Abstract
Background
Diabetes is rapidly rising all over the world at an alarming rate and has changed from a mild disorder to major causes of mortality and morbidity in the youth and middle-aged people, and the prevalence is seen especially in six inhabited continents of the globe. The present study aims to explore the antidiabetic, lipid lowering effect of Cassia auriculata L. flowers in alloxan-induced diabetes.
Methods
Diabetes was induced using alloxan monohydrate in experimental rats and subsequent therapeutic effects of C. auriculata extract and standard drug glibenclamide were monitored. Bioassay-directed fractionation using silica gel column chromatography was performed until pure fractions were isolated. The effect of the treatment was analyzed by hematological parameters and enzyme assays. The pure compounds were confirmed with thin layer chromatography and high performance liquid chromatography pattern and further subjected for characterization.
Results
The alterations in blood glucose were monitored throughout the study. There was a gradual fall in blood glucose and significant changes were observed in lipid profile and metabolic enzyme after treatment with C. auriculata. Bioassay fractionation represented that the C2 subfraction produced a dose-dependent fall in blood glucose and lipid profile and upon further purification yielded two pure compounds. The structure of the pure compound was elucidated using Fourier transform infrared, 1H nuclear magnetic resonance, 13C nuclear magnetic resonance, and mass spectral data.
Conclusion
The present study clearly indicated the significant antidiabetic effect of C. auriculata and lends support for its traditional usage without evident toxic effects.
Keywords: alloxan, Cassia auriculata, chromatography, hyperglycemia, hypolipidemic
1. Introduction
Diabetes mellitus is a chronic disease in humans caused by inherited and/or acquired deficiency in production of insulin by the pancreas, or by the ineffectiveness of the insulin action, associated with chronic hyperglycemia and alteration of carbohydrate, protein, and lipid metabolism. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels.1
The use of medicinal plants for the treatment of diabetes mellitus dates back from the Ebers Papyrus of about 1550 BC. Across the globe, about 1200 plants species are used in treating diabetes mellitus and most of them after laboratory testing showed effective hypoglycemic activity.2 Ayurvedic treating methodology has been practiced in rural India as a traditional system of medicine.
Cassia auriculata L. (Family: Caesalpiniaceae) is a shrub with large bright yellow flowers found growing wild in central and western India and cultivated in other areas of the country. The root cures tumors, skin diseases, and asthma; leaves are anthelmintic, good for ulcers, diarrhea, and leprosy; and the flowers are used in the treatment of urinary discharge, diabetes, and dysentery.3C. auriculata is also one of the major components of a beverage called “kalpa herbal tea” which has been widely consumed by people suffering from diabetes mellitus, constipation, and urinary tract diseases.4 An alternative preparation for diabetes medication is a mixture called “avarai panchaga choornam” which is prepared from dried and powdered plant parts and commonly used for opthalmia, conjunctivitis, diabetes, and urinary infections (equal amount of leaves, roots, flowers, bark, and unripe fruits).5
Despite the occurrence of some published preliminary work describing the phytochemical content of the plant, a systematic activity-directed isolation study on C. auriculata has not yet been carried out. The present study was carried out in rats to test the efficacy of methanol flower extract and column fractions of Ca on hyperglycemia and serum lipid profile changes associated with alloxan-induced diabetes mellitus in male albino rats.
2. Methods
2.1. Collection of plant material
The flowers of Ca (Caesalpineceae) were collected in and around Vellore District, Tamil Nadu, India. The plant materials were cleaned with distilled water, shade dried at room temperature, and authenticated by Dr A. Annadurai, Department of Botany, C. Abdul Hakeem College, Melvisharam, Vellore District, Tamil Nadu, and voucher specimens (CAHC-09/2009) were kept at the Department of Botany, C. Abdul Hakeem College, Melvisharam, Vellore District., Tamil Nadu, India. The dried flowers were coarsely powdered by using an electric blender and stored separately in an airtight container for further use.
2.2. Preparation of extract
Some 100 g of the dried powdered flowers of C. auriculata were taken separately and mixed with 500 mL of methanol and then magnetically stirred in a separate container overnight at room temperature. The residue was removed by filtration. The filtrate was concentrated under reduced pressure in a rotary evaporator at 60 ± 10 °C to yield 10 g of crude extract (10%) and the resultant extract was used for further studies.
2.3. Chemicals and solvents
Alloxan was procured from SD Fine Chem. Limited, Mumbai, India, for the present investigation. All other chemicals and solvents were of analytical grade and obtained from S.D. Fine Chemicals and Fischer Inorganic and Aromatic Limited, Chennai, India.
