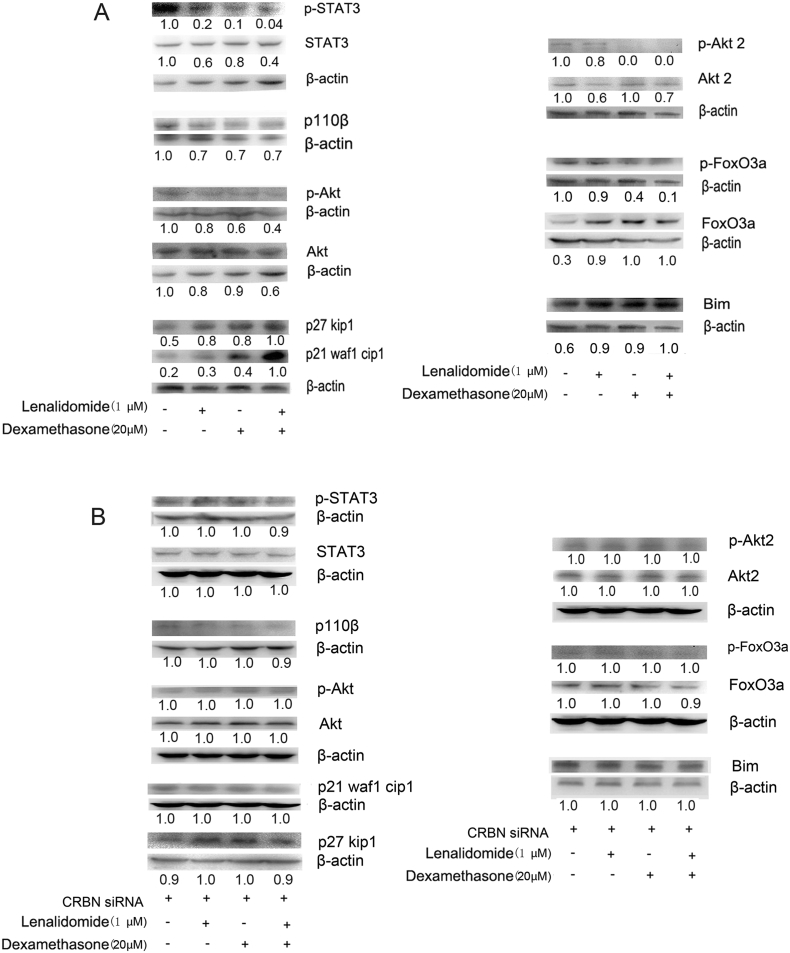
The IL-6/STAT3 axis is important for MCL cell growth, and lenalidomide and dexamethasone decreased STAT3 levels. Lenalidomide mainly reduced STAT3 expression, and dexamethasone mainly decreased phosphorylated STAT3 (p-STAT3); the drugs inhibited the IL-6/STAT3 axis synergistically (Fig. 4A).
Fig. 4.
Lenalidomide and dexamethasone shared signal pathways in cell cycle and apoptosis regulation.
(A) Western blot analysis of proteins from the PI3K/AKT2/FOXO3A and JAK/STAT3 pathways following treatment with lenalidomide (1 μM) and/or dexamethasone (20 μM). (B) Western blot analysis of PI3K/AKT and JAK/STAT3 pathways after CRBN silencing in the presence of lenalidomide (1 μM) and/or dexamethasone (20 μM).
The PI3K/Akt pathway is important for MCL cell growth and survival. Its inhibition completely or partially induces cell death (Lopez-Girona et al., 2012). P110 is the catalytic subunit of PI3K and AKT/PKB is a primary downstream target of PI3K. We assessed the effects of lenalidomide and/or dexamethasone on the expression and phosphorylation of the PI3K/AKT pathway, focusing on important kinases such as P110, AKT, P27KIP1, and P21WAF1/CIP1, which affect cell cycle status and apoptosis. Lenalidomide and dexamethasone synergistically reduced P110 and AKT expression while increased the expression of the cell cycle inhibitory factors p21WAF1/CIP1 and p27KIP1. Lenalidomide mainly reduced AKT expression; dexamethasone mainly decreased p-AKT (Fig. 4A). Following CRBN gene silencing, we examined the expression levels and phosphorylation status of kinases in the JAK/STAT3 and PI3K/AKT pathways. In contrast to the observations made when CRBN expression was unchanged, we did not observe altered expression levels or phosphorylation status following lenalidomide and dexamethasone treatment (Fig. 4B).
