Abstract
The purpose of this longitudinal study was to investigate whether there are distinct etiological processes explaining dual usage of alcohol and conventional cigarettes by mothers from pre-conception through the early parenting years. Data on 8,800 biological mothers were drawn from the Early Childhood Longitudinal Study-Birth Cohort (ECLS-B), representative of U.S. births in 2001. A general growth mixture model (GGMM) was used to empirically identify developmental trajectories of maternal smoking and drinking over the five-to-six year study period. Six classes defined by alcohol consumption and cigarette smoking were identified. These included a non-smoking, low probability of drinking class (41%) and two drinking classes displaying no smoking with either moderate (26%) or escalating high (8%) probability drinking. Additionally, two predominantly smoking classes were identified, one displaying temporary reduction in smoking during pregnancy and low probability of drinking (11%) and one following a trajectory of persistent heavy smoking with a declining probability of drinking (9%). The sixth class was described by temporary reduction in smoking during pregnancy with high probability of drinking (6%). Covariates differentially predicted class membership, e.g., having a high school degree but not further education predicted concurrent drinking and smoking, and breastfeeding for more than six months is protective against concurrent use. Prior to conception, during prenatal care, and in post-natal clinical visits, whether for personal or pediatric care, screening women of reproductive age via characteristics that predict heterogeneity in smoking and drinking trajectories may help guide prevention and treatment options.
Keywords: maternal smoking, maternal alcohol use, GGMM, concurrent trajectories, ECLS-B
INTRODUCTION
Alcohol use during pregnancy, discouraged by the American Academy of Pediatrics (Williams & Smith, 2015), is a leading cause of still birth, spontaneous abortion, and preterm delivery, as well as various child neurobehavioral problems, such as fetal alcohol syndrome (FAS) and deficits in attention, memory, and IQ (Meyer-Leu, Lemola, Daeppen, Deriaz, &Gerber, 2011). Longitudinal research suggests that even low level drinking during this critical period can be detrimental to a child’s neurocognitive development, although exposure did not necessarily dictate deleterious outcomes (Streissguth, 2007). Studies of maternal smoking during pregnancy also indicate negative maternal health impacts, including reduced fertility, spontaneous abortion, and preterm delivery, as well as detriments to the child’s health, including decreased birth weight and sudden infant death (Wigle et al., 2008). Such risks have inspired a growing body of epidemiological research examining the patterns of and risk factors for maternal prenatal smoking and/or alcohol consumption (Powers, McDermott, Loxton, & Chojenta, 2013) and have guided the development of effective prevention programs (Haynes et al., 2015).
Despite a significant decline in drinking and smoking from preconception to prenatal period, 52% of new mothers resume alcohol use and 24% resume smoking within a year post-delivery (Substance Abuse and Mental Health Services Administration, 2009). Evidence (Tsai et al. 2010, Powers, et al. 2013, Lange, Probst, Quere, Rehm, & Popova, 2015) suggests that 12–22% of women of reproductive age (WRA) and 8–14% of pregnant women consume both substances. Concurrent prenatal use of alcohol and cigarettes imposes added risk to maternal and child health in the form of preterm labor, low birth weight, and infant growth restrictions (Odendaal, Steyn, Elliott, & Burd, 2008). Continuing or resuming smoking and/or risky alcohol use postpartum poses threats to maternal and child health and well-being. Children’s exposure to secondhand smoke is associated with Sudden Infant Death Syndrome, respiratory ailments, and infections (U.S. Department of Health and Human Services 2006). Children raised by mothers with risky drinking behaviors risk exposure to unstable environments (Jester et al. 2000) with implications for subsequent emotional/behavioral problems (Eiden, Edwards, & Leonard 2007).
The multiple mechanisms — e.g., cross-tolerance, sociobehavioral, environmental, genetic, neurochemical (McKee & Weinberger, 2013) — informing additive and multiplicative effects of concurrent drinking and smoking underscore the importance of explicating concurrent use patterns. Despite a concerning level of concurrent use during the perinatal period and shared risk factors, e.g., mental disorders (Ingersoll, Hettema, Cropsey, & Jackson, 2011), being unmarried (Tsai, et al., 2010), curtailed breastfeeding (Jagodzinski & Fleming 2007), longitudinal patterns of maternal smoking (Mumford & Liu 2015) and drinking (Liu, Mumford, & Petras, 2015) have only been modeled separately. To inform public health and clinical efforts in better allocating prevention efforts, we investigate distinct trajectories representing single and dual usage of alcohol and cigarettes by mothers from pre-conception through the early parenting years, and the extent to which mothers’ baseline characteristics are associated with these patterns.
METHODS
Sample
Study data come from the Early Childhood Longitudinal Study (ECLS-B), a nationally representative study of over 10,000 children from the 2001 U.S. birth cohort and their parents. The sample was drawn from a list of registered births provided by the National Center for Health Statistics (NCHS), using a clustered list frame sampling design with counties and county groups as the primary sampling units (PSU) (Flanagan & West, 2004). This study examines biological mothers’ survey responses at four time points: child approximately 9 months old (2001–2, baseline), 2 years old (2003–04), 4 years old (2005–06, preschool), and 5 or 6 years old (2006–07, kindergarten). Trained interviewers visited the respondent (mother’s) home at each wave with a $30 respondent fee for mothers and a book for the child. Smoking and alcohol use measures at three months prior to conception and during the third trimester were retrospectively collected at baseline, with the advantage of circumventing potential antenatal underreporting due to respondent beliefs about socially undesirable behaviors. In population-based studies, retrospective reports of prenatal drinking have been more forthcoming and accurate than self-reported drinking during pregnancy (Alvik, Haldorsen, Groholt, & Lindemann, 2006). Similarly, retrospective reports of prenatal smoking have been shown to align with self-reports and cotinine measurements during pregnancy (Pickett, Kasza, Biesecker, Wright, & Wakschlag, 2009). Sample n’s are rounded to the nearest 50 in compliance with ECLS-B rules. Cases that missed all smoking/drinking measures (N=100) or any exogenous variables (N=1600) were excluded, resulting in an analytic sample of 8,800 (84% of the original sample) adult biological mothers. The selected sample is significantly different from the excluded cases on a number of study variables; however, the average magnitude of the difference is small, i.e., 2% for smoking measures, 6% for drinking measures, and 6% for exogenous variables (see Supplement Table 1 for details). In order to assess whether the exclusion of cases with missing data bias the study results, we conducted a sensitivity analysis and found that a 6 class model using all cases is similar in structure as the presented model (results available upon request).