2.4. Experimental animals
Adult male albino rats weighing around 180–200 g were purchased from Tamil Nadu Veterinary and Animal Sciences University, Chennai, India. The animals were kept in polypropylene cages (3 in each cage) at an ambient temperature of 25 ± 2 °C and 55–65% relative humidity. Animals were maintained in the animal house, acclimatized to the laboratory conditions, and were fed with commercially available rat chow (Hindustan Lever Ltd., Bangalore, India). They had free access to water. The experiments were designed and conducted in accordance with the institutional guidelines (Reg. No: 1011/c/06/CPCSEA).
2.5. Acute toxicity studies
The acute oral toxicity study was carried out according to the guidelines set by the Organization for Economic Co-operation Development (OECD).6
2.6. Experimental induction of diabetes
Diabetes was induced in the rats by the administration of a single intraperitoneal dose of alloxan monohydrate (150 mg/kg) in normal saline.7 Two days after alloxan injection, rats screened for diabetes having glycosuria and hyperglycemia with blood glucose level above 250 mg/dL were taken for the study.
2.7. Experimental design
Assessment of antihyperglycemic effect of C. auriculata-Metahnol flowers extract in normal and alloxan induced diabetic rats
The animals were divided into the following groups comprising six animals in each group. Group I: normal control rats, Group II: diabetic (alloxan-induced) control rats, Group III: diabetic induced rats treated with C. auriculata-M extract (250 mg/kg bw) for 25 days, Group IV: diabetic induced rats treated with C. auriculata-M extract (350 mg/kg bw) for 25 days, Group V: diabetic induced rats treated with C. auriculata-M extract (450 mg/kg bw) for 25 days, and Group VI: diabetic drug control rats treated with glibenclamide (600 μg/kg bw)8 for 25 days.
The extract and the drug glibenclamide were given in aqueous solution daily using an intragastric tube for 25 days.
Purification of C. auriculata
The methanol crude extract (60 g) was fractionated by column chromatography using silica gel (60–120 mesh). Elution was performed using hexane pure to yield two fractions A and B (500 mL each). Further elution of columns with different proportions of hexane:ethyl acetate (4:1, 2:1, 1:1, 0:1, 500 mL each fraction) was carried out to yield three fractions—C, D and E [fractions with similar Rf values in thin layer chromatography (TLC) pattern were pooled together]. All the collected fractions were dried and subjected to antidiabetic assay against alloxan-induced diabetic rats.
The fractions showing potent antidiabetic activity were selected for further separation by column chromatography. Among the collected fractions, fraction C showed potent antidiabetic activity and it was rechromatographed to obtain subfractions on elution with ethyl acetate:acetone 4:1, 2:1, 1:1, 0:1 (200 mL each). All the collected subfractions (C1, C2, C3, and C4) were air dried and tested for antidiabetic activity against alloxan-induced diabetic rats. Those subfractions showing 100% activity were alone taken for further purification by column chromatography.
All the fractions were monitored by TLC. Among all the subfractions obtained from the second column, fraction C2 showed potent antidiabetic activity and was subjected to subsequent repeated column chromatography using silica gel (230–400 mesh) to obtain various subfractions (C2a, C2b, C2c, C2d, C2e, C2f) on elution with pure methanol. After monitoring by TLC (precoated Plate 0.2 mm thick, E. Merck, Darmstadt, Germany) and high performance liquid chromatography (HPLC), the pure fractions (C2c and C2f) were carefully evaporated to dryness and subsequently characterized by spectral analysis.
Assessment of antihyperglycemic effect of different fractions of C. auriculata flowers in normal and alloxan induced diabetic rats
The animals were divided into the following groups comprising six animals in each group. Group I: normal control rats, Group II: diabetic (alloxan-induced) control rats, Group III: diabetic induced rats treated with C. auriculata fraction C (50 mg/kg) for 25 days, Group IV: diabetic induced rats treated with C. auriculata fraction C (100 mg/kg) for 25 days, Group V: diabetic induced rats treated with C. auriculata subfraction C2 (50 mg/kg) for 25 days, Group VI: diabetic induced rats treated with C. auriculata subfraction C2 (100 mg/kg) for 25 days, and Group VII: diabetic drug control rats treated with glibenclamide (600 μg/kg) for 25 days.
The column fractions and the drug glibenclamide were given in aqueous solution daily using an intragastric tube for 25 days.
C. auriculata fractions with the most potent antidiabetic activity were alone represented in the experimental design and in the results.
Sacrifice study
At the end of the experimental period, the animals were deprived of food overnight, anaesthetized, and then sacrificed by decapitation. Blood was taken from the jugular vein and collected in two tubes for serum and plasma separation. The liver was immediately dissected out, washed in ice-cold saline, weighed, and subjected for biochemical estimation.