Lenalidomide and dexamethasone act synergistically to promote AKT2/FOXO3A/BIM-mediated apoptosis. AKT2 activation promotes FOXO3A phosphorylation and degradation, downregulating the expression of the proapoptotic regulator, BIM, which induces apoptosis (Baraka and Salem, 2011). Lenalidomide mainly reduced AKT2 expression and increased FOXO3A expression; dexamethasone mainly decreased p-AKT2 and p-FOXO3A; the drugs inhibited the AKT2/FOXO3A pathway synergistically (Fig. 4A). Upon CRBN gene silencing, all changes to the corresponding factors disappeared (Fig. 4B).
These results collectively indicate that lenalidomide and dexamethasone share crucial signaling pathways in inducing MCL cell cycle arrest and apoptosis and that CRBN is a very important target of the signaling regulated by lenalidomide and dexamethasone.
3.5. Multi-drug Resistance and Resensitization of MCL Cells From Long-term Lenalidomide Exposure and Drug Removal Accompanied Altered CRBN Expression
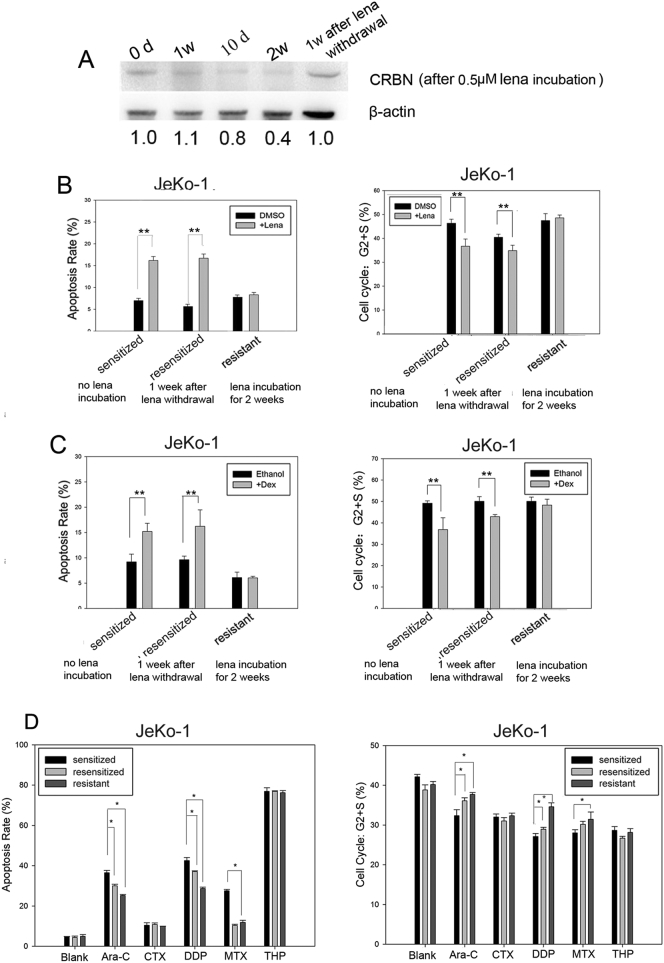
We then assessed the sensitivity of MCL cells to lenalidomide after long-term lenalidomide treatment. JeKo-1 cells were cultured with 0.5 μM lenalidomide for 2 weeks. The cells were collected at day 7, 10, and 14. A group of treated cells was then cultured for an additional week without lenalidomide, and the cells were collected for analysis of CRBN expression. We found that CRBN expression gradually decreased following lenalidomide exposure. However, one week after the removal of lenalidomide, CRBN expression increased to the pre-treatment level (Fig. 5A). Cells (lenalidomide-sensitive, lenalidomide-resistant, or lenalidomide-resensitized) were subjected to additional treatment with 1 μM lenalidomide for 3 days or 20 μM dexamethasone for 1 day and the cell cycle status and apoptosis were analyzed. We found that the cells became resistant to lenalidomide following a two-week lenalidomide treatment and that with these cells, lenalidomide-induced apoptosis and cell cycle arrest were markedly reduced. The cells became resensitized after one week following the removal of lenalidomide and their responses to lenalidomide-induced apoptosis and cell cycle arrest were similar to those of the original lenalidomide-sensitive cells (Fig. 5B). Similarly, the cells became resistant to dexamethasone following lenalidomide exposure, and were resensitized following the removal of lenalidomide (Fig. 5C). The lenalidomide-sensitive, -resistant, and -resensitized JeKo-1 cells were subsequently treated with additional chemotherapeutic drugs, namely 50 μM cyclophosphamide (CTX), 0.2 μM therarubicin (THP), 10 μM methotrexate (MTX), 20 μM cisplatin (DDP), or 20 μM cytarabine (ara-C). After 24 h, apoptosis and cell cycle status were examined in each treatment group. The lenalidomide-resistant cells were resistant to ara-C, DDP, and MTX, but remained sensitive to CTX and THP. The lenalidomide-resensitized cells partly recovered the sensitivity to ara-C and DDP, but not to MTX (Fig. 5D).
