Summary
Toxic leukoencephalopathy results from damage to the white matter caused by various toxins. It manifests itself as white matter signal abnormalities with or without the presence of restricted diffusion. These changes are often reversible if the insulting agent is removed early, with the exception of posthypoxic leukoencephalopathy that can manifest itself 1–2 weeks after the initial insult. However, many other potential causes of white matter signal abnormalities can mimic the changes of toxic leukoencephalopathy. Thus, familiarity with the causes, clinical presentation and particularly imaging findings of toxic leukoencephalopathy is critical for early treatment and improved prognosis. The purpose of this pictorial essay is to familiarize the reader with the various causes of toxic leukoencephalopathy along with its differential diagnoses and mimics.
MeSH Keywords: Brain Diseases, Metabolic; Magnetic Resonance Imaging; Toxic Actions
Background
Toxic leukoencephalopathies can have similar imaging appearances even when caused by different agents. Moreover, various mimics of toxic leukoencephalopathy can have similar imaging findings, delaying the correct diagnosis and appropriate treatment. Thus, differentiating these entities from each other is very important. In this pictorial essay, the known causes of toxic leukoencephalopathy will be listed (Table 1) and discussed along with numerous case examples. This article discusses the imaging findings of the various differential diagnoses and mimics of toxic leukoencephalopathy with corresponding case examples (Table 2).
Table 1.
Various causes of toxic leukoencephalopathy.
|
Table 2.
Toxic leukoencephalopathy mimics.
|
Toxic Leukoencephalopathies
Chemotherapeutic agents
A variety of chemotherapeutic agents are known to cause toxic leukoencephalopathy including melphalan, purine analogs, cisplatin and methotrexate - probably the best known offender (Table 1). Patients often present with acute neurotoxic effects such as transient ischemic attacks, seizures, encephalopathy and ataxia [1]. Spinal cord involvement presents as myelopathy. MRI findings include symmetric, focal to diffuse, increases in T2-FLAIR signal intensity (Figure 1), with transient diffusion restriction involving the periventricular white matter and centrum semiovale [1].
Figure 1.
A 15-year-old girl treated for acute lymphoblastic leukemia who presented with headache after methotrexate treatment initiation. Axial T2-FLAIR image (A) demonstrates slightly increased signal intensity with restricted diffusion involving periventricular white matter and centrum semiovale (white arrows) as seen on DWI (B) and ADC (C) images.
Radiation induced leukoencephalopathy
Radiation induced injury to the brain can be classified into three categories - acute, early delayed and late delayed, which are described as different phases of an evolution. Patients are often asymptomatic, however, impaired cognitive function is affected most commonly in clinically apparent patients. The acute injury occurs during therapy and has little prognostic significance. In the early delayed and late delayed phases, diffuse leukoencephalopathy can develop, which is manifested by an increased T2-FLAIR signal intensity in the periventricular white matter (Figure 2). Early in the process, there is relative sparing of the subcortical U-fibers. Diffusion restriction varies and is dependent upon the severity of the injury. As time progresses, associated cerebral atrophy becomes more evident. A more focal form of radiation-induced injury can result in necrosis and enhancement of the cortex and white matter [2].
Figure 2.
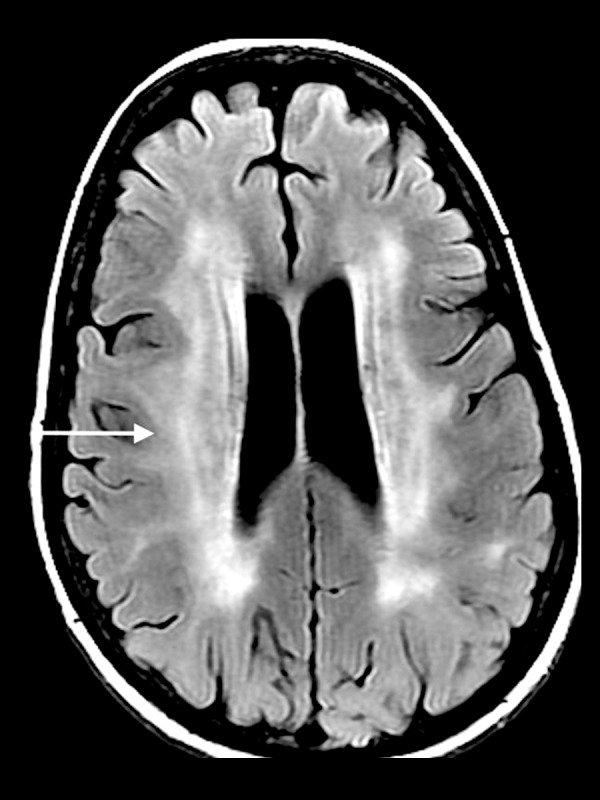
A 60-year-old man with whole-brain radiation therapy for brain metastasis from lung cancer. Axial T2-FLAIR image demonstrates increased signal in the periventricular white matter (white arrow) with relative sparing of the subcortical U-fibers.
