Abstract
The relationships of the sponge classes are controversial, particularly between the calcareous and siliceous sponges. Specimens of the putative calcarean Eiffelia globosa Walcott from the Burgess Shale show the presence of diagnostic hexactinellid spicules integrated into the skeletal mesh. The arrangement of these spicules in Eiffelia is shown to be precisely equivalent to that of early protospongioid hexactinellids, and sponge growth occurred through an identical pattern to produce identical skeletal body morphology. The difference in spicule composition of the classes is interpreted through the observation of taphonomic features of Eiffelia that suggest the presence of at least two mineralogically distinct layers within the spicules. These results support molecular analyses that identify the calcarean-silicisponge transition as the earliest major sponge branch and suggest that the heteractinids were paraphyletic with respect to the Hexactinellida.
Keywords: Cambrian, evolution, Porifera, stem group
Sponges (phylum Porifera) are widely acknowledged to be the most primitive extant metazoans and include some of the earliest metazoan fossils (1-3), but surprisingly little is known about their phylogenetic relationships. Anatomical distinctions have long distinguished the three principal classes, Hexactinellida, Demospongiae and Calcarea, and more recent molecular and biochemical analyses support the monophyly of the two silica-biomineralizing classes (4-7). The relationship of the Calcarea is more problematic, however, with molecular evidence suggesting it may be very close to the ctenophores and cnidarians (5) and perhaps even the sister group of “higher” metazoans (8), making sponges as a whole paraphyletic. Significant separation of the Calcarea from the Hexactinellida/Demospongiae (Silicispongea) clade is also implied by fundamental differences in morphology and development: unlike Silicispongea, calcareans lack morphologically distinct microscleres (minute spicules that rigidify soft tissue in contrast to skeletal megascleres), and their calcitic spicules are secreted intercellularly within an organic sheath (vs. formation of silica spicules onto an intracellularly secreted axial organic filament in Silicispongea).
By documenting the extinct stem-group members of extant taxa, the fossil record provides a unique, and potentially powerful, means of resolving such phylogenetic issues (9). By virtue of their mineralized skeletons and the highly conserved nature of spicule symmetry (3, 10, 11), the three sponge classes can be tracked well back into the Paleozoic. Indeed, the hexactinellids, with their primitively hexactine/tetraradiate (orthogonal symmetry) spicules, are recognized in the terminal Proterozoic (1) and occur widely in the early Paleozoic as the single-layered subrectilinear networks of protospongioids (12). Putative calcareans are reported from the Tommotian (13), but unambiguous members of the clade, with preserved mineralogy, have not been identified before the Silurian (14). However, a morphological sequence of heteractinids appears to extend the lineage to the Lower Cambrian (15). Heteractinids are a problematic group of Paleozoic sponges characterized by hexaradiate spicules, a pattern not seen in extant forms, but with obvious symmetry relationships with the triradial spicules of calcareans; combined with the preserved carbonate mineralogy and bilaminar structure of late Paleozoic forms (14), they have been widely accepted as calcareans.
The principle representative of Cambrian heteractinids is Eiffelia Walcott, 1920, known primarily from articulated specimens from the Middle Cambrian Burgess Shale (16, 17) but with morphologically indistinguishable spicules also widely reported from the Cambrian to Carboniferous (e.g., 12, 18-20). Although Eiffelia is a heteractinid by definition, its higher level relationships have yet to be examined critically. In this study we reexamine Burgess Shale Eiffelia and show it to have a mosaic of calcarean and hexactinellid characters. As such, it is not accommodated by either crown group and sheds important light on the interrelationships of basal metazoans.
Materials and Methods
We examined all 30 catalogued specimens of Burgess Shale Eiffelia in the U.S. National Museum (USNM), Washington, D.C., and two previously unstudied specimens housed at the Royal Ontario Museum (ROM), Toronto. This study is based on the two ROM specimens (ROM 57022 and 57023) and six USNM specimens, including the type specimen (USNM 66523, 200648, 200653, 200638, 200654, and 200656). There is no stratigraphic information available for any of the USNM specimens nor for ROM 57023, which was collected from scree; ROM 57022 was collected 210 cm below the base of the “Phyllopod Bed” within the Walcott Quarry Shale Member of the Burgess Shale Formation (21).
All of the selected fossils were studied by using a binocular stereoscope with camera lucida attachment. ROM 57022 was also analyzed by using backscattered scanning electron microscopy and electron-dispersive Fourier analysis, with parts studied in a petrographic thin section or treated locally with concentrated hydrofluoric acid (HF). In addition to documenting the morphology of the constituent spicules, we consider the geometry of spicule arrangement in Eiffelia and develop a model of mineralogical transition in early sponge evolution.