Fig. 5.
CRBN expression correlated positively with the LD regimen; long-term exposure to lenalidomide induced multi-drug resistance.
(A) CRBN expression in JeKo-1 cells after treatment with 0.5 μM lenalidomide followed by 1-week removal of drug pressure; protein levels were normalized to β-actin. (B) Apoptosis and cell cycle status in lenalidomide-sensitive, -resistant, and -resensitized JeKo-1 cells after follow-up treatment with 1 μM lenalidomide. (C) Apoptosis and cell cycle status in lenalidomide-sensitive, -resistant, and -resensitized JeKo-1 cells after follow-up treatment with 20 μM dexamethasone. Data are the mean ± SD (**p < 0.01). (D) Apoptosis and cell cycle detection of JeKo-1 cells in the presence of ara-C (20 μM), CTX (50 μM), DDP (20 μM), MTX (10 μM), or THP (1 μM) in lenalidomide-sensitized, -resensitized, and -resistant JeKo-1 cells. Sensitized: No exposure to lenalidomide; resensitized: 1-week escape from lenalidomide; resistant: 2-week exposure to lenalidomide.
4. Discussion
MCL has poor prognosis and is often difficult to treat. Clinical trials have attempted to explore better regimens for maintenance therapy of MCL. Various clinical trials have shown that lenalidomide is effective for treating MCL (Leonard et al., 2012). Lenalidomide monotherapy was also efficacious in heavily pretreated patients with aggressive, relapsed/refractory non-Hodgkin lymphoma, even after autologous stem cell transplantation (Goy et al., 2013). In experimental models, lenalidomide blocked tumor cell proliferation and angiogenesis, and stimulated T-cell and natural killer (NK) cell-mediated cytotoxicity (Wiernik et al., 2008, Witzig et al., 2011, Habermann et al., 2009, Zinzani et al., 2012, Geha et al., 2007, Gunnellini and Falchi, 2012). Our study revealed that lenalidomide and dexamethasone acted synergistically in vitro, increasing lenalidomide-induced apoptosis and cell cycle arrest by 2–3 folds in multiple MCL cell lines. The synergy is likely achieved by lenalidomide and dexamethasone exerting their effects through common target CRBN and signaling pathways. Lenalidomide and dexamethasone inhibited IL-6 secretion and IL-6R expression, reducing the activities of STAT3, PI3K/Akt and AKT2/FOXO3A pathways. Lenalidomide tended to reduce the expression of important kinases in these pathways, while dexamethasone tended to decrease the expression of phosphorylated kinases.
CRBN is the target of lenalidomide. Following direct binding, lenalidomide activates the E3 ligase activity of CRBN, resulting in the rapid ubiquitination and degradation of Ikaros and Aiolos, downregulating interferon regulatory factor 4 (IRF4), which is involved in regulating different molecular pathways such as cell cycle arrest and apoptosis (Reddy et al., 2008, Wu et al., 2008, Zhu et al., 2014). Dexamethasone might also target CRBN, whose expression correlated with sensitivity to the LD regimen, though more direct evidence needs to be obtained. CRBN positivity is very high in the MCL cells among patients with relapsed MCL in this study, which probably accounts for the effectiveness of the LD regimen in the maintenance therapy of MCL.