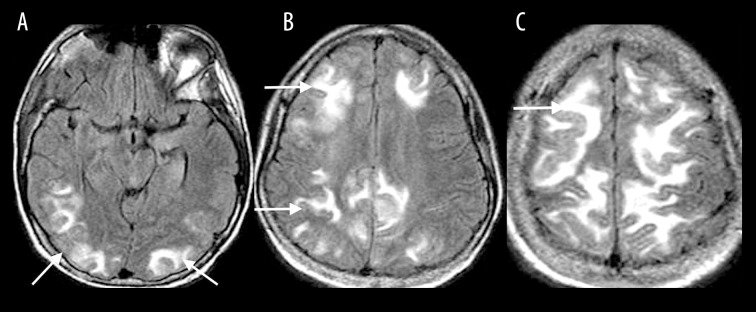
Metabolic disorders
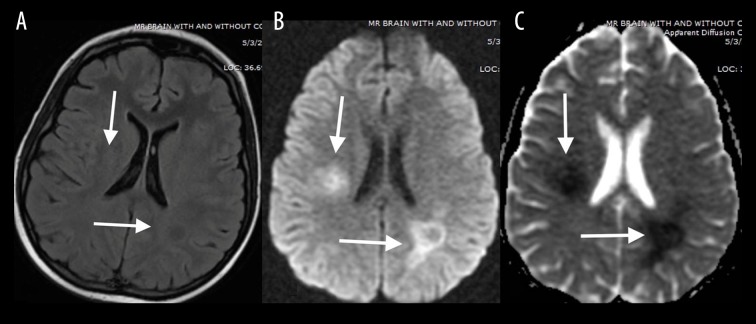
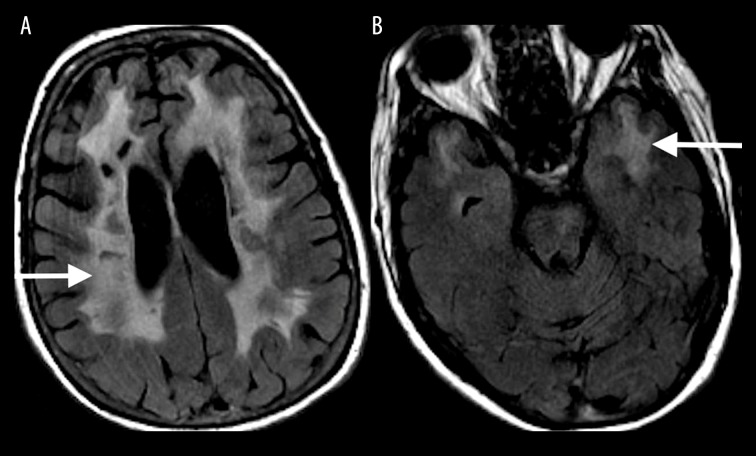
Uremia is commonly associated with the posterior reversible encephalopathy syndrome (PRES); however, there are reports of uremia resulting in cytotoxic edema [3]. The MRI findings typically include confluent, increased T2-FLAIR signal intensity (Figure 3A) and diffusion restriction involving the supratentorial white matter (Figure 3B, 3C).
Figure 3.
A 48-year-old woman with chronic renal failure and uremia presented with altered mental status. Coronal T2-FLAIR (A) MR image shows confluent increased T2-FLAIR signal (white arrow) with a corresponding diffusion restriction involving bilateral supratentorial white matter on DWI (B) and ADC map (C).
Phenylketonuria (PKU) is an inborn error of metabolism resulting in an impaired metabolism of phenylalanine. It manifests on MRI as areas of increased T2 signal intensity (Figure 4A) with a corresponding diffusion restriction (Figure 4B, 4C) in the periventricular parietal and occipital white matter. The signal abnormalities can extend to the frontal subcortical white matter in more severe cases [4].
Figure 4.
A 24-year-old man with phenylketonuria. Axial T2-FLAIR (A), DWI (B) images and ADC map (C) showing increased T2-FLAIR signal with a corresponding diffusion restriction in the periventricular parietal, and occipital white matter (white arrows).