2.8. Biochemical assay
Blood glucose was measured using a Gluco Chek glucose estimation kit [Aspen diagnostic (P) Ltd. Delhi, India]. The body weight of all experimental animals was recorded using a digital weighing scale. Insulin was estimated using a Radio Immuno Assay (RIA) kit supplied by Linco Research Inc., Stat diagnostic, Mumbai, India. Total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and triglycerides (TG) were estimated using standard kits purchased from TransAsia Bio Medical Limited, Mumbai, India. For the determination of very low density lipoprotein cholesterol (VLDL-C) and low density lipoprotein cholesterol (LDL-C) Friedewald’s formula was used which states: VLDL cholesterol = TG/5 and LDL cholesterol = TC − (VLDL-C + HDL-C).9 Hexokinase,10 glucose-6-phosphatase,11 fructose-1,6-bisphosphatase,12 and glycogen content13 were assayed. The inorganic phosphate liberated was estimated by the method of Fiske and Subbarow.14
2.9. Characterization of isolated compounds
The pure compounds were subjected to HPLC – Agilient, Fourier transform infrared (IR) – Shimadzu, 1H and 13C nuclear magnetic resonance (NMR) – Bruker, and liquid chromatography-mass spectrometry analysis – Agilient. HPLC was recorded in an Agilent 1200 series instrument, using Phenomex C18 column (200 mm × 3.9 mm id, particle size 5 μm), Fourier transform IR spectra were recorded on a Shimadzu IR affinity instrument. The 1H and 13C NMR was recorded in an Avance Bruker 400 MHz instrument using DMSO-d6 with tetramethylsilane as internal standard. The mass spectra were recorded in Agilent 1200 MS series 2000 liquid chromatography-mass spectrometry instrument.
2.10. Statistical analysis
All the results were expressed as the mean ± standard deviation and all the grouped data were statistically evaluated with SPSS\16.0 software (IBM, Chicago, SPSS Inc.). Hypothesis testing methods included one way analysis of variance (ANOVA) followed by least significant difference test; significance levels at p < 0.01 and p < 0.001 were considered to indicate statistical significance and Tukey’s test.
3. Results
3.1. Acute toxicity studies
Acute toxicity study was carried out according to the guidelines of OECD. A minimum dose of 50 mg to a maximum dose of 5000 mg/kg was given orally to mice and observed continuously for 30 minutes and periodically for the next 24 hours, and then for subsequent days until the end of the study period. All the observations such as changes in skin, fur, eyes, behavioral pattern, convulsions, diarrhea, lethargy, and mortality were systematically recorded individually for all the animals. There was no lethality nor were any toxic reactions found at any of the doses selected until the end of the study period.
3.2. Effect of C. auriculata-M extract on body weight, blood glucose, and insulin in normal and alloxan-induced experimental diabetes in rats
Table 1 explicates the body weight, blood glucose, and plasma insulin levels in normal and experimental animal groups. In alloxan-induced diabetic animals, the body weight decreased significantly by 36.28% and significant elevation of blood glucose was observed when compared to the levels in normal animals. After administration of the flower extract of C. auriculata-M at a gradient dose of 250 mg/kg, 350 mg/kg, and 450 mg/kg, the animals regained their body weight, and significant reductions in the level of glucose by 9.20%, 42.0%, and 57.62% (p < 0.001) were observed. The results were on a par with glibenclamide-treated animals
Table 1.
The effect of Cassia auriculata-M extract on body weight, blood glucose, and insulin levels of control and alloxan-induced experimental diabetes in rats.
| Groups | Bodyweight (g) | Blood glucose (mg%) | Plasma insulin (μU/mL) |
|---|---|---|---|
| I Normal control | 192.25 ± 3.81 | 117.83 ± 3.78 | 14.16 ± 0.52 |
| II Diabetic control (DC) | 122.50 ± 6.29* | 323.33 ± 24.17* | 6.13 ± 0.45* |
| III C. auriculata-M treated (250 mg/kg bw) | 140.17 ± 5.35 | 235.81 ± 14.61* | 8.10 ± 0.45* |
| IV C. auriculata-M treated (350 mg/kg bw) | 155.0 ± 7.63* | 187.50 ± 13.88* | 9.38 ± 0.44* |
| V C. auriculata-M treated (450 mg/kg bw) | 172.50 ± 9.10* | 137.00 ± 4.69* | 12.96 ± 0.58* |
| VI Drug treated | 175.00 ± 7.63* | 139.17 ± 6.50* | 12.72 ± 0.61* |
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, **p < 0.001, ***p < 0.0001.
The level of insulin in Group II alloxan-induced diabetic animals was decreased by 56.70% when compared with Group I normal animals. There was a dose-dependent increase in C. auriculata-treated groups when compared with alloxan-induced diabetic animals. A similar effect was also observed in glibenclamide administrated group VI animals.
From the analysis of the results, it was evident that there was a progressive fall of blood sugar level and significant increase in body weight and insulin after the intake of C. auriculata-M extract. These results were on a par with the standard drug.