Measures
Growth model indicators: Longitudinal measures of maternal smoking and drinking
Smoking is measured as the average quantity smoked in terms of cigarettes per day (CPD). A four-level categorical ordinal variable was created at each time point: never smokers, ≤5 CPD, 5–10 CPD, and >10 CPD (heavy smoking). Drinking quantity is measured in categories of the number of alcoholic beverages consumed in an average week (<1, 1–3, 4–6, 7–13, 14–19, and 20 or more). We collapsed the original measures of alcohol consumption into a four-level ordinal variable per Brown, Olson, & Croninger (2010): no alcohol, <1 drink per week, 1–3 drinks per week, and 4+ drinks per week, thereby managing low response frequencies that pose problems for model estimation (e.g., across waves, 0.1% to 7.2% of mothers drank 4+ drinks per week).
Exogenous variables
Covariates collected at baseline include: age (18–25, 26–35, and 36+) (Meschke, Holl, & Messelt, 2013); education (less than high school; high school degree; some college; college or graduate degree) (Kandel, Griesler, & Schaffran 2009); race/ethnicity (white, black, Hispanic, Asian or other) (Kandel et al. 2009); postpartum depression measured with a modified 12-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (severe or moderate depression vs. low or no depression per Paulson et al. (2009)); marital status (married or cohabitating vs. others)(Meschke et al. 2013); household income-poverty (<poverty line; 100–130% poverty line; 130–185% poverty line; >185% poverty line), reflecting common federal aid eligibility requirements and prior research (Mumford, Hair, Yu, & Liu, 2014); employment status (full-time, part-time, not employed)(Cooklin, Donath, & Amir, 2008); breastfeeding (never, breastfed ≤6 months, breastfed >6 months per Ogbuanu et al. (2011)); planned pregnancy (yes, no; (Edwards & Werler, 2006)).
Analytic Plan
General growth mixture models (GGMM) (Muthén 2004) were used to empirically identify developmental trajectories of women’s smoking and drinking before, during and after pregnancy using Mplus version 7.11. GGMM uses a categorical latent class variable in combination with continuous growth factors to explore population heterogeneity in the change process of the outcome of interest, i.e., whether the study population consists of two or more discrete classes of individuals with varying growth trajectories (Muthén 2004). Trajectory classes are characterized by concurrent longitudinal development of both drinking and smoking. Modeling sequence followed the guidelines for growth models with ordered categorical outcomes. The origin of time was set at baseline to support the association of baseline covariates with subsequent drinking and smoking measures (Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004). The determination of the functional form was conducted separately for smoking and alcohol consumption. A series of growth models was first estimated with fixed or random intercept and slope factors, and compared for model fit (e.g. likelihood ratio test for nested models and the Bayesian Information Criterion (BIC), parsimony and interpretability. For both smoking (LL=−19938.874 (10), BIC=39968.53) and drinking (LL=−33355.568 (10), BIC=66801.92), a model with a random intercept and a random slope best fit the data (Table 1, details available online). Heterogeneity in the longitudinal development of maternal drinking and smoking was then explored by estimating models with increasing numbers of classes. Deciding on the number of longitudinal latent classes is based on BIC as well as substantive evaluation of the classes. Entropy was obtained as a measure of classification quality. Final class membership was regressed on exogenous variables via multinomial logistic regression.
Table 1.
Parameters estimated in the functional form for smoking and drinking in Logit coeffients*
| Intercept | Slope | Thresholds for during pregnancy (2nd time point) | Thresholds for all other time points | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking | |||||||||
|
| |||||||||
| Mean | Variance | Mean | Variance | #1 | #2 | #3 | #1 | #2 | #3 |
| 0.000 | 46.030 | −0.280 | 0.274 | 9.258 | 11.163 | 13.492 | 6.311 | 7.869 | 10.291 |
|
| |||||||||
| Drinking | |||||||||
|
| |||||||||
| Mean | Variance | Mean | Variance | #1 | #2 | #3 | #1 | #2 | #3 |
| 0.000 | 8.421 | −0.113 | 0.189 | 6.240 | 7.536 | 10.248 | 1.217 | 2.959 | 5.569 |
Results based on a model a random intercept and a random slope as it best fits the data (for details, please see online supplement material). All parameters presented in the table were statistically significant (P-value<.01).
Missing data on the outcome measures were accounted for by using the full information maximum likelihood (FIML) estimation method (Schafer and Graham 2002). Over 60% of the study sample had valid information on all six smoking/drinking measures and another 20% missed only one smoking/drinking measure. Complex sampling design was accounted for by computing robust standard errors using a sandwich estimator (White 1980). Results were weighted to represent the 2001 birth cohort.
RESULTS
Over half (50.6%) of the women in the sample gave birth between age 26 and 35. The majority were White (60.8%), married or cohabitating (82.1%), employed (20.8% full time and 32.8% part time) with household income higher than 185% poverty line (54.6%). Over half of the women in the sample had at least a college degree (29.6% had some college degree and 26.0% had a college or graduate degree). Over half (50.7%) of the women planned their pregnancy, and two-thirds of the sample breastfed for at least 6 months (42.6% ≤ 6 months and 27.1%> 6 months). Table 2 presents the weighted distribution of maternal alcohol and cigarette use before, during and after pregnancy.
Table 2.