Description. In life, Eiffelia was globose, up to 6 cm in diameter, with a lattice of spicules loosely arranged into a single layer (16, 17). Spicules occur in multiple size orders with the largest (first-order) spicules defining the overall geometry and smaller order spicules progressively filling the intervening spaces. Both Walcott (16) and Rigby (17, 22) identified the spicules as hexaradiate, but our reexamination reveals the additional presence of tetraradiate, including hexactine, spicules. As with most nonmineralized and lightly mineralized structures in the Burgess Shale, Eiffelia typically occurs flattened on bedding planes, the individual elements defined by reflective films with very slight relief.
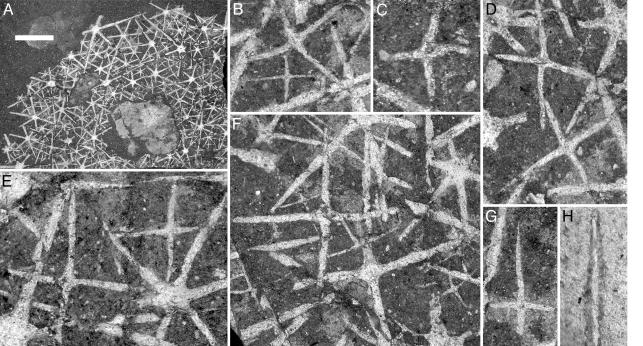
ROM 57023. ROM 57023 (Fig. 1) is an articulated but incomplete Eiffelia with an estimated body diameter of ≈25 mm. Spicules vary widely in size and appear to fall into five distinct size orders, although, on the assumption of an incremental size-order ratio of 1.3-1.4, we infer a total of seven. The largest measured spicules have a ray length of >4.5 mm, a central disk diameter of 0.65 mm, and a basal ray diameter of 0.18 mm; the smallest have ray lengths of just 0.2-0.3 mm.
Fig. 1.
Morphology of Eiffelia globosa (ROM 57023). (A) Whole specimen. (B-G) Details of A, showing tetraradiate spicules. Note the quadruled arrangement of tetraradiates in E and F, and evidence of a central vertical ray in C and G.(H) Spicule showing evidence of bilayered construction. (Scale bar: A, 3 mm; F, 0.50 mm; D, 0.45 mm; B, E, and G, 0.35 mm; C and H, 0.20 mm.)
All of the first-order spicules in this specimen are hexaradiate and appear to lack perpendicular rays. Second-order spicules are also predominantly hexaradiate; however, the smaller size orders are increasingly dominated by tetraradiates. In the smallest size class, for example, tetraradiates outnumber hexaradiates by a ratio of 10:1. The largest certain tetraradiate has rays 3.5 mm long, equivalent to the second-order hexaradiates.
As the name implies, adjacent rays of tetraradiate spicules in ROM 57023 tend to diverge at angles of 90°, although this value can vary by up to 10° (Fig. 1B). At least some also preserve evidence of a compressed ray (or rays) oriented perpendicular to the plane of the other four (Fig. 1D). By contrast, the hexaradiate spicules exhibit generally regular 60° radial symmetry and show no evidence of perpendicular rays (ref. 16, p. 324). The tetraradiates also differ from adjacent, similarly sized hexaradiates in having a smaller basal disk (see Fig. 1 B and D).
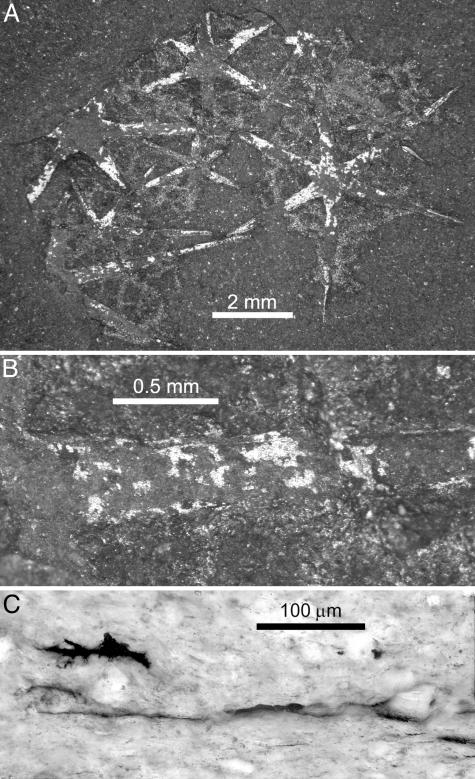
ROM 57022. ROM 57022 (Fig. 2) was collected as a small fragment exposing five articulated hexaradiate spicules and comprising just three size orders (maximum ray length 5 mm). Preservation of this specimen is excellent, however, and reveals a number of significant features. The spicules show slight relief, and the surface of each spicule is defined by a thin (<1 μm), HF-resistant, reflective carbonaceous film. The interior of the spicules (i.e., the material lying beneath the carbonaceous film and responsible for the slight topographic relief) is composed of diagenetic aluminosilicates, readily distinguished from the surrounding shale by its distinct cation composition (unpublished results) and differential response to HF.