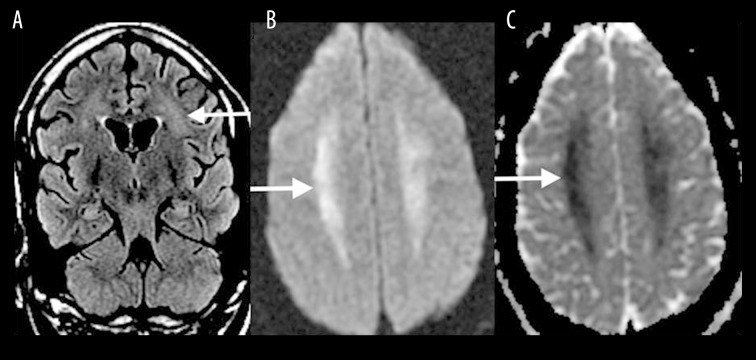
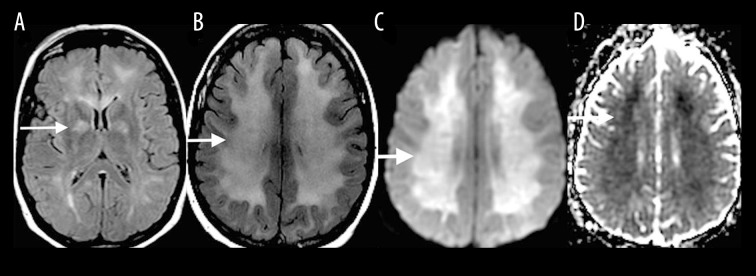
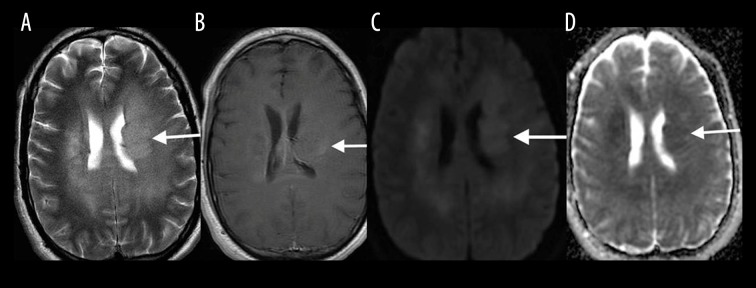
Delayed posthypoxic leukoencephalopathy
Delayed posthypoxic leukoencephalopathy typically occurs 1–2 weeks after the initial insult, whether resulting from cardiorespiratory compromise or other toxic causes [5]. MRI findings are similar to other toxic leukoencephalopathies and include high T2-FLAIR signal intensity in the periventricular white matter and centrum semiovale (Figure 5A) with associated diffusion restriction (Figure 5B, 5C).
Figure 5.
A 40-year-old woman imaged 2 weeks following hypoxic-anoxic injury. Axial T2-FLAIR (A), DWI (B) and ADC map (C) show high T2-FLAIR signal in the periventricular white matter and centrum semiovale with associated diffusion restriction (white arrows).
Carbon monoxide
The most common site affected by CO poisoning is the globus pallidus, which presents on MRI as increased T2 and T2-FLAIR signal intensity (Figure 6A) along with corresponding diffusion restriction in the acute phase. The caudate, putamen, thalami, cerebellum and brainstem may also be affected, but much less commonly than the globus pallidus. Carbon monoxide poisoning can also result in diffuse hypoxic-ischemic encephalopathy or focal cortical necrosis with diffuse or focal cortical diffusion restriction, respectively [6]. Cerebral white matter demyelination occurs after the acute stage and is manifested by increased T2-FLAIR signal intensity with diffusion restriction in the periventricular white matter, centrum semiovale, and in severe cases in the subcortical white matter, corpus callosum and internal capsule (Figure 6B–6D).
Figure 6.
A 35-year-old man with carbon monoxide poisoning. Axial T2-FLAIR image (A) on the day of poisoning shows increased T2-FLAIR signal in the globus pallidus (white arrow) along with a corresponding diffusion restriction (not shown). One month after carbon monoxide exposure, axial T2-FLAIR (B), DWI (C) and ADC map (D) show increased T2-FLAIR signal with diffusion restriction in the periventricular white matter and centrum semiovale (white arrows).