Weighted distribution of maternal alcohol and cigarette use before, during and after pregnancy (N=8,800), ECLS-B
| Maternal Alcohol Use | Maternal Smoking | ||
|---|---|---|---|
| 3 months before pregnancy | |||
|
| |||
| No alcohol use | 60.2% | No smoking | 75.9% |
| Less than 1 drink a week | 16.3% | Smoking <5 CPD | 6.0% |
| 1–3 drinks a week | 16.6% | Smoking 6–10 CPD | 8.4% |
| 4 or more drinks a week | 7.0% | Smoking >10 CPD | 9.8% |
|
| |||
| Last 3 months of pregnancy | |||
|
| |||
| No alcohol use | 96.7% | No smoking | 88.7% |
| Less than 1 drink a week | 2.2% | Smoking <5 CPD | 5.4% |
| 1–3 drinks a week | 1.1% | Smoking 6–10 CPD | 3.7% |
| 4 or more drinks a week | 0.1% | Smoking >10 CPD | 2.2% |
|
| |||
| 9 month post- partum | |||
|
| |||
| No alcohol use | 63.1% | No smoking | 80.1% |
| Less than 1 drink a week | 20.0% | Smoking <5 CPD | 5.7% |
| 1–3 drinks a week | 13.2% | Smoking 6–10 CPD | 7.3% |
| 4 or more drinks a week | 3.7% | Smoking >10 CPD | 6.9% |
|
| |||
| 2 years post- partum | |||
|
| |||
| No alcohol use | 68.7% | No smoking | 80.7% |
| Less than 1 drink a week | 15.6% | Smoking <5 CPD | 5.4% |
| 1–3 drinks a week | 11.2% | Smoking 6–10 CPD | 7.3% |
| 4 or more drinks a week | 4.5% | Smoking >10 CPD | 6.6% |
|
| |||
| Child in Preschool | |||
|
| |||
| No alcohol use | 64.0% | No smoking | 81.3% |
| Less than 1 drink a week | 14.6% | Smoking <5 CPD | 4.6% |
| 1–3 drinks a week | 15.0% | Smoking 6–10 CPD | 7.2% |
| 4 or more drinks a week | 6.4% | Smoking >10 CPD | 6.9% |
|
| |||
| Child in Kindergarten | |||
|
| |||
| No alcohol use | 64.8% | No smoking | 82.8% |
| Less than 1 drink a week | 13.5% | Smoking <5 CPD | 5.0% |
| 1–3 drinks a week | 14.6% | Smoking 6–10 CPD | 6.1% |
| 4 or more drinks a week | 7.2% | Smoking >10 CPD | 6.1% |
Heterogeneity in smoking and drinking patterns
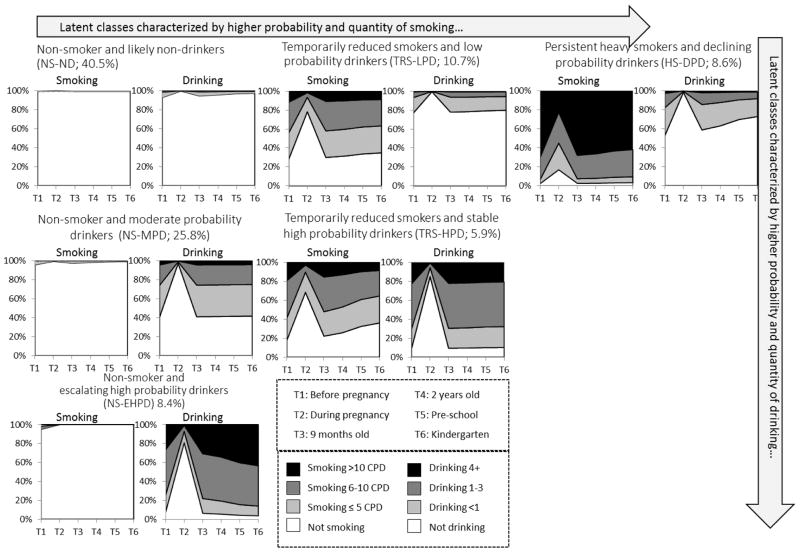
A 6-class solution (LL=−54462.96 (39), BIC=109279.97) was selected (Table 3). While the 9-class solution presents the lowest BIC, the reduction in BIC from a 4 to a 5-class solution (1815.88) or from a 5 to a 6-class solution (1484.80) is considerably greater than for the other models, indicating diminishing returns when adding additional classes. Figure 1 presents the model estimated time-specific probability of endorsing each category of smoking and drinking, given class membership. Over 40% of mothers (non-smokers and likely non-drinkers; NS-ND) exhibited a pattern of very low probability of smoking (<0.01) and drinking (<0.05). Nearly all NS-ND mothers reduced or quit both substances by the third trimester of pregnancy.
Table 3.
Determining the number of classes in GGMM (N=8,800), ECLS-B
| Model | LL | # of parameters | BICa | Entropyb |
|---|---|---|---|---|
| 1 class model | −71759.190 | 14 | 143645.474 | 1 |
| 2 class model | −61631.708 | 19 | 123435.901 | 0.962 |
| 3 class model | −57202.961 | 24 | 114623.799 | 0.886 |
| 4 class model | −56158.689 | 29 | 112580.645 | 0.881 |
| 5 class model | −55228.056 | 34 | 110764.769 | 0.861 |
| 6 class model | −54462.962 | 39 | 109279.972 | 0.859 |
| 7 class model | −54093.646 | 44 | 108586.732 | 0.863 |
| 8 class model | −53863.760 | 49 | 108172.350 | 0.856 |
| 9 class model | −53688.713 | 54 | 107867.648 | 0.857 |
Bayesian Information Criterion, lower values indicating better fit.
Entropy ranges from 0 to 1 and indicates a better classification of individuals as values approach 1.
Figure 1.
Conditional 6 Class Model of Maternal Smoking and Drinking, ECLS-B*
*The trend during pregnancy may not be reflected in class labels due to limited space. Specifically, mothers in the TRS-LPD class significantly reduced smoking and quit drinking during pregnancy. A similar trend is reflected in the HS-DPD class. Mothers in the NS-MPD class and the NS-EHPD class reduced or quit drinking, and mothers in the TRS-HPD class also reduced both smoking and drinking during pregnancy (see Result section for details).
Two classes featured non-smoking patterns with various levels of drinking. A quarter (25.8%) in the class non-smokers and moderate probability drinkers (NS-MPD) remained abstinent from smoking while displaying a steady probability (about 0.6) of drinking any alcohol and a very low (<0.05) probability of drinking 4+ drinks per week. Non-smokers and escalating high probability drinkers (NS-EHPD) (8.4%) are presented with a near-zero probability of smoking and a steadily high probability (0.9) of drinking any alcohol outside of pregnancy with an increasing trend in quantity through early parenting. NS-EHPD mothers had a probability as high as 0.4 of consuming 4+ drinks per week by the time the child entered kindergarten.
Another two classes displayed low-level drinking with moderate to high levels of smoking. One tenth (10.7%) of the mothers (temporary reduction smokers and low probability drinkers; TRS-LPD) exhibited a probability of any smoking as high as close to 0.8, which dropped to 0.3 during pregnancy. While we observe a slight trend toward decreasing smoking over time, TRS-LPD mothers had about a 0.7 probability of smoking by the time their child entered kindergarten. At the same time, TRS-LPD mothers displayed a steady, low probability (<0.2) of drinking (limited to <3 drinks per week) outside of pregnancy (during which most quit drinking). By comparison to TRS-LPD mothers, the persistent heavy smokers and declining probability drinkers (HS-DPD; 8.6%) displayed a very high and stable probability (>0.9) of any smoking throughout the study period with a temporary reduction during pregnancy and a clear decline in drinking over time despite a slightly higher probability (0.4) of drinking any alcohol postpartum. Further, the probability of HS-DPD mothers reporting heavy smoking prior to conception and postpartum was as high as 0.7. Finally, temporarily reduced smokers and stable high probability drinkers (TRS-HPD; 5.9%) displayed a similar smoking trend compared to the TRS-LPD class, with a significant trend toward declining smoking probability. However, their drinking behavior closely resembled the NS-EHPD class, with a lower probability (<0.2) of 4+ weekly drinks.