Fig. 2.
Morphology of Eiffelia globosa (ROM 57022). (A) Bedding-plane view, reflective patches are fragments of HF-resistant carbonaceous film. Note the slight color/textural difference between the shale matrix and spicules where the film has peeled away (both aluminosilicate). The poorly defined material interspersed between the spicules is also HF-resistant. (B) Detail of spicule ray showing bilayered structure and fragments of carbonaceous film. (C) Perpendicular-to-bedding thin section through spicule ray showing divergence of the carbonaceous film in the cortical layer.
Close examination of the margins of spicules in ROM 57022 shows a distinct bilayered construction, the outer region consistently 200-300 μm wide (Fig. 2B). Walcott (16) noted this finding in the type material (see Fig. 1C), and it appears to have been the basis of his interpretation that the spicules contained a central canal. Certainly the same double-walled spicules can be seen in the type material, but the original constitution has been obscured by pervasive diagenesis; we assume that Eiffelia spicules were originally solid, like those of later heteractinids (14). In a cross section, the outer layer of Eiffelia spicules is distinguished by thickening and the divergence of the otherwise collapsed, essentially coalesced, carbonaceous film (Fig. 2C). Although there is no remnant of the original mineralogy, the conspicuously different responses of these two spicule layers to diagenesis points to marked differences in composition.
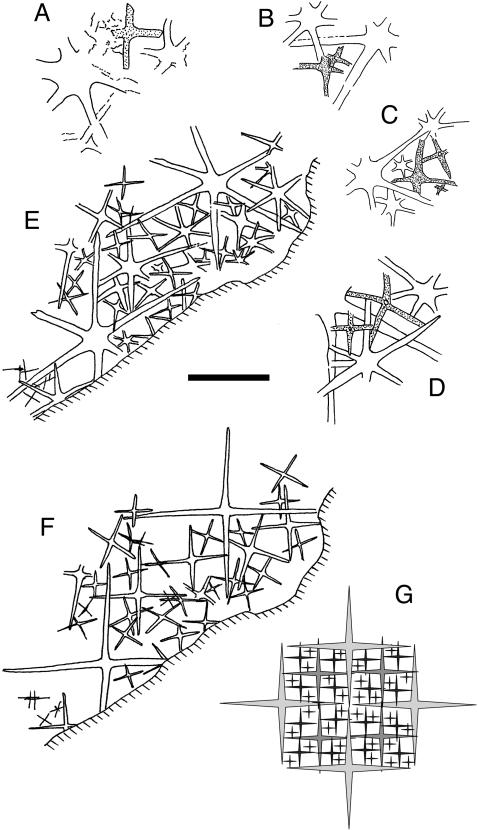
USNM-Type Material. Only six of 30 Eiffelia specimens in the USNM-type collection were sufficiently well preserved to allow detailed analysis of smaller spicules and sufficiently large to distinguish true absence of tetraradiate spicules from sampling error. Of these six, all except USNM 66523 include tetraradiate spicules, and in the case of USNM 200656, they comprise more than one-half of all spicules (Fig. 3E). In all instances, the largest spicules are hexaradiate, whereas most tetraradiates are third-order or smaller (Fig. 3 A-E).
Fig. 3.
Camera lucida drawings from the type material of Eiffelia globosa showing tetraradiate spicules. (A) USNM 200648. (B) USNM 200653. (C and D) USNM 200638. Tetraradiate spicules are shaded in A-D.(E) USNM 200656. (F) Artificial construction obtained by tracing of E with all remaining hexaradiates replaced by tetraradiates, revealing an irregular quadruling habit (see Discussion). (G) Illustration of perfect quadruling as seen in Protospongia. (Scale bar: A, 1 mm; B-F, 2 mm.)
In USNM 200656, 25 of 38 visible spicules are tetraradiate and allow a determination of their larger-scale arrangement in the Eiffelia skeleton. Beginning with a camera lucida tracing of the specimen (Fig. 3E), we assumed a fixed orientation for the majority of spicules but replaced the few remaining hexaradiates with tetraradiates of equal size, orthogonal to the fixed orientation, or rotated to reflect the original orientation of each hexaradiate if this orientation differed from the fixed direction (Fig. 3F). The result is a pattern of irregular quadruling (quadruling is a rectangular grid subdivided by orthogonal spicules, with members of each smaller order positioned in the center of spaces between the previous order; see Fig. 3G) identical to that of early protospongioids.