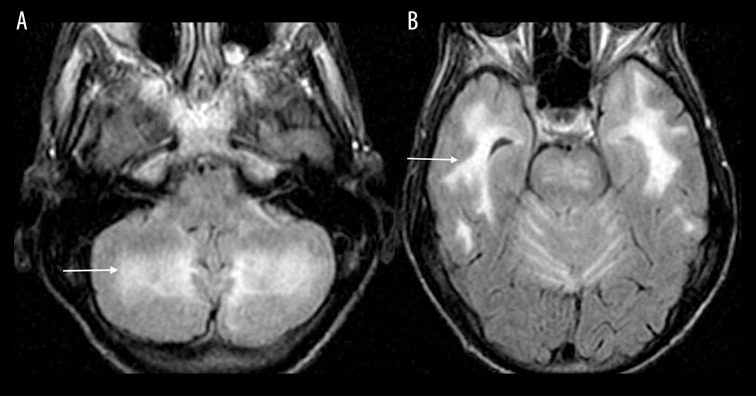
Drugs of abuse
Drugs of abuse, including heroin, toluene, cocaine, methamphetamine among others, are a well-known cause of toxic leukoencephalopathy. “Chasing the Dragon” refers to a method of heroin administration where the heroin is heated to a liquid form and the vapors are inhaled. MRI findings are characteristic and involve symmetrically increased T2 and T2-FLAIR signal intensity in the cerebellar white matter (Figure 7A, 7B) and the posterior limb of the internal capsule. There is also symmetrically increased T2 and T2-FLAIR signal intensity involving the supratentorial white matter as seen with other leukoencephalopathies [7].
Figure 7.
A 48-year-old woman with a history of heroin abuse. Axial T2-FLAIR images through the cerebellum (A) and temporal lobes (B) show increased signal involving the cerebellar and bilateral temporal white matter (white arrows).
Toxic Leukoencephalopathy Mimics
Demyelinating disease
Demyelinating diseases include a wide array of diseases. Multiple sclerosis is the most common of the demyelinating diseases that affect the supra- and infratentorial white matter. A careful inspection of these lesions will help differentiate it from toxic leukoencephalopathies. Classically, the high-signal T2-FLAIR lesions of MS have a predilection for the periventricular white matter. The lesions follow the medullary veins and are oriented perpendicular to the ventricular surface (Figure 8). Further inspection may reveal high T2-FLAIR signal lesions affecting the corpus callosum, subcortical white matter, brainstem, cerebellum, optic nerves and spinal cord. In the acute phase, MS plaques exhibit contrast enhancement [8].
Figure 8.
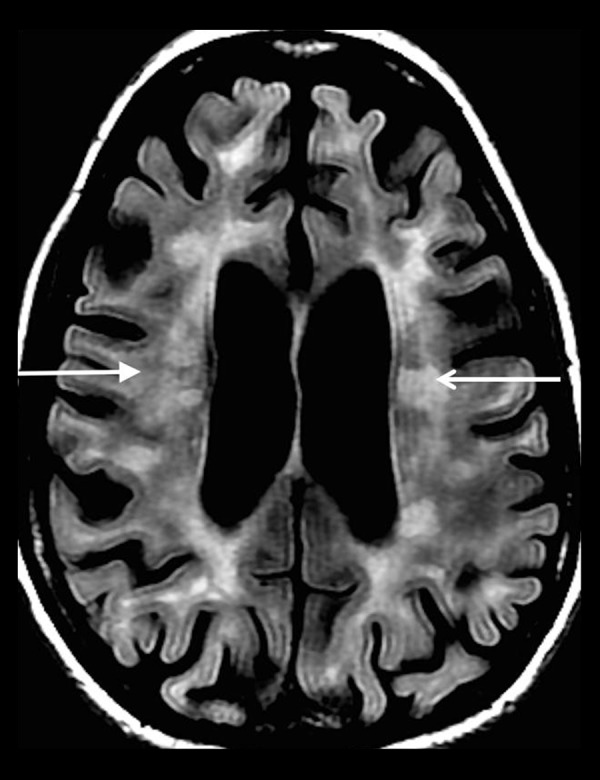
A 52-year-old woman with a long-standing history of multiple sclerosis. Axial T2-FLAIR image shows areas of increased T2-FLAIR signal in the bilateral periventricular white matter (white arrows) oriented perpendicular to the ventricular surfaces.
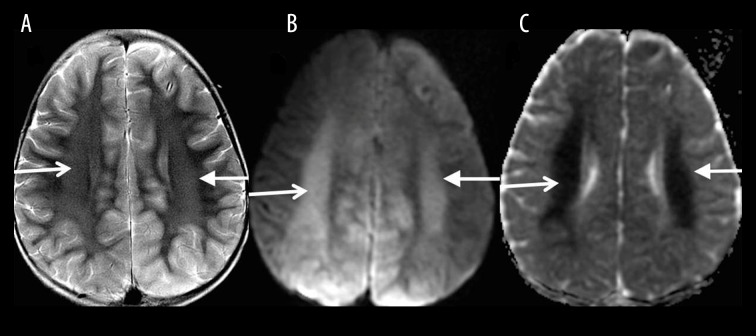
Posterior reversible encephalopathy syndrome (PRES)
The MRI features of PRES classically demonstrate symmetric T2-FLAIR hyperintensities involving the cortical and subcortical white matter of the parietal and occipital lobes, and less commonly the frontal lobes, temporal lobes, cerebellum and brainstem (Figure 9). FLAIR signal abnormalities become more confluent as the disease progresses, however, they resolve when the offending agent is removed. Diffusion restriction is uncommon [9].