Covariate effects using the non-use NS-ND class as reference
Table 4a presents the effect of covariates on the log odds of being in each of the five classes, using the non-use NS-ND class as the reference. When compared to their peers who gave birth between 26 and 35, mothers younger than 25 were twice as likely (AOR=2.04) to be in the predominately smoking TRS-LPD class, while 80% less likely (AOR=0.17) to be in the drinking NS-EHPD class. By contrast, mothers who gave birth past age 35 were more than twice as likely (AOR=2.21) to be in the drinking NS-EHPD class, when compared to their 26–35 year-old peers. Non-white mothers, compared to whites, were consistently less likely (AORs range from 0.02 to 0.49) to be in any of the five smoking/drinking classes. Marriage or cohabitation had a protective effect (AORs range from 0.46 to 0.72) from being in each of the smoking/drinking classes (with the exception of the drinking NS-EHPD class). Mothers of higher levels of education, compared to those with less than a high school diploma, were more likely (AORs range from 1.56 to 2.48) to be in the drinking NS-MPD class while less likely (AORs range from 0.04 to 0.55) to be in the predominately smoking HS-DPD class. In addition, compared to mothers with the least education, those with at least a college degree were over five times more likely (AOR=5.40) to be in the drinking NS-EHPD class; those with some college (AOR=0.60) or at least a college degree (AOR=0.16) were less likely to be in the predominately smoking TRS-LPD class; and those with a high school degree were twice as likely (AOR=1.88) to be in concurrent use TRS-HPD class. Having a household income higher than 185% of the poverty line significantly increased the probability (AORs range from 2.25 to 6.92) of being in the two drinking classes, NS-MPD and NS-EHPD, and concurrent use TRS-HPD classes. Mothers from this income bracket were only 40% as likely (AOR=0.63) to be in the predominately smoking HS-DPD class, when compared to those who earned an income below the poverty line.
Table 4a.
Covariate a effects on Maternal Smoking and Drinking Trajectories (N=8,800), ECLS-B (Reference class: NS-ND)
| Drinking Classes | Concurrent Users | ||||
|---|---|---|---|---|---|
|
| |||||
| Predominantly Smokers | Both Alc/Cigs | ||||
|
| |||||
| NS-MPD (25.8%) | NS-EHPD (8.4%) | TRS-LPD (10.7%) | HS-DPD (8.6%) | TRS-HPD (5.9%) | |
|
|
|||||
| AORb | AOR | AOR | AOR | AOR | |
| Maternal Age | |||||
| Age 18–25 | – | 0.17** | 2.04** | – | – |
| Age 36+ | – | 2.21** | – | – | – |
| Maternal Race/Ethnicity | |||||
| Black | 0.45** | 0.19** | 0.27** | 0.03** | 0.40** |
| Hispanic | 0.37** | 0.26** | 0.23** | 0.02** | 0.14** |
| Asian and Others | 0.42** | 0.21** | 0.41** | 0.28** | 0.49** |
| Mother Married or Cohabiting with Partner | |||||
| Yes | 0.58** | – | 0.72* | 0.65* | 0.46** |
| Maternal Education | |||||
| High school | 1.56* | – | – | 0.55** | 1.88* |
| Some college | 2.18** | – | 0.60* | 0.29** | – |
| College or grad school | 2.48** | 5.40* | 0.16** | 0.04** | – |
| Household Income (proportion of federal poverty line) | |||||
| 100–130% | – | – | – | – | – |
| 130–185% | – | – | – | – | – |
| >185% | 2.43** | 6.92* | – | 0.63* | 2.25** |
| Moderate or Severe Postpartum Depression | |||||
| Depressed | – | – | 1.45** | 2.21* | – |
| Breastfeeding | |||||
| ≤ 6 months | 1.37* | 1.76* | – | 0.76* | – |
| > 6 months | – | – | 0.32** | 0.22** | 0.40** |
| Maternal Employment Status | |||||
| Part-time | 1.55** | 1.69** | 1.35* | – | 1.76** |
| Full-time | 1.51** | – | – | 1.40* | – |
| Planned pregnancy | – | – | 0.73* | 0.65** | 0.69* |
P-value <0.05
P-value<0.01
The choice of reference category for each covariates were carefully selected based on theory, empirical distribution, as well as past literature. The reference category for Maternal Age is 26–35, for Maternal Race/Ethnicity is White, for Maternal Education is Less than high school, for Household Income is Lower than poverty line, for Breastfeeding is Never breastfeed, and for Employment Status is Not employed.
Adjusted Odds Ratio; AOR greater than 1 represents increasing log odds of being assigned to a given class compared to the NS-ND class.
Postpartum depression had a significant impact on being classified as predominately smokers, TRS-LPD (AOR=1.45) or HS-DPD (AOR=2.21). The impact of depression on the three higher drinking probability classes (NS-MPD, NS-EHPD, and TRS-HPD) failed to reach the 0.05 significance level. Compared to those who did not breastfeed, breastfeeding for at least 6 months had a protective effect (AORs range from 0.22 to 0.40) from being predominately smokers, TRS-LPD or HS-DPD, or concurrent users TRS-HPD. Despite the protective effect of breastfeeding for up to 6 months from being in the predominately smoking HS-DPD class (AOR=0.76), it increased the likelihood of being in the two drinking, NS-MPD (AOR=1.37) or NS-EHPD (AOR=1.76) classes. Further, both part-time (AOR=1.55) and full-time (AOR=1.51) employment increased the likelihood of classification in the drinking NS-MPD class. While part-time employment increased the likelihood of being classified in the drinking NS-EHPD (AOR=1.69) class, predominately smoking TRS-LPD (AOR=1.35), or the concurrent use TRS-HPD (AOR=1.76) classes, full-time employment increased the likelihood of being classified as predominately smokers HS-DPD (AOR=1.40). Finally, those who planned pregnancy were less likely to be in the two predominately smoking classes, TRS-LPD (AOR=0.73) and HS-DPD (AOR=0.65) or the concurrent use TRS-HPD class (AOR=0.69).
Covariate effects using alternative reference classes
Table 4b presents the effects of covariates using the concurrent use, TRS-HPD class as the reference, showing the distinction between the concurrent use class and the drinking or predominately smoking classes. For example, being married or cohabitating, breastfeeding for at least 6 month and planned pregnancy all had a protective effect against being in the concurrent use class. Married or cohabitated mothers were more likely to be in predominately smoking TRS-LPD (AOR=1.56) class or the non-use NS-ND class (AOR=2.52). Compared to those who did not breastfeed, breastfeeding for at least 6 months were more likely to be in either of the two drinking classes, NS-MPD (AOR=2.63) or NS-EHPD (AOR=3.16) as well as the non-use, NS-ND class (AOR=2.53). Mothers who planned their pregnancy were more likely to be in the predominately drinking NS-EHPD class (AOR=1.93) or the non-use NS-ND class (AOR=1.46).
Table 4b.