3.3. Effect of C. auriculata-M extract on lipid levels in normal and alloxan-induced experimental diabetes in rats
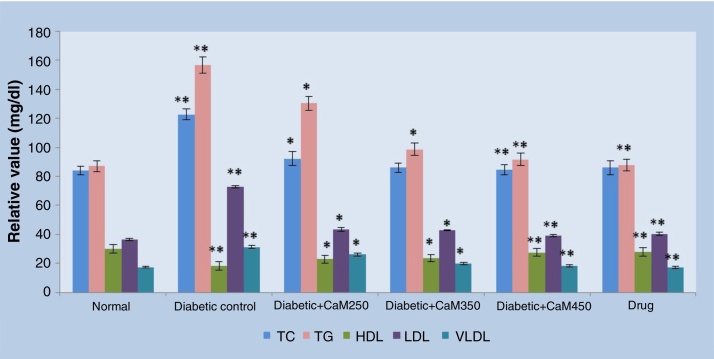
Fig. 1 summarizes the levels of lipid profile such as TC, TG, LDL-C, VLDL-C, and HDL-C in normal and experimental animals in each group. Alloxan induction resulted in significant (p < 0.01) elevation of TC, TG, LDL-C, VLDL-C, and reduction of HDL-C levels as compared to the normal control rats. After administration of C. auriculata-M extract to Group III, Group IV, and Group V, there was depletion in TC by 24.73%, 29.89%, and 31.52%, TG by 16.81%, 37.02%, and 41.49%, LDL-C by 40.22%, 41.15%, and 47.06%, and VLDL-C by 16.82%, 37.02%, and 41.49%, respectively. Also, there was a significant increase in HDL-C by 25.71%, 29.40%, and 51.43%, respectively, as compared with Group II diabetic rats. As expected in glibenclamide-treated Group VI animals, the increased levels of TC, TG, LDL-C, VLDL-C and decreased HDL-C reverted to near normal values.
Fig. 1.
The effect of Cassia auriculata-M extract on lipid profile of control and alloxan-induced experimental diabetes in rats.
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, **p < 0.001, ***p < 0.0001.
3.4. Effect of C. auriculata-M extract on hexokinase, glucose-6-phosphatase, fructose-1,6-bisphospatase, and glycogen in normal and alloxan-induced experimental diabetes in rats
The levels of hexokinase, glucose-6-phosphatase, fructose-6-phosphatase, and glycogen in normal and experimental animals in each group are summarized in Table 2. A significant decrease (p < 0.001) in hepatic hexokinase and glycogen levels was noted, and a simultaneous increase (p < 0.001) in glucose-6-phosphatase and fructose-6-phosphatase level was observed in alloxan-induced diabetic rats. Administration of C. auriculata-M extract and glibenclamide normalized the altered levels.
Table 2.
The effect of Cassia auriculata-M extract on hexokinase, glucose-6-phosphatase, fructose-1,6-bisphospatase, and glycogen of control and alloxan-induced experimental diabetes in rats.
| Groups | Hexokinase (μmol of glucose phosphorylated/min/g protein) | Glucose-6-phosphatase (μmol of Pi liberated/min/mg protein) | Fructose-1,6-bisphospatase (μmol of Pi liberated/h/mg protein) | Glycogen (mg/100 g tissue) |
|---|---|---|---|---|
| I Normal control | 11.63 ± 0.31 | 0.12 ± 0.17 | 0.27 ± 0.02 | 11.75 ± 0.38 |
| II Diabetic control (DC) | 8.40 ± 0.30** | 0.28 ± 0.03** | 0.42 ± 0.03** | 6.41 ± 1.23* |
| III C. auriculata-M treated (250 mg/kg bw) | 8.51 ± 0.24 | 0.25 ± 0.02 | 0.30 ± 0.02 | 8.25 ± 0.38 |
| IV C. auriculata-M treated (350 mg/kg bw) | 9.05 ± 0.31* | 0.18 ± 0.03* | 0.28 ± 0.03* | 9.03 ± 0.40* |
| V C. auriculata-M treated (450 mg/kg bw) | 10.31 ± 0.41* | 0.16 ± 0.02* | 0.27 ± 0.02* | 10.23 ± 0.46* |
| VI Drug treated | 10.45 ± 0.42* | 0.13 ± 0.01* | 0.28 ± 0.02* | 10.20 ± 0.53* |
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, †p < 0.001, ‡p < 0.0001.
Pi: inorganic phosphate.
3.5. Effect of C. auriculata fractions on body weight, blood glucose insulin, and lipid levels of control and alloxan-induced experimental diabetes in rats
Oral administration of C. auriculata fraction C to experimental groups showed potent antidiabetic activity. There was a significant rise in body weight (12.66% and 40.62%) and insulin levels (54.02% and 65.87%) while there was a gradual fall in glucose levels (50.62% and 55.25%) after administration of C. auriculata fraction and the results are shown in Table 3. The other fractions showed moderate change in alloxan-induced diabetic rats, hence data are not shown.