Based on the effect of lenalidomide and dexamethasone, what should be the appropriate treatment schedule of the LD regimen? Should we use it until disease progression? We found that long-term lenalidomide treatment downregulated CRBN expression and induced multi-drug resistance in vitro, including resistance to ara-C, DDP, and MTX. Following the withdrawal of lenalidomide, MCL cells recovered sensitivity to lenalidomide and dexamethasone. Long-term treatment with lenalidomide also results in the upregulation of T regulatory cells (Wu et al., 2008, Raja et al., 2014), the presence of which is detrimental to tumor cell clearance (Zhu et al., 2014). We suggest rotating the LD regimen with other regimens such as interferon-α (IFN-α) in the maintenance therapy of MCL.
As 25 mg lenalidomide per day in myeloma treatment may be intolerable in maintenance therapy due to the adverse effects, there is also the question of whether low-dose lenalidomide (10 mg per day) is more tolerable (Aue et al., 2009). We found that low-dose lenalidomide was sufficient for killing MCL cells, but did not achieve good effects in multiple myeloma cells (data not shown), and it had synergistic effects with high-dose dexamethasone on MCL cells. Low-dose lenalidomide was sufficient for exerting its immunoregulatory effects by activating dendritic cells, T cells, NK cells, and NKT cells, which are helpful in eliminating residual tumor cells (Chang et al., 2008). Therefore, we used 10 mg lenalidomide orally per day for days 1–21, combined with 40 mg per week dexamethasone orally for 6 months in rotation with IFN-γ 3 × 105 IU twice a week for 6 months in the maintenance therapy of patients with MCL at our medical center, which greatly improved the PFS and OS of a small cohort of patients with relapsed MCL. We are also trying Rituximab instead of IFN-γ rotated with the LD regimen in the maintenance of MCL patients, and expect to publish the new data in the future. We also established that, in cases of lenalidomide resistance, alkylating agents and anthracycline drugs should be used, and platinum and anti-metabolic drugs such as ara-C and MTX should be avoided.
In the present study, the LD regimen elicited good responses in patients with relapsed MCL. Nevertheless, the number of patients in this study is limited. Our observations should be followed up with clinical trials and further studies.
Conflict of Interest
The authors declare there is no conflict of interest.
Author Contributions
Jiexian Ma, Yanhui Xie and Youhua Xie designed the study, wrote and revised the manuscript. Jiexian Ma and Kefei Wu performed the experiments and collected patients' data. Xiaoxian Cui and Weiya Bai help with some experiments. Yanchen contributed to the patients' lymphoma pathological examination.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 81600143], the Shanghai Sailing Program of Shanghai Science and Technology Committee [grant number 15YF1403700], the Shanghai Key Laboratory of Clinical Geriatric Medicine [grant number 13DZ2260700], and the Shanghai Key Developing Disciplines Program [grant number 2015ZB0501].
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.037.
Contributor Information
Youhua Xie, Email: yxie@fudan.edu.cn.
Yanhui Xie, Email: yanhuixie@163.com.
Appendix A. Supplementary Data
Supplementary material
References
- Aue G., Njuguna N., Tian X., Soto S., Hughes T., Vire B. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94(9):1266–1273. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraka A., Salem H.M. Clinical significance of T-regulatory cells in B-cell non-Hodgkin's lymphoma. Egypt J. Immunol. 2011;18(2):23–30. [PubMed] [Google Scholar]
- Broyl A., Kuiper R., van Duin M., van der Holt B., el Jarari L., Bertsch U. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood. 2013;121(4):624–627. doi: 10.1182/blood-2012-06-438101. [DOI] [PubMed] [Google Scholar]
- Carbone A., Gloghini A., Castagna L., Santoro A., Carlo-Stella C. Primary refractory and early-relapsed Hodgkin's lymphoma: strategies for therapeutic targeting based on the tumour microenvironment. J. Pathol. 2015;237(1):4–13. doi: 10.1002/path.4558. [DOI] [PubMed] [Google Scholar]
- Chang D.H., Liu N., Klimek V., Hassoun H., Mazumder A., Nimer S.D. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Br. J. Haematol. 2008;140(1):36–45. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R.S., Notarangelo L.D., Casanova J.L., Chapel H., Conley M.E., Fischer A. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J. Allergy Clin. Immunol. 2007;120(4):776–794. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy A., Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit. Rev. Oncol. Hematol. 2011;80(1):69–86. doi: 10.1016/j.critrevonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Goy A., Sinha R., Williams M.E., Kalayoglu Besisik S., Drach J., Ramchandren R. Single agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J. Clin. Oncol. 2013;31(29):3688–3695. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnellini M., Falchi L. Therapeutic activity of lenalidomide in mantle cell lymphoma and indolent non-Hodgkin's lymphomas. Adv. Hematol. 2012;2012:523842. doi: 10.1155/2012/523842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann T.M., Lossos I.S., Justice G., Vose J.M., Wiernik P.H., McBride K. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br. J. Haematol. 2009;145(3):344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- Leonard J.P., Jung S.H., Johnson J., Pitcher B.N., Bartlett N.L., Blum K.A. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (alliance) J. Clin. Oncol. 2012;33(31):3635–3640. doi: 10.1200/JCO.2014.59.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A., Mendy D., Ito T., Miller K., Gandhi A.K., Kang J. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Jung S., Johnson J. CALGB 50803(ALLIANCE): a phase 2 trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. Hematol. Oncol. 2013;31(Suppl. I) (117 Abstract 063; ICML Abstracts) [Google Scholar]
- McKay P., Leach M., Jackson R., Cook G., Rule S. Guidelines for the investigation and management of mantle cell lymphoma. Br. J. Haematol. 2012;159(4):405–426. doi: 10.1111/bjh.12046. [DOI] [PubMed] [Google Scholar]
- Morrison V.A., Jung S.H., Johnson J., LaCasce A., Blum K.A., Bartlett N.L. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: final results of a phase II trial (CALGB 50501) Leuk. Lymphoma. 2014;56(4):958–964. doi: 10.3109/10428194.2014.938333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Galán P., Dreyling M., Wiestner A. Mantle cell lymphoma: biology, pathogenesis and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja K.R., Plasil M., Rihova L., Pelcova J., Adam Z., Hajek R. Flow Cytometry-based enumeration and functional characterization of CD8 T regulatory cells in patients with multiple myeloma before and after lenalidomide plus dexamethasone treatment. Cytometry B Clin. Cytom. 2014;86(4):220–228. doi: 10.1002/cyto.b.21109. [DOI] [PubMed] [Google Scholar]
- Reddy N., Hernandez-Ilizaliturri F.J., Deeb G., Roth M., Vaughn M., Knight J. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br. J. Haematol. 2008;140(1):36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- Sansone P., Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30(9):1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Huang J., Huang X.Y., Zhang J.J. A signal transducer and activator of transcription 3_nuclear factor κB (Stat3-NFκB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-α. J. Biol. Chem. 2014;289(43):30082–30089. doi: 10.1074/jbc.M114.591719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose J.M. Mantle cell lymphoma: 2012 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2012;87(6):604–609. doi: 10.1002/ajh.23176. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Li Y., Wen Y., Chen Y.F., Na L.X., Li S.T. Curcumin, a potential inhibitor of up-regulation of TNF-α and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-κB and JNK pathway. Biomed. Environ. Sci. 2009;22(1):32–39. doi: 10.1016/S0895-3988(09)60019-2. [DOI] [PubMed] [Google Scholar]
- Wiernik P.H., Lossos I.S., Tuscano J.M., Justice G., Vose J.M., Cole C.E. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J. Clin. Oncol. 2008;26(30):4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- Witzig T.E., Vose J.M., Zinzani P.L., Reeder C.B., Buckstein R., Polikoff J.A. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann. Oncol. 2011;22(7):1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- Wu L., Adams M., Carter T., Chen R., Muller G., Stirling D. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20 + tumor cells. Clin. Cancer Res. 2008;14(14):4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- Zaja F., De Luca S., Vitolo U., Orsucci L., Levis A., Salvi F. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: clinical results and effects on microenvironment and neo-angiogenic biomarkers. Haematologica. 2012;97(3):416–422. doi: 10.3324/haematol.2011.051813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang J., Qian J., Li H., Romaguera J.E., Kwak L.W. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120(18):3783–3792. doi: 10.1182/blood-2012-04-424630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.X., Braggio E., Shi C.X., Kortuem K.M., Bruins L.A., Schmidt J.E. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014;124(4):536–545. doi: 10.1182/blood-2014-02-557819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani P.L., Vose J.M., Czuczman M.S., Reeder C.B., Haioun C., Polikoff J. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann. Oncol. 2012;24(11):2892–2897. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani P.L., Vose J.M., Czuczman M.S., Reeder C.B., Haioun C., Polikoff J. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann. Oncol. 2013;24(11):2892–2897. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material