Covariate a effects on Maternal Smoking and Drinking Trajectories (N=8,800), ECLS-B (Reference class: TRS-HPD)
| Concurrent Users | |||||
|---|---|---|---|---|---|
|
|
|||||
| Drinking Classes | Predominantly Smokers | Non-use class | |||
|
| |||||
| NS-MPD (25.8%) | NS-EHPD (8.4%) | TRS-LPD (10.7%) | HS-DPD (8.6%) | NS-ND (40.5%) | |
|
| |||||
| AORb | AOR | AOR | AOR | AOR | |
| Maternal Age | |||||
| Age 18–25 | 0.70* | 0.13** | 1.51* | -- | -- |
| Age 36+ | -- | -- | -- | -- | -- |
| Maternal Race/Ethnicity | |||||
| Black | -- | -- | -- | 0.07** | 2.52** |
| Hispanic | 2.75** | -- | -- | 0.13** | 7.40** |
| Asian and Others | -- | 0.44* | -- | -- | 2.04** |
| Mother Married or Cohabiting with Partner | |||||
| Yes | -- | -- | 1.56* | -- | 2.17* |
| Maternal Education | |||||
| High school | -- | -- | 0.45** | 0.29** | 0.53* |
| Some college | -- | -- | 0.39** | 0.19** | -- |
| College or grad school | 2.82** | 6.15* | 0.18** | 0.05** | -- |
| Household Income (proportion of federal poverty line) | |||||
| 100–130% | -- | -- | 0.44** | -- | -- |
| 130–185% | -- | -- | -- | -- | -- |
| >185% | -- | -- | -- | 0.28** | 0.44** |
| Moderate or Severe Postpartum Depression | |||||
| Depressed | -- | -- | -- | 1.74** | -- |
| Breastfeeding | |||||
| ≤ 6 months | -- | -- | -- | 0.63* | -- |
| > 6 months | 2.63** | 3.16** | -- | -- | 2.53** |
| Maternal Employment Status | |||||
| Part-time | -- | -- | -- | 0.50** | 0.57** |
| Full-time | -- | -- | -- | -- | -- |
| Planned pregnancy | -- | 1.93** | -- | -- | 1.46* |
P-value <0.05
P-value<0.01
The choice of reference category for each covariates were carefully selected based on theory, empirical distribution, as well as past literature. The reference category for Maternal Age is 26–35, for Maternal Race/Ethnicity is White, for Maternal Education is Less than high school, for Household Income is Lower than poverty line, for Breastfeeding is Never breastfeed, and for Employment Status is Not employed.
Adjusted Odds Ratio; AOR greater than 1 represents increasing log odds of being assigned to a given class compared to the TRS-HPD class.
Table 4c presents covariate effects using the predominately smoking TRS-LPD class as the reference, showing the comparison between the three smoking classes, TRS-LPD, HS-DPD, and TRS-HPD with different quitting rate during pregnancy. Younger age, being Black or Hispanics, and having a higher education all had a protective effect against continuous smoking during pregnancy. Compared to their peers, mothers younger than 25 were 60% less likely (AOR=0.42), Black mothers were 90% less likely (AOR=0.11), Hispanic mothers were over 90% less likely (AOR=0.08), and mothers of higher education levels were less likely (AORs ranged from 0.26 to 0.65) to continue smoking during pregnancy. In contrast, postpartum depression increased the likelihood of continuous smoking through pregnancy by 50% (AOR=1.52).
Table 4c.
Covariate a effects on Maternal Smoking and Drinking Trajectories (N=8,800), ECLS-B (Reference class: TRS-LPD)
| Concurrent Users | |||||
|---|---|---|---|---|---|
|
|
|||||
| Drinking Classes | Predominantly Smokers | Both Alc/Cigs | Non-user class | ||
|
| |||||
| NS-MPD (25.8%) | NS-EHPD (8.4%) | HS-DPD (8.6%) | TRS-HPD (5.9%) | NS-ND (40.5%) | |
|
| |||||
| AORb | AOR | AOR | AOR | ||
| Maternal Age | |||||
| Age 18–25 | 0.46** | 0.08** | 0.42** | 0.66* | 0.49** |
| Age 36+ | -- | 2.31** | -- | -- | -- |
| Maternal Race/Ethnicity | |||||
| Black | 1.70* | -- | 0.11** | -- | 3.76** |
| Hispanic | 1.61* | -- | 0.08** | -- | 4.34** |
| Asian and Others | -- | 0.52* | -- | -- | 2.41** |
| Mother Married or Cohabiting with Partner | |||||
| Yes | -- | -- | -- | 0.64* | 1.39* |
| Maternal Education | |||||
| High school | 1.86** | -- | 0.65* | 2.23** | -- |
| Some college | 3.63** | -- | 0.49* | 2.54** | 1.66* |
| College or grad school | 15.30** | 33.35** | 0.26* | 5.41** | 6.17** |
| Household Income (proportion of federal poverty line) | |||||
| 100–130% | -- | -- | -- | -- | -- |
| 130–185% | -- | -- | -- | -- | -- |
| >185% | 2.47** | 7.02* | -- | 2.29* | -- |
| Moderate or Severe Postpartum Depression | |||||
| Depressed | -- | -- | 1.52* | -- | 0.69** |
| Breastfeeding | |||||
| ≤ 6 months | -- | 1.69* | 0.73* | -- | -- |
| > 6 months | 3.29** | 3.95** | -- | -- | 3.16** |
| Maternal Employment Status | |||||
| Part-time | -- | -- | -- | -- | 0.74* |
| Full-time | 1.59** | -- | -- | -- | -- |
| Planned pregnancy | -- | 1.81** | -- | -- | 1.37* |
P-value <0.05
P-value<0.01
The choice of reference category for each covariates were carefully selected based on theory, empirical distribution, as well as past literature. The reference category for Maternal Age is 26–35, for Maternal Race/Ethnicity is White, for Maternal Education is Less than high school, for Household Income is Lower than poverty line, for Breastfeeding is Never breastfeed, and for Employment Status is Not employed.
Adjusted Odds Ratio; AOR greater than 1 represents increasing log odds of being assigned to a given class compared to the TRS-LPD class.