Table 3.
The effect of Cassia auriculata fractions on body weight, blood glucose and insulin levels of control and alloxan-induced experimental diabetes in rats.
| Groups | Body weight (g) | Blood glucose (mg%) | Plasma insulin (μU/mL) |
|---|---|---|---|
| I Normal control | 193.15 ± 3.91 | 118.18 ± 3.78 | 13.96 ± 0.52 |
| II Diabetic control (DC) | 121.60 ± 6.27* | 324.03 ± 24.17* | 6.33 ± 0.45* |
| III C. auriculata-C treated (50 mg/kg bw) | 137.0 ± 7.63 | 160.0 ± 7.63* | 9.75 ± 0.53* |
| IV C. auriculata-C treated (100 mg/kg bw) | 171.0 ± 7.63* | 145.0 ± 5.34* | 10.50 ± 0.45* |
| V C. auriculata-C2 treated (50 mg/kg bw) | 140.0 ± 7.63* | 139.0 ± 5.72* | 11.33 ± 0.53* |
| VI C. auriculata-C2 treated (100 mg/kg bw) | 174.50 ± 6.87* | 130.0 ± 7.63* | 13.25 ± 0.38‡ |
| VII Drug treated | 175.60 ± 7.63* | 134.47 ± 6.61* | 13.12 ± 0.63* |
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, †p < 0.001, ‡p < 0.0001.
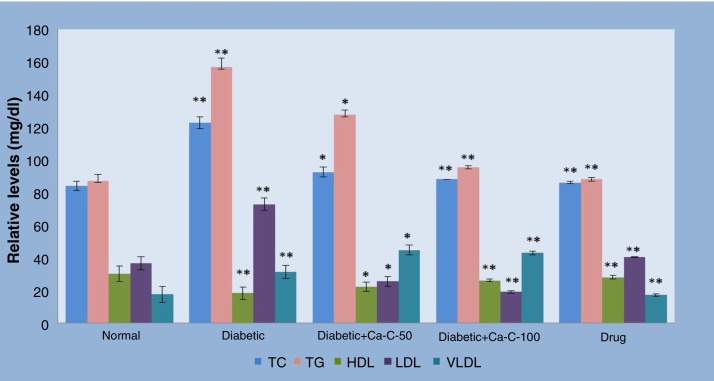
Fig. 2 explicates the levels of lipid profile such as TC, TG, LDL-C, VLDL-C, and HDL-C in normal and experimental animals in each group. Alloxan induction resulted in significant (p < 0.01) elevation of TC, TG, LDL-C, VLDL-C, and reduction of HDL-C levels compared to the normal control rats. After administration of C. auriculata fraction C to Group III and Group IV, there was a significant fall in TC (24.73% and 28.29%), TG (18.61% and 39.04%), LDL-C (38.53% and 41.01%), and VLDL-C (18.60% and 39.03%). Also, there was an increase of HDL-C (22.02% and 43.17%) in comparison with diabetic control rats, whereas in glibenclamide-treated Group VII animals, the increased levels of TC, TG, LDL-C, VLDL-C and decreased HDL-C reverted to near normal values.
Fig. 2.
The effect of Cassia auriculata C-fraction on lipid profile of control and alloxan-induced experimental diabetes in rats.
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, **p < 0.001, ***p < 0.0001.
The active fraction C was rechromatographed and four subfractions (C1, C2, C3, and C4) were obtained. All the subfractions were tested for antidiabetic activity. Oral administration of C. auriculata fraction C2 to Group V and Group VI showed a significant rise in body weight (14.28% and 42.44%) and insulin levels (85.24% and 116.15%) while there was a gradual fall in glucose levels (57.0% and 59.79%) after administration of C. auriculata fraction and these results are shown in Table 2. The other fractions did not show much change in alloxan-induced diabetic rats, hence data are not shown.
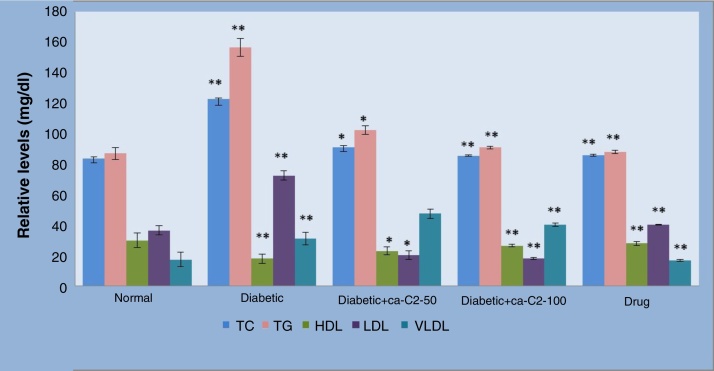
Alloxan induction resulted in significant (p < 0.01) elevation of TC, TG, LDL-C, VLDL-C, and reduction of HDL-C levels compared to the normal control rats. After administration of C. auriculata fraction C2 to Group V and Group VI, a significant fall in TC by 25.54% and 30.38%, TG by 34.57% and 41.91%, LDL-C by 34.40% and 44.21%, and VLDL-C by 34.56% and 41.90%, respectively, was observed. Also, there was an increase of HDL-C by 27.53% and 46.80%, respectively in comparison with diabetic control rats, whereas in glibenclamide-treated Group VI animals, the increased levels of TC, TG, LDL-C, VLDL-C and decreased HDL-C reverted to near normal values (Fig. 3).