Figure 9.
A 31-year-old woman with SLE and hypertension presented with headache and change in vision progressing to a generalized seizure. Axial T2-FLAIR images (A–C) show symmetric T2-FLAIR hyperintensities involving the cortical and subcortical white matter of the parietal and occipital lobes, and to a lesser extent also the frontal lobes, temporal lobes, cerebellum and brainstem (white arrows), consistent with PRES.
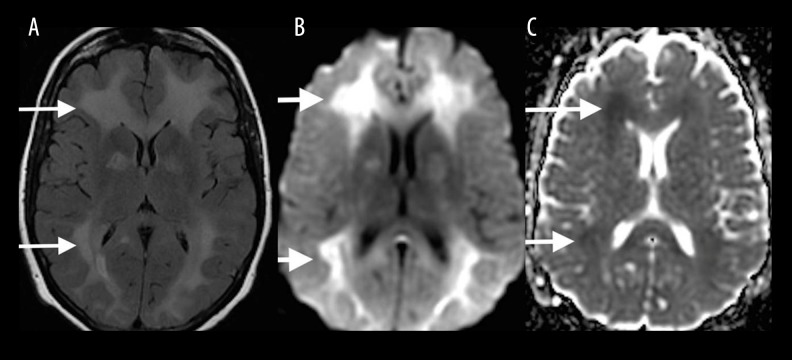
AIDS dementia complex
The imaging findings of the AIDS Dementia Complex include diffuse cerebral atrophy that is out of proportion to the patient’s age, and symmetrically increased T2 signal intensity in the periventricular and deep white matter (Figure 10). Diffusion restriction is never found, in contrast to the acute phase of toxic leukoencephalopathies [10].
Figure 10.
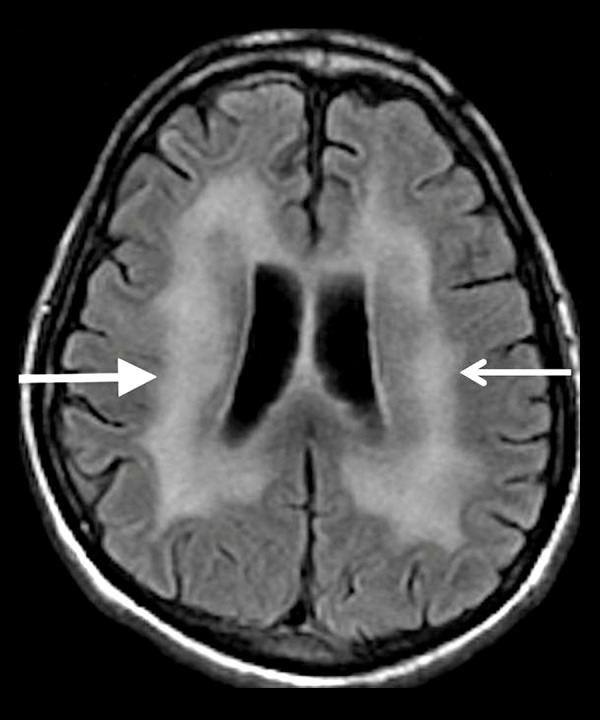
A 40-year-old man with a history of AIDS. Axial T2-FLAIR image shows diffuse cerebral atrophy that is out of proportion to the patient’s age, and symmetrically increased T2 signal in the periventricular and deep white matter (white arrows) sparing the subcortical U-fibers.
Progressive multifocal leukoencephalopathy (PML)
PML demonstrates multifocal areas of increased T2 signal intensity involving the periventricular and subcortical white matter along with the subcortical U-fibers (Figure 11). These areas of abnormal T2 signal are typically asymmetric which can distinguish them from toxic leukoencephalopathies. Usually, there is no mass effect or enhancement [10,11].
Figure 11.