DISCUSSION
Women who engage in smoking and/or risky alcohol consumption threaten their own health, and for those women contemplating motherhood, their children’s health as well. Understanding the concurrent patterns of women’s drinking and smoking during this critical period of time and identifying early risk factors for concurrent use constitutes the first step to inform public health and clinical efforts to prevent harmful effects. This is the first study that explicitly identifies women’s trajectories of concurrent drinking and smoking over an extended period spanning from pre-conception through the child’s entry to kindergarten. The large nationally representative sample allows for the identification of rare patterns. Across the full study period, our sample of U.S. mothers fell into six smoking/alcohol consumption patterns. Overall, consistent with other national data sources (Substance Abuse and Mental Health Services Administration 2014), abstinence from drinking during pregnancy was more common than from smoking, which was reportedly continued by at least one in 10 women in the third trimester of pregnancy. Education about the risks of maternal drinking during pregnancy has come from numerous sources since the 1970s (Warren 2015). Moreover, nicotine is highly addictive and pregnant women may have a harder time quitting smoking than quitting drinking (Hellerstedt, Pirie et al. 1998). Incongruously, the probability of drinking during the third trimester of pregnancy was near zero for the persistent heavy smokers (HS-DPD), but it was close to 0.2 for those who temporarily cut back on smoking during pregnancy (TRS-HPD) and those who did not smoke (NS-EHPD). Perhaps there is a behavioral time constraint, a financial constraint, or a self-moderation out of concerns of social stigma related to consuming both cigarettes and alcohol in larger quantities for women at this point in their life cycle. As discussed below, external factors may be augmenting stressful conditions, limiting access to health services, undermining quit efforts, or supporting continued smoking and/or alcohol consumption during pregnancy. Research is needed to untangle where these factors interact with individual dependency and behavior.
Overall, the prevalence of smoking and/or drinking both re-approached preconception levels by 9 months after child birth and thereafter remained relatively stable, with some variations from this pattern. In sum, the six classes of mothers defined by alcohol consumption and cigarette smoking over the study period not only reflect the expected patterns of each substance use (Mumford et al. 2014, Liu, Mumford, & Petras, 2016), but also identify differential patterns informing better discernment for public health education and for clinical treatment. These results identify classes of women who may benefit from closer attention to the interaction of alcohol and tobacco use to prevent amplification of deleterious outcomes (McKee & Weinberger 2013).
Results relating maternal characteristics to these patterns highlight the importance of clinical implications of risk factors for the complex spectrum of concurrent behavioral choices. Results are largely consistent with the literature regarding the effect of maternal age at childbirth (Meschke et al., 2013); education (Laborde & Mair, 2012); marital status (Tsai et al., 2010); income (Jagodzinski & Fleming, 2007); employment (Mumford & Liu, 2015); postpartum depression (Munafo, Heron, & Araya, 2008); and breastfeeding (Jagodzinski & Fleming, 2007). This study adds to the literature by examining how maternal smoking and drinking fluctuate concurrently in concert with the context of demographic and behavioral correlates.
Some particularly interesting relationships are worth highlighting. For over half of the women followed through this perinatal and parenting period, drinking and smoking reports may not be reliable reciprocal predictors, adding value to knowledge of the distinguishing correlates of separate and concurrent drinking and smoking trajectories. Younger maternal age increased the likelihood of concurrent drinking and smoking behavior, while age is more related to smoking than to drinking. However, compared to older mothers, younger mothers were more likely to temporarily quit smoking during pregnancy. Higher education consistently predicted higher likelihood of drinking and lower likelihood of smoking, and women with at a least a high school degree tended to quit smoking during pregnancy. Women who earned a high school degree but did not have further education faced an elevated risk of both smoking and drinking, as they are least likely to stay abstinence across this time period. Although employment and income overall are stronger predictors of drinking than of smoking, part-time employment suggests greater risks than full-time employment of resuming smoking post-partum and of escalating or higher probabilities of drinking. Women with higher income were also more likely to resume smoking postpartum while continuously engaging in high probability drinking. Contextual mechanisms associated with part-time work (e.g., disposable income, available free time for socializing with friends, or possibly the stressors of managing both childcare and work responsibilities) may be related to ongoing smoking and drinking and suggest opportunities for preventive health services. Postpartum depression was more related to smoking than drinking, showing particularly strong relationship to the predominately smoking classes. In that breastfeeding is negatively related to both smoking and drinking, postnatal women’s health care and pediatric visits may support women maintaining reduced alcohol consumption and smoking abstinence by encouraging breastfeeding for at least six months. Finally, the finding that planned pregnancy is more protective against smoking than drinking may reflect persistent misconceptions by women of reproductive age, that is, there are some safe options for drinking during pregnancy (Elek et al., 2013), with implications for the educational work that remains in communicating the guidelines of the American College of Obstetricians and Gynecologists (ACOG) regarding drinking during pregnancy.
This study suggests a new direction to reach the Healthy People 2020 target of minimizing maternal drinking and smoking during pregnancy, as well as during the preconception and postpartum periods. Expanding prevention of maternal smoking and alcohol misuse may require redesigning both research and clinical approaches to explicitly target all women of reproductive age, simultaneously broadening and focusing public health efforts. Building on improved screening tools, clinicians more than ever need effective intervention and treatment regimens. Unfortunately, screening and treatment guidance for women’s substance use tends to be limited to the period of pregnancy. ACOG explicitly targets their guidance for providers and patients regarding alcohol use and smoking to the period of pregnancy, leaving out critical periods preconception (as women may drink alcohol before they realize they are pregnant) and during the early years of parenting. Even during pregnancy, systematic screening is not yet the reality (e.g., Oser, Biebel, Harris, Klein, & Leukefeld, 2011), suggesting an opportunity to improve provider training before, during, and after the perinatal period.
Similar to the limited focal time period for screening women for smoking and drinking behavior, interventions have also targeted a narrow window. While there is some evidence of intervention effectiveness during pregnancy on drinking (Gilinsky, Swanson, & Powers, 2011) and smoking (Lumley et al., 2009) abstinence, limited attention has been paid to the prenatal and postpartum periods. Recent reviews of postpartum home visits found no evidence of effectiveness in reducing alcohol misuse (Yonemoto, Dowswell, Nagain, & Mori, 2013) and only limited evidence for the effectiveness in promoting smoking cessation (Likis et al., 2014). However, recent success in pediatric settings of parental smoking cessation (Winickoff et al., 2013) and risky drinking interventions (Jonas et al., 2012), in concert with this study’s findings of concurrent use patterns, suggest screening for concurrent maternal smoking and alcohol consumption patterns in pediatric settings may be warranted.