Fig. 3.
The effect of Cassia auriculata C2-fraction on lipid profile of control and alloxan-induced experimental diabetes in rats.
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, **p < 0.001, ***p < 0.0001.
3.6. Effect of C. auriculata fractions on hexokinase, glucose-6-phosphatase, fructose-1,6-bisphospatase and glycogen in control and alloxan-induced experimental diabetes in rats
Table 4 represents the activity of hexokinase, glucose-6-phosphatase, fructose-6-phosphatase, and glycogen levels. The hexokinase enzyme activity was suppressed in alloxan-induced diabetic rats when compared to normal rats. This activity was restored after treatment with C. auriculata fractions (C and C2) and glibenclamide in experimental diabetic rats. Glycogen content was decreased significantly in diabetic control compared to normal control; similarly, there were elevated levels of glucose-6-phosphatase and fructose-6-phosphatase in diabetic control rats, which were brought back to normal levels after treatment with C. auriculata fractions (C and C2).
Table 4.
The effect of Cassia auriculata fractions on hexokinase, glucose-6-phosphatase, fructose-1,6-bisphospatase, and glycogen of control and alloxan-induced experimental diabetes in rats.
| Groups | Hexokinase (μmoles of glucose phosphorylated/min/g protein) | Glucose-6-phosphatase (μmol of Pi liberated/min/mg protein) | Fructose-1,6-bisphospatase (μmol of Pi liberated/h/mg protein) | Glycogen (mg/100 g tissue) |
|---|---|---|---|---|
| I Normal control | 11.42 ± 0.38 | 0.12 ± 0.19 | 0.29 ± 0.02 | 11.91 ± 0.41 |
| II Diabetic control (DC) | 8.43 ± 0.29** | 0.30 ± 0.09** | 0.42 ± 0.02** | 6.49 ± 0.94** |
| III C. auriculata-C treated (50 mg/kg bw) | 8.80 ± 0.30 | 0.15 ± 0.02 | 0.28 ± 0.02 | 9.33 ± 0.47 |
| IV C. auriculata-C treated (100 mg/kg bw) | 9.66 ± 0.39* | 0.12 ± 0.02* | 0.27 ± 0.02* | 10.03 ± 0.44* |
| V C. auriculata-C2 treated (50 mg/kg bw) | 10.75 ± 0.38* | 0.14 ± 0.01* | 0.28 ± 0.02* | 9.96 ± 0.48* |
| VI C. auriculata-C2 treated (100 mg/kg bw) | 10.76 ± 0.40* | 0.13 ± 0.01* | 0.27 ± 0.03* | 10.11 ± 0.53* |
| VII Drug treated | 10.39 ± 0.40* | 0.12 ± 0.03* | 0.29 ± 0.03* | 11.01 ± 0.73* |
Data are expressed as mean ± SEM and each value represents six individual observations, evaluated by one- way ANOVA followed by Tukey’s test.
Diabetic control was compared with normal control and treated groups were compared with diabetic control.
*p < 0.01, **p < 0.001, ***p < 0.0001.
Pi: inorganic phosphate.
The most potent fraction C2 was further purified by column chromatography yielding two compounds C2c and C2f.
3.7. Characterization of isolated compound from methanol extract of C. auriculata
3.7.1. Characterization of C2c fraction of C. auriculata
IR (νcm−1): 3421, 1620, 1514, 1H NMR (DMSO-d6): δ 8.2(-CHO), 6.58–7.29(Ar-H), 4.8(H-6), 5.2 (H-21), 1.7–3.5(H-2, 3, 4, 5), 2.0(-OH), 13C NMR (DMSO-d6): 158.7(C-1), 158.4(C-13), 157.4(C-19), 156.1 (C-7), 146.1(C-17), 145.9(C-15), 128.1(C-11), 122.5(C-9), 119.3(C-16), 116.3(C-10), 115.5(C-12), 113.2(C-20), 107.9(C-8), 106.2(C-18), 40.6 (C-2), 40.4(C-4), 40.2(C-5), 39.9(C-21), 39.6(C-3), 39.4(C-6), 34.8(C-14), LCMS: 834, molecular formula − C42H42O18.