There are sufficient near-complete specimens of USNM Eiffelia, including those with poor preservation of small spicules, to follow Botting's (23) approach to analyzing growth patterns. The results show a proportional increase in maximum spicule ray length with sponge diameter, indistinguishable from the pattern observed in primitive hexactinellids and demosponges, but subsequently modified in various ways in almost every sponge lineage (23).
Discussion
Skeletal Structure of Hexactinellids and Calcareans. Primitive, early Paleozoic hexactinellids (“Reticulosa”; refs. 12 and 23-27) possessed a broadly consistent body-wall spiculation composed of hexactine-based tetraradial spicules arranged in up to nine morphologically similar size orders; adjacent size orders typically differ by a factor of ≈1.3 but can vary significantly (23). Spicules grew throughout life, to a limit, with smaller orders appearing sequentially between larger spicules (23), eventually giving rise to the idealized “quadruled” arrangement of Protospongia sensu stricto (24). Most early members of this clade, however, express the irregularly quadruled pattern seen in Eiffelia, suggesting that this design was the ancestral architecture among hexactinellids (12, 28). By the Late Paleozoic, most hexactinellids possessed thickened body walls composed of multiple spicule layers and no longer exhibited the discrete size ordering of earlier forms (e.g., ref. 29). Differentiated hexactinellid microscleres are present by at least the Ordovician but are rarely preserved in situ (30).
Like derived hexactinellids, most extant calcareans are characterized by thickened body walls and irregularly arranged spicules. The simplest (“ascon”) forms, however, are structurally equivalent to the protospongioids, with axial symmetry and an outer wall composed of a single layer of spicules. Although morphologically distinct microscleres are absent, calcarean spicules often occur in two or more sizes, either of two distinct magnitudes (e.g., ref. 31) or varying over a range. In the latter case, the sequence can sometimes be separated into successive size orders, related by a constant geometric factor that is typically 1.1-1.5, often near 1.3 (J.P.B., unpublished data); such size ordering has not been observed in demosponges. Interestingly, first-order spicules in the simplest extant calcareans (Clathrinida) and early Paleozoic hexactinellids (Protospongioidea) are both arranged rhomboidally, with the quadruled arrangement generated by positioning of subsequent orders.
The triradial symmetry of extant Clathrinida spicules is potentially a simple modification of hexaradiate spicules of Paleozoic heteractinids, a possibility also supported by the presence of abundant triradiates in the Late Paleozoic wewokellid heteractinids (e.g., ref. 32) and the similarity of heteractinid spicule structure to that of modern calacareans (14). The Paleozoic Eiffeliidae are widely regarded as the most basal heteractinids (29), and some authors have regarded the lineage as probable stem Calcarea (11). There are no known extant calcareans with hexactine (rather than hexaradiate) spicules or their derivatives and only a single fossil species, the enigmatic Canistrumella Rigby (17), also from the Burgess Shale. In the absence of data on microscleres or soft tissue, the only real distinctions between eiffeliids and protospongioids are spicule symmetry and mineralogy; our results show that even these differences do not hold in Eiffelia (Table 1).
Table 1. Comparison of presence and absence of primary characteristics of siliceous sponges (Hexactinellida plus Demospongiae), Calcarea, and Eiffelia.
| Characteristic | Silicispongea | Eiffelia | Calcarea |
|---|---|---|---|
| Axial organic filament | 1 | 0? | 0 |
| Hexactine spicules | 1 | 1 | 0 |
| Opal-A in spicules | 1 | 1? | 0 |
| Rhomboidal first-order grid | 1* | 1 | 1* |
| Multiple size orders | 1* | 1 | 1* |
| Axial symmetry | 1* | 1 | 1* |
| ACC/Mg-calcite in spicules | 0 | 1? | 1 |
| Organic spicule sheath | 0 | 1? | 1 |
| Hexaradiate spicules | 0 | 1 | 1* |
1, presence; 0, absence; 1*, present in ancestral forms but subsequently lost in at least some extant taxa; ?, character state probably as given, based on our observations, but could not be categorically confirmed with the available material.
Implications for Phylogeny. On the basis of its preserved morphology and growth patterns, Eiffelia could be regarded either as a peculiar hexactinellid with hexaradiate spicules or a peculiar calcarean with tetraradiate spicules, although there is no compelling evidence to prefer one interpretation over the other (Table 1). Such mosaics of morphological features are common in the early fossil record and increasingly are being recognized, not as bizarre experiments in early evolution, but as the stem-group “intermediate” stages linking extant higher-order taxa (9). In this light, Eiffelia is readily interpreted as intermediate between the heteractinid calcareans and the protospongioid hexactinellids.