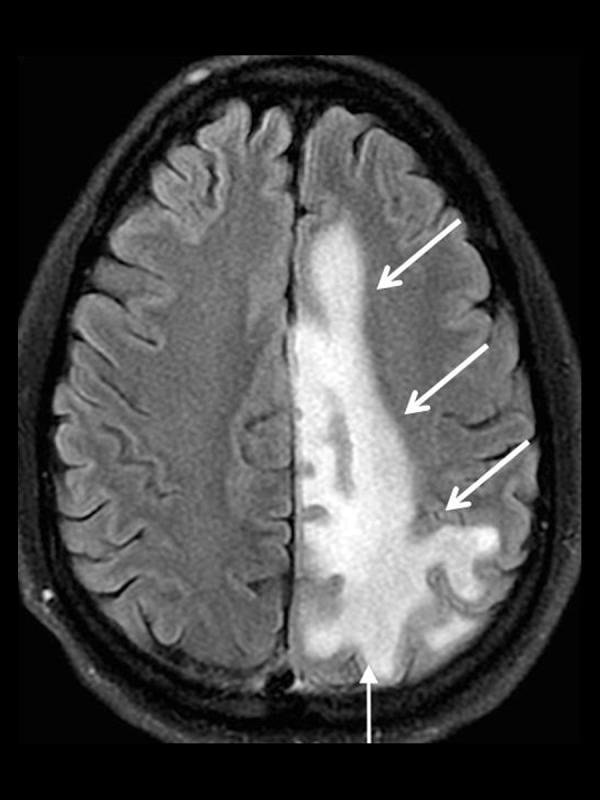
A 45-year-old man with Progressive Multifocal Leukoencephalopathy (PML). Axial T2-FLAIR image shows a large area of increased T2 signal involving the periventricular and subcortical white matter including the subcortical U-fibers (white arrows).
Genetic leukodystrophies
It is beyond the scope of this pictorial essay to describe in detail the genetic leukodystrophies; however, it is imperative that the interpreting radiologist is aware of these entities in the differential diagnosis of toxic leukoencephalopathy. Many of the leukodystrophies begin early in life making the patient’s history vital, although late-onset leukodystrophies are not uncommon. The X-linked adrenoleukodystrophy, one of the more common varieties, typically manifests itself as increased T2 signal intensity in the peritrigonal regions and extends across the splenium of the corpus callosum [12]. As the disease progresses, the abnormal white matter signal becomes more confluent and spreads outward and cephalad until most of the cerebral white matter is affected (Figure 12).
Figure 12.
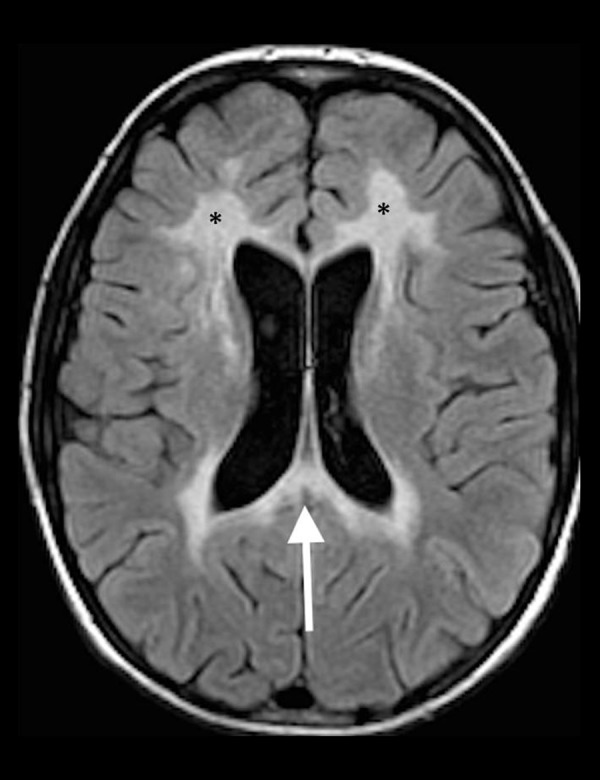
A 14-year-old boy with X-linked adrenoleukodystrophy. Axial T2-FLAIR image shows increased T2 signal in the peritrigonal regions extending across the splenium of the corpus callosum (white arrow) as well as the involvement of the frontal lobe white matter bilaterally (black asterisks).
Vascular disease
Chronic small-vessel disease can lead to changes on MRI that may mimic a toxic leukoencephalopathy. The findings usually include patchy areas of increased T2-FLAIR signal intensity in the periventricular, deep and subcortical white matter, without a corresponding diffusion restriction, unless seen in the acute phase. In severe cases, the areas of abnormal T2-FLAIR signal can often become more confluent, possibly resulting in a diagnostic dilemma.