3.7.2. Characterization of C2f fraction of C. auriculata
IR (νcm−1): 3421, 1620, 1558, 1H NMR (DMSO-d6): δ 9.72(-CHO), 6.2-7.2(Ar-H), 4.9(H-6), 4.79(H-21), 3.37–4.16(H-2, 3, 4, 5), 2.35(H-22), 2.0(-OH), 13C NMR(DMSO-d6): 158.9(C-1), 158.3(C-13), 156(C-19), 154.74(C-07), 154.37(C-17), 146.05(C-15), 128.07(C-11), 127.5(C-9), 122.1(C-16), 119.1(C-10), 116.19(C-12), 115.4(C-20), 113.2(C-8), 120.9(C-18), 46.2 (C-2), 40.5(C-4), 40.3(C-5), 40.9(C-21), 39.9(C-3), 39.3(C-6), 34.8(C-14), 21.5(C-22), LCMS: 818, molecular formula—C42H42O17.
4. Discussion
The present study was undertaken to evaluate the antidiabetic and lipid lowering effect of methanol extract and purified fractions of C. auriculata on alloxan-induced diabetic rats. The results of the present study indicated that C. auriculata-M extract significantly reduced the risk of diabetic complications in experimental diabetic rats and proved the nontoxic effect on intake of C. auriculata.
4.1. Effect of C. auriculata-M extract and fractions on body weight, blood glucose, and insulin in normal and alloxan-induced experimental diabetes in rats
The decrease in body weight with diabetes mellitus has been attributed to gluconeogenesis, i.e., catabolism of proteins and fats, which is associated with the characteristic loss of body weight due to increased muscle wasting and loss of tissue proteins.15 When diabetic rats were treated with C. auriculata-M flower extract and fractions, the weight loss was found to be reversed. The capability of the flower extract to prevent loss of body weight seems to be the result of its ability to prevent the onset of diabetes (Table 1, Table 3).
Diabetes affects glucose and lipid metabolism; many studies have shown that the level of blood glucose was elevated in experimental diabetic rats. Previous reports suggest that the reduction in the insulin levels in alloxan-induced rats might be attributed to the reduced secretion of the hormone, which might be due to the damage of beta cells of the pancreas. It is evident that alloxan selectively destroys the pancreatic cells and induces hyperglycemia. Administration of C. auriculata-M extract and fractions to the experimental groups showed an increase in body weight and insulin levels following a gradual decrease in blood glucose levels (Tables 1 and 3).
Administration of C. auriculata fraction C and C2 (100 mg/kg) showed a significant increase in the levels of insulin by 65.87% and 109.32%, respectively. This increase was similar to that of drug-treated groups, which is represented in Table 3. Plants may act on blood glucose through a different mechanism; some of them may have insulin-like substances, some may inhibit insulinase activity, and others may cause increase in the number of beta cells in the pancreas by activating regeneration of these cells.16
Hence, the results from previous studies explicated that C. auriculata extract and purified fractions C and C2 showed a significant alteration in diabetic complications.
The present study is in line with previous reports suggesting that the hypoglycemic action of C. auriculata may be by potentiating the insulin effects on plasma, by increasing either the pancreatic secretion of insulin from the existing beta cells or by its release from the bound form, or by regenerating the beta cells in alloxan-induced diabetes.17
4.2. Effect of C. auriculata-M extract and fractions on lipid levels in normal and alloxan-induced experimental diabetes in rats
Diabetes mellitus often involves abnormal lipid metabolism, which is a metabolic disorder in diabetic complications.18 Saxena et al19 reported that hyperglycemia produces a marked increase in serum TG and TC. It is well known that the major risk factors of cardiovascular disease are high levels of TC and LDL cholesterol, whereas increased HDL-C reduces the risk of cardiovascular disease. In the present study, there was an increase in TC, TG, LDL-C, VLDL-C and decrease in HDL-C levels in alloxan induced diabetic rats, which may be the result of increased breakdown of lipids and mobilization of free fatty acids from the peripheral depots.
The positive risk factor for atherosclerosis is a significant reduction in HDL-C in the diabetic rats.20 Regular administration of C. auriculata-M extract and fractions revealed significant changes of the lipid profile in experimental diabetic animals (Fig. 1, 2). These results were in line with the previous findings of other medicinal plants, which have antihyperglycemic and hypolipidemic activity with a stimulatory effect on insulin release.21
A reduced atherogenic index (TC/HDL) was recorded in the plant extract fed animals. The dose of 450 mg/kg body weight of C. auriculata-M extract (Fig. 1), C. auriculata-C2 at a dose of 100 mg/kg (Fig. 2) not only lowered the TC, TG, LDL-C, and VLDL-C, but also enhanced the HDL-C which is known to play an important role in the transport of cholesterol from peripheral cells to the liver by a pathway termed “reverse cholesterol transport”, and is considered to be a cardioprotective lipid. Thus, C. auriculata has a significant impact in improving the imbalance in lipoprotein metabolism.