Whether Eiffelia is a stem-group hexactinellid or a stem-group calcarean depends on accurate phylogenetic reconstruction of the extant crown groups. Unfortunately, molecular phylogenies have failed to resolve the relationships and polarity of the sponge classes, although two scenarios have received most of the support: (i) (Hexactinellida plus Demospongea) (Calcarea plus Eumetazoa) (5, 6, 33-37) in which Silicspongea is monophyletic, and (ii) Hexactinellida (Demospongea (Calcarea plus Eumetazoa)) (8, 38-41), which gives a paraphyletic Silicispongea. The balance of recent molecular phylogenies, based on a range of genes, is approximately equal (42), but with an increasing shift toward silicisponge monophyly [albeit with Homoscleromorpha excluded from both Demospongiae (43) and Silicispongea (44)], particularly in light of corroborative biochemical (7), paleontological (45), and of course mineralogical data. Molecular analyses also consistently identify a close relationship between Calcarea and Ctenophora plus Cnidaria (e.g., ref. 8), usually interpreted as poriferan paraphyly with respect to Eumetazoa.
The discovery of an evolutionary intermediate between hexactinellids and calcareans argues strongly against a basal Hexactinellida because this result would require repeated derivation of siliceous spicules secreted onto axial filaments, in hexactinellids and demosponges, and perhaps also Homoscleromorpha. By providing evidence of a direct link between Calcarea and Hexactinellida, it also categorically excludes some less well supported topologies such as that of Adams (5), in which Calcarea are nested among Cnidaria and Ctenophora. In contrast, the hypothesis of silicisponge monophyly that is emerging prominently from neontological work is entirely consistent with our data.
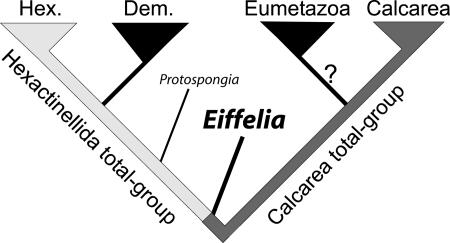
Recognition of a probable (Hexactinellida plus Demospongea) (Calcarea plus Eumetazoa) topology is interesting, but molecular studies do little to constrain character polarity and, thus, the nature of the common ancestral node. By contrast, our analysis of Eiffelia provides direct evidence for both the derivation of demosponges from total-group (probably stem) hexactinellids (cf. ref. 45) and the derivation of Eumetazoa from (probably stem) calcareans (see Fig. 4). Unfortunately, calcarean/hexactinellid polarity remains unresolved because of the lack of unequivocal outgroup comparisons, although we offer a calcarean-to-hexactinellid trajectory in light of the mineralogical transition suggested by Eiffelia (see below). Future fossil discoveries of demonstrable stem-group poriferans with preserved or implied mineralogy and symmetry will be needed to disprove or corroborate this hypothesis.
Fig. 4.
Proposed phylogenetic relationships of extant sponge classes, Eumetazoa, and Eiffelia globosa. Eiffelia is shown as a stem hexactinellid (Silicispongea) and a derived member of the “Heteractinida” (stem Calcarea). Eiffelia and early hexactinellids share tetraradial hexactines and a quadruled spicule geometry, but the spicules of the latter are entirely siliceous.
Mineralogical Transition: An Hypothesis. The evolution of silicisponges from a primitive calcarean requires a number of fundamental shifts in spicule structure, including a transition from an external organic sheath to an internal filament (46, 47) and from a composite magnesium calcite/amorphous calcium carbonate (ACC) (48) mineralogy to opal. Given the consistent positioning and size-order relationships of spicules between Eiffelia and early hexactinellids, it appears that spicules underwent a transformation rather than a loss and subsequent replacement.
The bilayered construction of Eiffelia spicules (Figs. 1C and 2B) clearly identifies two components of distinctly different chemical and/or physical properties. Comparison might be made with the differentiated magnesium calcite/amorphous calcium carbonate composition of extant calcarean spicules (cf. ref. 48), but the similar diagenetic lability of these two carbonate phases is unlikely to account for the observed differences. Moreover, it is the outer layer that is more substantially preserved in the fossils, but in modern calcareans it is the core that is composed of the more stable magnesium calcite. Insofar as a mineralogical transition is demanded in most transformational scenarios between calcareans and silicisponges, and given the widespread capacity for combined calcium carbonate and silica biomineralization among extant and fossil sponges (49), we suggest that the two layers of Eiffelia spicules represent distinct carbonate and opaline silica phases. Assuming a Calcarea to Hexactinellida polarity, the spicule core is likely to have been calcareous and surrounded by a secondary layer of opaline silica.