Results should be interpreted in the context of the following limitations. First, this study relies on self-reports of the ECLS-B measures of smoking and alcohol consumption. Self-reports of perinatal smoking behavior, at least, are highly correlated with maternal (Pickett et al., 2009) and co-resident children’s urinary cotinine levels (Ino, Ohtani et al. 2011). Second, two time points of the outcome measures are collected restrospectively, but retrospective measures of smoking (Dietz et al., 2011) and drinking (Alvik et al., 2006) during pregnancy have been shown to exceed underreporting that may have reflected awareness of social desirability of maternal pregnancy behaviors. Third, the data do not support investigation of whether greater quantities of weekly alcohol consumption are clustered in sessions of binge drinking or are spread out as more moderate daily consumption. Fourth, while there is no evidence of secular changes in women’s smoking behavior in the years since the ECLS-B data were collected (Centers for Disease Control and Prevention, 2011), there has been some decline in women’s drinking during pregnancy(Substance Abuse and Mental Health Services Administration, 2002, 2014). Likewise, it is possible that the pattern of concurrent alcohol use and smoking by women at this developmental point in their life course has changed significantly in the years from the results presented here. Fifth, the current study does not account for a range of social and environmental factors that may be related to women’s smoking and drinking behavior at this point in their life course. For example, while low income is a known risk factor for smoking behavior, food insecurity and social networks within disadvantaged neighborhoods may also merit investigation for impact on women’s concurrent use behavior (Castro, Heck, Forster, Widome, & Cubbin, 2015). Addressing individual risk behaviors, particularly in advance of conception, in the context of engaged community structures and attention to environmental influences (e.g., education, employment, access to health services, housing) as a model for programming (e.g., Preconception Stress and Resiliency Pathways (PSRP) model; see Ramey et al. 2015) as well as research regarding concurrent substance use pathways is warranted.
Compliance with Ethical Standards
This research was funded by the National Institute on Drug Abuse (1R01DA030496).
The authors declare that they have no conflict of interest.
All procedures were in accordance with the ethical standards of the NORC IRB and 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this secondary data analysis study, formal consent is not required.
Supplementary Material
References
- Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcoholism-Clinical and Experimental Research. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psychological Methods. 2004;9:30–52. doi: 10.1037/1082-989x.9.1.30. [DOI] [PubMed] [Google Scholar]
- Brown CW, Olson HC, Croninger RG. Maternal Alcohol Consumption During Pregnancy and Infant Social, Mental, and Motor Development. Journal of Early Intervention. 2010;32:110–126. doi: 10.1177/1053815110366654. [DOI] [Google Scholar]
- Castro Y, Heck K, Forster JL, Widome R, Cubbin C. Social and Environmental Factors Related to Smoking Cessation among Mothers: Findings from the Geographic Research on Wellbeing (GROW) Study. American Journal of Health Behavior. 2015;39:809–822. doi: 10.5993/AJHB.39.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. PRAMS and Smoking Data Tables. 2011 Retrieved from http://www.cdc.gov/prams/data-tobaccotables.htm.
- Cooklin AR, Donath SM, Amir LH. Maternal employment and breastfeeding: results from the longitudinal study of Australian children. Acta Pædiatrica. 2008;97:620–623. doi: 10.1111/j.1651-2227.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Homa D, England LJ, Burley K, Tong V, Dube SR, Bernert JT. Estimates of Nondisclosure of Cigarette Smoking Among Pregnant and Nonpregnant Women of Reproductive Age in the United States. American Journal of Epidemiology. 2011;173:355–359. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- Edwards EM, Werler MM. Alcohol Consumption and Time to Recognition of Pregnancy. Maternal and Child Health Journal. 2006;10:467–472. doi: 10.1007/s10995-006-0083-1. [DOI] [PubMed] [Google Scholar]
- Elek E, Harris SL, Squire CM, Margolis M, Weber MK, Dang EP, Mitchell B. Women’s Knowledge, Views, and Experiences Regarding Alcohol Use and Pregnancy: Opportunities to Improve Health Messages. American Journal of Health Education. 2013;44:177–190. doi: 10.1080/19325037.2013.768906. http://dx.doi.org/10.1080/19325037.2013.768906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Edwards EP, Leonard KE. A conceptual model for the development of externalizing behavior problems among kindergarten children of alcoholic families: role of parenting and children’s self-regulation. Developmental Psychology. 2007;43:1187–1201. doi: 10.1037/0012-1649.43.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan KD, West J. Children Born in 2001: First Results from the Base Year of the Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) U.S Department of Education, National Center for Education Statistics; 2004. (NCES 2005036) Retrieved from http://nces.ed.gov/pubs2005/2005036.pdf. [Google Scholar]
- Gilinsky A, Swanson V, Power K. Interventions delivered during antenatal care to reduce alcohol consumption during pregnancy: A systematic review. Addiction Research & Theory. 2011;19:235–250. doi: 10.3109/16066359.2010.507894. [DOI] [Google Scholar]
- Haynes GW, Neuman D, Hook C, Haynes DC, Steeley JM, Kelley M, … Paine M. Comparing Child and Family Outcomes Between Two Home Visitation Programs. Family and Consumer Sciences Research Journal. 2015;43:209–228. doi: 10.1111/fcsr.12098. [DOI] [Google Scholar]
- Hellerstedt WL, Pirie PL, Lando HA, Curry SJ, McBride CM, Grothaus LC, Nelson JC. Differences in preconceptional and prenatal behaviors in women with intended and unintended pregnancies. Amermical Journal of Public Health. 1998;88:663–666. doi: 10.2105/ajph.88.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll KS, Hettema JE, Cropsey KL, Jackson JP. Preconception Markers of Dual Risk for Alcohol and Smoking Exposed Pregnancy: Tools for Primary Prevention. Journal of Women’s Health. 2011;20:1627–1633. doi: 10.1089/jwh.2010.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Ohtani T, Yoshimi I. Urinary Biomarkers for Secondhand Smoke. Journal of Clinical Laboratory Analysis. 2011;25:354–358. doi: 10.1002/jcla.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen ML, Sørensen NO, Broberg L, Damm P, Hedegaard M, Tabor A, Hegaard HK. Alcohol consumption and binge drinking in early pregnancy. A cross-sectional study with data from the Copenhagen Pregnancy Cohort. BMC Pregnancy and Childbirth. 2015;15:1–10. doi: 10.1186/s12884-015-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodzinski T, Fleming MF. Correlates of postpartum alcohol use. WMJ: official publication of the State Medical Society of Wisconsin. 2007;106:319–325. [PubMed] [Google Scholar]