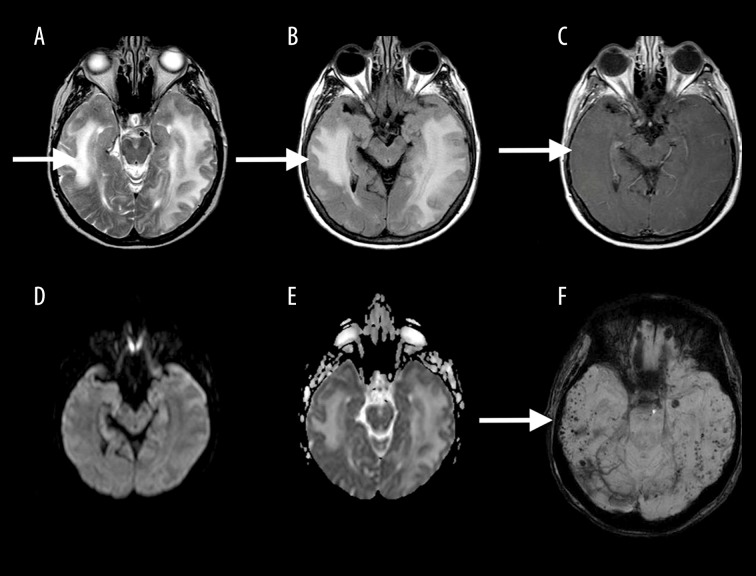
Also included in the vascular mimics of toxic leukoencephalopathy is cerebral amyloid angiopathy (CAA)-related inflammation. MRI findings of CAA-related inflammation reveal confluent zones of increased T2 signal with localized mass effect that is usually confined to the subcortical white matter; however, cortical involvement has been described. The key to making this diagnosis is the presence of multiple punctate hypointense foci on GRE and SWI-weighted sequences (Figure 13) that are characteristic of cerebral amyloid angiopathy [13].
Figure 13.
A 70-year-old man with amyloid angiopathy. Axial T2W (A), T2-FLAIR (B), contrast-enhanced T1W (C), DWI mages (D) and ADC map (E) show confluent zones of increased T2 signal (white arrow) without restricted diffusion, with the presence of multiple punctate hypointense foci on SWI (F), characteristic of cerebral amyloid angiopathy (white arrow).
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is also a vascular etiology that can mimic toxic leukoencephalopathy. MRI findings include focal and confluent areas of high T2-FLAIR signal affecting the subcortical white matter and centrum semiovale with a particular predilection for the frontal, parietal and anterior temporal regions along with the external capsule (Figure 14). Corresponding diffusion restriction is present in the acute phase of the infarcts [14].
Figure 14.
A 40-year-old woman with CADASIL. Axial T2-FLAIR images show focal and confluent areas of high T2-FLAIR signal affecting the subcortical white matter and centrum semiovale with particular predilection for the frontal (A), parietal and anterior temporal regions (B).
Gliomatosis cerebri
Gliomatosis cerebri is a diffuse infiltrating neoplasm that by definition involves at least three lobes. MRI findings reveal increased T2-FLAIR signal affecting the cerebral white matter and less commonly the cortex (Figure 15A). There is typically no to minimal enhancement (Figure 15B), and diffusion restriction is uncommon, although the case that we present does have corresponding diffusion restriction (Figure 15C, 15D). One of the key features that distinguish gliomatosis cerebri from toxic leukoencephalopathy is the associated mass effect [15].
Figure 15.
A 50-year-old man with Gliomatosis cerebri. Axial T2W (A), post-gadolinium T1W (B), DWI (C) and ADC map (D) and mages show increased T2-FLAIR signal affecting the cerebral white matter with mass effect, mild contrast enhancement and mildly restricted diffusion (white arrows).
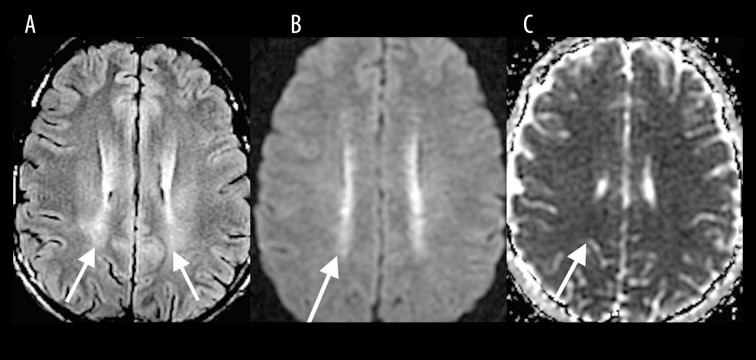
Postictal
In the immediate postictal period, imaging findings usually include abnormal cortical and subcortical signal abnormalities associated with restricted diffusion. However, in the late postictal phase after one week, increased T2 signal intensity (Figure 16A) with a corresponding diffusion restriction (Figure 16B, 16C) in the centrum semiovale bilaterally, similar to the findings in toxic leukoencephalopathy, can also be seen [16].