The organic sheath surrounding modern calcarean spicules consists of dense bands of collagen fibrils, each up to a maximum of 130 nm in diameter (50), the sheath with a total thickness of ≈0.5 μm (ref. 50; Fig. 2) to 2.0 μm (ref. 47; Fig. 1). This measurement is comparable with the carbonaceous film associated with Eiffelia spicules (Figs. 1 and 2), although whether the two structures are directly homologous remains to be seen. In Eiffelia, for example, the sheath appears to envelope the whole of the spicule rather than just the core. Moreover, the ability of extant demosponges to produce unmineralized spicules (e.g., Darwinella) (51) and the presence of conspicuous, apparently carbonaceous films around spicules of otherwise unproblematic Burgess Shale demosponges (e.g., Pirania; unpublished data) points to alternative origins of organic constituents in sponge spicules.
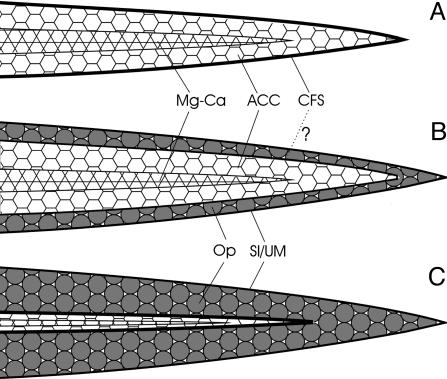
With an external selection pressure to produce a stronger mesh, the dominant trend among Paleozoic hexactinellids (e.g., ref. 12), we suggest that the transformation from hexagonal to tetragonal spicule symmetry was followed to allow greater mesh regularity and spicule interlocking. Thus, the original kinetic advantage of correlated ray orientation and optic axes in Calcarea (47) was largely superceded by the mechanical superiority offered by siliceous spiculation. The initial stage of magnesium calcite secretion could then have been progressively reduced, eventually leading to an axial organic filament surrounded by opal (Fig. 5). Implicit in this model is the homology of the organic sheath of calcarean spicules and the organic axial filament of silicisponges, a relationship that is also suggested by the presence of a mineralized core within the axial filament of at least some extant demosponges [e.g., Crambe (52)].
Fig. 5.
Proposed transition from magnesium calcite (Calcarea) to opal (Silicospongea) spicules based on the bilayered structure exhibited by Eiffelia. (A) Calcarean spicule with outer sheath of collagen fibrils. (B) Secondary precipitation of opal onto the collagenous sheath (as represented by Eiffelia).(C) Further increase in opal precipitation, accompanied by reduction of the calcaean sheath to an axial filament and loss of hexaradial symmetry. Mg-Ca, magnesium calcite; ACC, amorphous calcium carbonate; CFS, collagen fibril sheath; Op, opal; Sl/Um, silicalemma/unit membrane or second collagen fibril sheath.
Conclusion
As a stem-group hexactinellid, Eiffelia globosa joins a growing list of problematic Cambrian fossils recognized as key intermediate stages linking higher-order taxa (e.g., refs. 53 and 54). In this instance, the mosaic of calcarean and hexactinellid characters documents the morphological transition between two poriferan “classes” (Table 1), with the peculiarly bilayered spicules suggesting a heuristic, possibly even correct, model for understanding the mineralogical transition between the Calcarea and Silicispongea (Fig. 5).
The identification of the stem-group status of Eiffelia also invites reconsideration of other early sponges and sponge-like organisms. Thin-walled, irregularly quadruled protospongioids, for example, differ considerably from extant hexactinellids and show marked similarities to Eiffelia, they almost certainly represent forms that diverged before the crown group (12, 23). The recently described heteractinid Eiffelospongia (22) possessed an external layer of monaxon spicules and differs significantly from Eiffelia, but the regularity of the primary spicule mesh imitates that of an early hexactinellid even more closely. We are also intrigued by the highly reflective, probably carbonaceous, films that define the spicules of the apparent Burgess Shale demosponge Pirania (see ref. 17) and whether these films might be homologous with the organic film surrounding the spicules of Eiffelia and/or the spicules of extant Calcarea.
Chancelloriids are problematic Cambrian fossils with a sponge-like form, including multirayed, lightly mineralized sclerites surrounded by a thin, reflective, carbonaceous film (51) similar to that associated with Eiffelia and Pirania (but also most other organically preserved fossils). Unlike sponge spicules, however, chancelloriid sclerites are “hollow” and constructed of multiple elements, making it difficult [although not impossible (51)] to accommodate them within the poriferan bodyplan (55, 56). Even so, it may be worth considering their structure with reference to our model of mineralogical transition: in this case the originally calcitic core may simply have abandoned mineralization but retained the original form of the “calcarean” organic sheath (rather than having it condense to an axial filament as we propose for the transition to Silicispongea). In other words, the bipartite constitution of chancelloriid rays (the hollow core and lightly mineralized wall) conceivably corresponds to the bipartite constitution of Eiffelia spicules.