- Jester JM, Jacobson SW, Sokol RJ, Tuttle BS, Jacobson JL. The Influence of Maternal Drinking and Drug Use on the Quality of the Home Environment of School-Aged Children. Alcoholism: Clinical and Experimental Research. 2000;24:1187–1197. doi: 10.1111/j.1530-0277.2000.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Garbutt JC, Amick HR, Brown JM, Brownley KA, Council CL, … Harris RP. Behavioral Counseling After Screening for Alcohol Misuse in Primary Care: A Systematic Review and Meta-analysis for the US Preventive Services Task Force. Annals of Internal Medicine. 2012;157:645–654. doi: 10.7326/0003-4819-157-9-201211060-00544. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug & Alcohol Dependence. 2009;104:S24–S33. doi: 10.1016/j.drugalcdep.2008.12.005. S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde N, Mair C. Alcohol Use Patterns Among Postpartum Women. Maternal & Child Health Journal. 2012;16:1810–1819. doi: 10.1007/s10995-011-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Quere M, Rehm J, Popova S. Alcohol use, smoking and their co-occurrence during pregnancy among Canadian women, 2003 to 2011/12. Addictive Behaviors. 2015;50:102–109. doi: 10.1016/j.addbeh.2015.06.018. http://dx.doi.org/10.1016/j.addbeh.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Likis F, Andrews J, Fonnesbeck C, Hartmann K, Jerome R, Potter S, … McPheeters M. Smoking Cessation Interventions in Pregnancy and Postpartum Care: Evidence Report/Technology Assessment No.214. 2014 (AHRQ Publication No. 14-E001-EF). Retrieved from Rockville, MD: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- Liu W, Mumford E, Petras H. Maternal Alcohol Consumption During the Perinatal and Early Parenting Period: A Longitudinal Analysis. Maternal and Child Health Journal. 2016;20:376–85. doi: 10.1007/s10995-015-1836-5. [DOI] [PubMed] [Google Scholar]
- Liu W, Mumford EA, Petras H. Maternal patterns of postpartum alcohol consumption by age: A longitudinal analysis of adult urban mothers. Prevention Science. 2015;16:353–363. doi: 10.1007/s11121-014-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2009;(3):Cd001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH. How Can We Use Our Knowledge of Alcohol-Tobacco Interactions to Reduce Alcohol Use? Annual Review of Clinical Psychology. 2013;9:649–674. doi: 10.1146/annurev-clinpsy-050212-185549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschke LL, Holl J, Messelt S. Older Not Wiser: Risk of Prenatal Alcohol Use by Maternal Age. Maternal & Child Health Journal. 2013;17:147–155. doi: 10.1007/s10995-012-0953-7. [DOI] [PubMed] [Google Scholar]
- Meyer-Leu Y, Lemola S, Daeppen JB, Deriaz O, Gerber S. Association of Moderate Alcohol Use and Binge Drinking During Pregnancy with Neonatal Health. Alcoholism: Clinical and Experimental Research. 2011;35:1669–1677. doi: 10.1111/j.1530-0277.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- Mumford EA, Hair EC, Yu TC, Liu W. Women’s longitudinal smoking patterns from preconception through child’s kindergarten entry: profiles of biological mothers of a 2001 US birth cohort. Maternal and Child Health Journal. 2014;18:810–820. doi: 10.1007/s10995-013-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford EA, Liu W. Growth models of maternal smoking behavior: Individual and contextual factors. Substance Use & Misuse. 2015;50:1261–1273. doi: 10.3109/10826084.2014.998234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research. 2008;10:1609–1620. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Odendaal HJ, Steyn DW, Elliott A, Burd L. Combined Effects of Cigarette Smoking and Alcohol Consumption on Perinatal Outcome. Gynecologic and Obstetric Investigation. 2008;67:1–8. doi: 10.1159/000150597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbuanu C, Glover S, Probst J, Liu JH, Hussey J. The Effect of Maternity Leave Length and Time of Return to Work on Breastfeeding. Pediatrics. 2011;127:E1414–E1427. doi: 10.1542/peds.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser C, Biebel E, Harris M, Klein E, Leukefeld C. Gender Differences in Provider’s Use of a Standardized Screening Tool for Prenatal Substance Use. Journal of Addiction Medicine. 2011;5:36–42. doi: 10.1097/ADM.0b013e3181ccec2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. Journal of Child Psychology and Psychiatry. 2009;50:254–262. doi: 10.1111/j.1469-7610.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine & Tobacco Research. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. ntp117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J, McDermott L, Loxton D, Chojenta C. A Prospective Study of Prevalence and Predictors of Concurrent Alcohol and Tobacco Use During Pregnancy. Maternal and Child Health Journal. 2013;17:76–84. doi: 10.1007/s10995-012-0949-3. [DOI] [PubMed] [Google Scholar]
- Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Shalowitz M, … Raju TNK. The Preconception Stress and Resiliency Pathways Model: A Multi-Level Framework on Maternal, Paternal, and Child Health Disparities Derived by Community-Based Participatory Research. Maternal and Child Health Journal. 2015;19:707–719. doi: 10.1007/s10995-014-1581-1. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Streissguth A. Offspring Effects of Prenatal Alcohol Exposure from Birth to 25 Years: The Seattle Prospective Longitudinal Study. Journal of Clinical Psychology in Medical Settings. 2007;14(2):81–101. doi: 10.1007/s10880-007-9067-6. [DOI] [Google Scholar]
- Streissguth AP, Barr HM, Bookstein FL, Sampson PD, Olson HC. The long-term neurocognitive consequences of prenatal alcohol exposure: A 14-year study. Psychological Science. 1999;10:186–190. doi: 10.1111/1467-9280.00131. [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results From the 2001 National Household Survey on Drug Abuse: Volume I, Summary of National Findings. 2002. Retrieved from Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Substance Use among Women During Pregnancy and Following Childbirth. 2009 Retrieved from Rockville, MD: http://www.oas.samhsa.gov/2k9/135/PregWoSubUseHTML.pdf.
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. 2014. Retrieved from Rockville, MD. [PubMed] [Google Scholar]
- Tsai J, Floyd R, Green P, Denny C, Coles C, Sokol R. Concurrent Alcohol Use or Heavier Use of Alcohol and Cigarette Smoking Among Women of Childbearing Age with Accessible Health Care. Prevention Science. 2010;11:197–206. doi: 10.1007/s11121-009-0158-5. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke:A Report of the Surgeon General. Atlanta, GA: 2006. Retrieved http://www.surgeongeneral.gov/library/reports/secondhandsmoke/fullreport.pdf. [Google Scholar]
- Warren KR. A Review of the History of Attitudes Toward Drinking in Pregnancy. Alcoholism: Clinical and Experimental Research. 2015;39:1110–1117. doi: 10.1111/acer.12757. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: Journal of the Econometric Society. 1980;48:817–838. [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2008;11:373–517. doi: 10.1080/10937400801921320. 793024706 [pii] [DOI] [PubMed] [Google Scholar]
- Williams JF, Smith VC. Fetal Alcohol Spectrum Disorders. Pediatrics. 2015 doi: 10.1542/peds.2015-3113. [DOI] [PubMed] [Google Scholar]
- Winickoff JP, Nabi-Burza E, Chang Y, Finch S, Regan S, Wasserman R, … Rigotti NA. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics. 2013;132:109–117. doi: 10.1542/peds.2012-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemoto N, Dowswell T, Nagai S, Mori R. Home visits in the early period after the birth of a baby. Cochrane Database of Systematic Reviews. 2013;(7) doi: 10.1002/14651858.CD009326.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.