Figure 16.
A 12-year-old boy 2 weeks following status epilepticus. Axial T2W (A), DWI (B) and ADC map (C) images show increased signal in the centrum semiovale bilaterally with restricted diffusion (white arrows).
Summary/Conclusions
In addition to the known toxic causes of leukoencephalopathy, there are several diseases that can present with a similar constellation of signs and symptoms. As a result, the imaging findings of toxic leukoencephalopathy are often nonspecific and there are many other disease entities that can mimic the findings, resulting in a diagnostic dilemma for the interpreting radiologist. It is imperative that a detailed clinical history is kept in mind and that the interpreting radiologist be aware of the differential diagnoses as there are many potential mimics.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Vázquez E, Delgado I, Sánchez-Montañez A, et al. Side effects of oncologic therapies in the pediatric central nervous system: Update on neuroimaging findings. Radiographics. 2011;31:1123–39. doi: 10.1148/rg.314105180. [DOI] [PubMed] [Google Scholar]
- 2.Valk PE, Dillon WPL. Radiation injury of the brain. Am J Neuroradiol. 1991;12(1):45–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Kang E, Jeon SJ, Choi S. Uremic encephalopathy with atypical magnetic resonance features on diffusion-weighted images. Korean J Radiol. 2012;13(6):808–11. doi: 10.3348/kjr.2012.13.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moritani T, Smoker WRK, Sato Y, et al. Diffusion-weighted imaging of acute excitotoxic brain injury. Am J Neuroradiol. 2005;26(2):216–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. Am J Neuroradiol. 2005;27(8):1763–65. [PMC free article] [PubMed] [Google Scholar]
- 6.Lo CP, Chen SY, Lee KW, et al. Brain injury after acute carbon monoxide poisoning: Early and late complications. Am J Roentgenol. 2007;189(4):W205–11. doi: 10.2214/AJR.07.2425. [DOI] [PubMed] [Google Scholar]
- 7.Tamrazi B, Almast J. Your brain on drugs: Imaging of drug-related changes in the central nervous system. Radiographics. 2012;32(3):701–19. doi: 10.1148/rg.323115115. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y. Multiple sclerosis: The role of MR imaging. Am J Neuroradiol. 2006;27(6):1165–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. Am J Neuroradiol. 2008;29(6):1036–42. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AB, Smirniotopoulos JG, Rushing EJ. Central nervous system infections associated with human immunodeficiency virus infection: Radiologic-pathologic correlation. Radiographics. 2008;28(7):2033–58. doi: 10.1148/rg.287085135. [DOI] [PubMed] [Google Scholar]
- 11.Gourineni VC, Juvet T, Kumar Y, et al. Progressive multifocal leukoencephalopathy in a 62-year-old immunocompetent woman. Case Rep Neurol Med. 2014;2014:549271. doi: 10.1155/2014/549271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheon J, Kim I, Hwang YS, et al. Leukodystrophy in children: A pictorial review of MR imaging features. Radiographics. 2002;22(3):461–76. doi: 10.1148/radiographics.22.3.g02ma01461. [DOI] [PubMed] [Google Scholar]
- 13.Auer DP, Pütz B, Gössl C, et al. Differential lesion patterns in CADASIL and sporadic subcortical arteriosclerotic encephalopathy: MR imaging study with statistical parametric group comparison. Radiology. 2001;218(2):443–51. doi: 10.1148/radiology.218.2.r01fe24443. [DOI] [PubMed] [Google Scholar]
- 14.Savoiardo M, Erbetta A, Storchi G, Girotti F. Case 159: Cerebral amyloid angiopathy – related inflammation. Radiology. 2010;256(1):323–27. doi: 10.1148/radiol.10091170. [DOI] [PubMed] [Google Scholar]
- 15.Shin YM, Chang KH, Han MH, et al. Gliomatosis cerebri: Comparison of MR and CT features. Am J Roentgenol. 1993;161(4):859–62. doi: 10.2214/ajr.161.4.8372774. [DOI] [PubMed] [Google Scholar]
- 16.Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: A focused review. Seizure. 2016;38:63–67. doi: 10.1016/j.seizure.2016.04.007. [DOI] [PubMed] [Google Scholar]