Although the polarity of the transition cannot be established from the available molecular data, we suggest the possibility that Calcarea are paraphyletic with respect to Silicispongea; the definitive resolution of this issue depends on clarification of many aspects of basal metazoan phylogeny. However the relationships are eventually resolved, Eiffelia now joins Protospongia (12), Canistrumella (17), and various other problematic Paleozoic sponges in its exclusion from any class-level crown group. As such, it offers key insights into the deep interrelationships of the Porifera, particularly in light of the conflicting results arising from recent molecular analyses.
Acknowledgments
We thank E. Valiulis (USNM) and D. Collins (ROM) for access to specimens; S. Finney and U. Balthasar for technical assistance in preparation and chemical analysis; and the two anonymous referees for constructive comments. J.P.B. was funded by a Junior Research Fellowship at Christ's College, Cambridge. This work is Cambridge Earth Science Contribution no. 8076.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HF, hydrofluoric acid; ROM, Royal Ontario Museum; USNM, U.S. National Museum.
References
- 1.Brasier, M. D., Green, O. & Shields, G. (1997) Geology 25, 303-306. [Google Scholar]
- 2.Gehling, J. G. & Rigby, J. K. (1996) J. Paleontol. 70, 185-195. [Google Scholar]
- 3.Debrenne, F. & Reitner, J. (2001) in The Ecology of the Cambrian Radiation, eds. Zhuravlev, A. Y. & Riding, R. (Columbia Univ. Press, New York), pp. 301-325.
- 4.Zrzavy, J., Mihulka, S., Kepka, P., Bezdek, A. & Tietz, D. (1998) Cladistics 14, 149-185. [DOI] [PubMed] [Google Scholar]
- 5.Adams, C. L., McInerney, J. O. & Kelly, M. (1999) Mem. Queensl. Mus. 44, 33-43. [Google Scholar]
- 6.Medina, M., Collins, A. G., Silberman, J. D. & Sogin, M. L. (2001) Proc. Natl. Acad. Sci., USA 98, 9707-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiel, V., Blumenberg, M., Hefter, J., Pape, T., Pomponi, S., Reed, J., Reitner, J., Worheide, G. & Michaelis, W. (2002) Naturwissenschaften 89, 60-66. [DOI] [PubMed] [Google Scholar]
- 8.Borchiellini, C., Manuel, M., Alivon, E., Boury-Esnault, N., Vacelet, J. & Le Parco, Y. (2001) J. Evol. Biol. 14, 171-179. [DOI] [PubMed] [Google Scholar]
- 9.Budd, G. & Jensen, S. (2000) Biol. Rev. 75, 253-295. [DOI] [PubMed] [Google Scholar]
- 10.Reiswig, H. M. (1971) Cah. Biol. Mar. 12, 505-514. [Google Scholar]
- 11.Bergquist, P. (1978) Sponges (Hutchinson, London).
- 12.Botting, J. P. (2004) J. Syst. Palaeontol. 2, 31-63. [Google Scholar]
- 13.Kruse, P. D., Zhuravlev, A. Y. & James, N. P. (1995) Palaios 10, 291-321. [Google Scholar]
- 14.Mehl, D. & Reitner, J. (1996) Berliner Geowiss. Abh. 18, 243-255. [Google Scholar]
- 15.Johns, R. A. (1994) Geol. Soc. Am. Abstr. Prog. 26, 426. [Google Scholar]
- 16.Walcott, C. D. (1920) Smithson. Misc. Collect. 67, 261-364. [Google Scholar]
- 17.Rigby, J. K. (1986) Palaeontogr. Can. 2, 1-105. [Google Scholar]
- 18.Culver, S. J., Repetski, J. E., Pojeta, J. Jr., & Hunt, D. (1996) J. Paleontol. 70, 1-6. [Google Scholar]
- 19.Bengtson, S., Conway Morris, S., Cooper, B. J., Jell, P. A. & Runnegar, B. N. (1990) Mem. Assoc. Australas. Palaeontol. 9, 1-364. [Google Scholar]
- 20.Dilliard, K. A. & Rigby, J. K. (2001) Brigham Young Univ. Geol. Stud. 46, 1-11. [Google Scholar]
- 21.Fletcher, T. P. & Collins, D. H. (1998) Can. J. Earth Sci. 35, 413-438. [Google Scholar]
- 22.Rigby, J. K. & Collins, D. H. (2004) R. Ont. Mus. Contrib. Sci. 1, 1-156. [Google Scholar]
- 23.Botting, J. P. (2003) Lethaia 36, 41-53. [Google Scholar]
- 24.Rigby, J. K. (1966) J. Paleontol. 40, 549-554. [Google Scholar]
- 25.Mehl, D. (1991) in Fossil and Recent Sponges, eds. Reitner, J. & Keupp, H. (Springer-Verlag, Berlin), pp. 43-53.
- 26.Mehl, D. (1996) Geol.-Paläeont. Mitt. Univ. Innsbruck 4, 1-55. [Google Scholar]
- 27.Mehl-Janussen, D. (1999) Münchner Geowiss. Abh. Reihe A 37, 1-72. [Google Scholar]
- 28.Chen Z., Hu J., Zhou C.-M., Xiao S.-H. & Yuan, X.-L. (2004) Chin. Sci. Bull. 49, 1625-1628. [Google Scholar]
- 29.Finks, R. M. (1970) Symp. Zool. Soc. London 25, 3-22. [Google Scholar]
- 30.Kozur, H. W., Mostler, H. & Repetski, J. (1996) Geol.-Paläont. Mitt. Univ. Innsbruck 21, 201-221. [Google Scholar]
- 31.Borojevic, R. & Klautau, M. (2000) Zoosystema 22, 187-201. [Google Scholar]
- 32.Rigby, J. K. & Church, S. B. (1993) J. Paleontol. 67, 909-916. [Google Scholar]
- 33.Manuel, M., Borchiellini, C., Alivon, E., Le Parco, Y., Vacelet, J. & Boury-Esnault, N. (2003) Syst. Biol. 52, 311-333. [DOI] [PubMed] [Google Scholar]
- 34.Cavalier-Smith, T., Allsopp, M. T., Chao, E. E., Boury-Esnault, N. & Vacelet, N. (1996) Can. J. Zool. 74, 2031-2045. [Google Scholar]
- 35.Lafay, B., Boury-Esnault, N., Vacelet. J. & Christen, R. (1992). Biosystems 28, 139-151. [DOI] [PubMed] [Google Scholar]
- 36.Collins, A. G. (1998) Proc. Nat. Acad. Sci. USA 95, 15458-15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson, K. J. & Eernisse, D. J. (2001) Evol. Dev. 3, 170-205. [DOI] [PubMed] [Google Scholar]
- 38.Kruse, M., Leys, S. P., Müller, I. M. & Müller, W. E. G. (1998) J. Mol. Evol. 46, 721-728. [DOI] [PubMed] [Google Scholar]
- 39.Schütze, J., Krasko, K., Custodio, M. R., Efremova, S. M., Müller, I. M. & Müller, W. E. G. (1999) Proc. R. Soc. London Ser. B 266, 63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchiellini, C., Boury-Esnault, N., Vacelet, J. & Le Parco, Y. (1998) Mol. Biol. Evol. 15, 647-655. [DOI] [PubMed] [Google Scholar]
- 41.Müller, W. E. G. & Müller, I. M. (1999) Mem. Queensl. Mus. 44, 381-397. [Google Scholar]
- 42.Reiswig, H. (2004) Boll. Mus. Ist. Biol. Univ. Genova 68, 71-84. [Google Scholar]
- 43.Borchiellini, C., Chombard, C., Manuel, M., Alivon, E., Vacelet, J. & Boury-Esnault, N. (2004) Mol. Phylogent. Evol. 32, 823-837. [DOI] [PubMed] [Google Scholar]
- 44.Nichols, S. (2005) Mol. Phylog. Evol., 34, 81-96. [DOI] [PubMed] [Google Scholar]
- 45.Botting, J. P. (2003) Lethaia 36, 335-344. [Google Scholar]
- 46.Jones, W. C. (1967) Nature 214, 365-368. [Google Scholar]
- 47.Jones, W. C. (1970) Symp. Zool. Soc. London 25, 91-123. [Google Scholar]
- 48.Aizenberg, J., Weiner, S. & Addadi, L. (2003) Connect. Tissue Res. 44, 20-25. [PubMed] [Google Scholar]
- 49.Reitner, J. & Wörheide, G. (2002) in Systema Porifera, eds. Hooper, J. N. A. & Van Soest, R. M. W. (Kluwer/Plenum, New York), pp. 52-68.
- 50.Ledger, P. (1974) Tissue Cell 6, 385-389. [DOI] [PubMed] [Google Scholar]
- 51.Butterfield, N. J. & Nicholas, C. J. (1996) J. Paleontol. 67, 758-787. [Google Scholar]
- 52.Uriz, M. J., Turon, X. & Becerro, M. (2000) Cell Tissue Res. 301, 299-309. [DOI] [PubMed] [Google Scholar]
- 53.Budd, G. E. (2002) Nature 417, 271-275. [DOI] [PubMed] [Google Scholar]
- 54.Shu D.-G., Conway Morris, S., Han, J., Zhang, Z.-F. & Liu, J.-N. (2004) Nature 430, 422-428. [DOI] [PubMed] [Google Scholar]
- 55.Bengtson, S. & Hou, X. (2001) Acta Palaeontol. Pol. 46, 1-22. [Google Scholar]
- 56.Janussen, D., Steiner, M. & Zhu Maoyan. (2002) J. Paleontol. 76, 596-606. [Google Scholar]