4.3. Effect of C. auriculata-M extract and fractions on hexokinase, glucose-6-phosphatase, fructose-1,6-bisphospatase, and glycogen in normal and alloxan-induced experimental diabetes in rats
Hexokinase is an insulin-dependent and insulin-sensitive enzyme and is almost completely inhibited or inactivated in diabetic rat liver in the absence of insulin.22 Administration of C. auriculata and fractions to alloxan-induced diabetic rats reverted the suppressed activity of hexokinase in liver. The increased activity of hexokinase causes an increase in glycolysis and utilization of glucose for energy production; these results are on a par with the drug glibenclamide and explicates that hexokinase plays an important role in the maintenance of glucose homeostasis.18
Glucose-6-phosphatase and fructose-1,6-bisphophatase play an important role in glucose homeostasis.23 Insulin deficiency results in the activation of gluconeogenic enzymes during diabetes. C. auriculata administration significantly decreased the activity of gluconeogenic enzymes in diabetic rats. The reduction in enzyme activity corresponded to the decrease in serum glucose as less glucose was being produced and released into the bloodstream. The level of plasma insulin was found to increase significantly in diabetic rats treated with C. auriculata, which may be a possible reason for the significant reduction in the level of gluconeogenic enzymes.
Glycogen is the primary intracellular storable form of glucose and its levels in various tissues, especially skeletal muscle, are a direct reflection of insulin activity.24 Diabetes mellitus impairs the normal capacity of the liver to synthesize glycogen. Various studies have reported that the decrease in hepatic glycogen observed in this study may be due to lack of insulin in the diabetic state and these type of results are probably due to the inactivation of the glycogen synthetase system. Treatment of diabetic rats with C. auriculata and fractions significantly stimulates the secretion of insulin, thereby improving glycogen content of liver.
The fraction C2 was subjected to subsequent column chromatography to obtain various fractions. All the fractions and the subfractions were continuously monitored using a precoated TLC plate. The subfraction C2 yielded two pure compounds C2c and C2f. The fractions C2c and C2f obtained were golden brown in color. The TLC pattern showed a single spot indicating that it was a pure compound and the chromatogram data represented a single peak. The isolated pure compound was subjected to further characterization to identify its structure.
4.4. Characterization of C2c fraction of C. auriculata
The spectral data of the isolated compound was in full agreement with the proposed structure. In general, IR spectra revealed the presence of −OH, >C O, and —C C by the formation of strong absorption bands at 3441 cm−1, 1627 cm−1 and, 1514 cm−1, respectively.
In the 1H NMR spectra, the signals of the respective protons of the isolated compound were verified on the basis of their chemical shifts, multiplicities, and coupling constants. The spectra showed the aromatic protons at 5.9–7.1 ppm, methylene proton at 5.2; 4.8 ppm, C-H protons at 1.7–3.5 ppm, aldehyde protons at 8.2 ppm and hydroxy proton at 2.0 ppm. The 13C NMR spectrum showed the >C O signals at 158.7 ppm, the hydroxyl carbon at 39.6–40.6 ppm, and the aromatic carbon at 106.2–128.1 ppm.
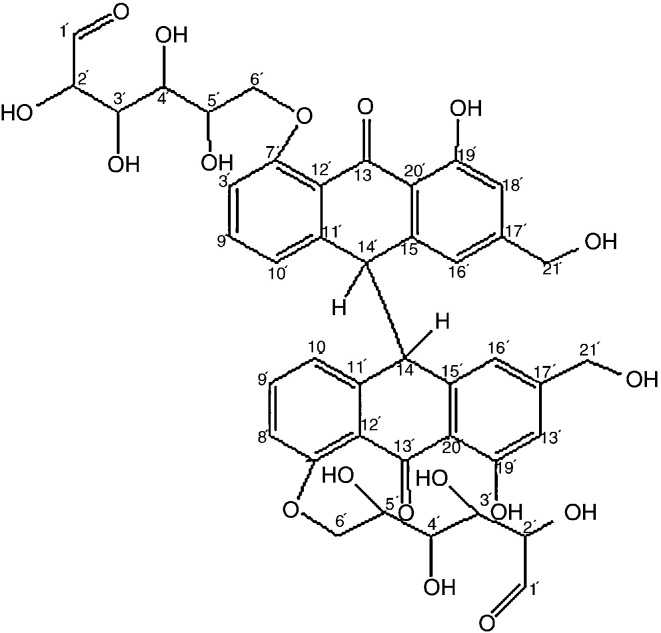
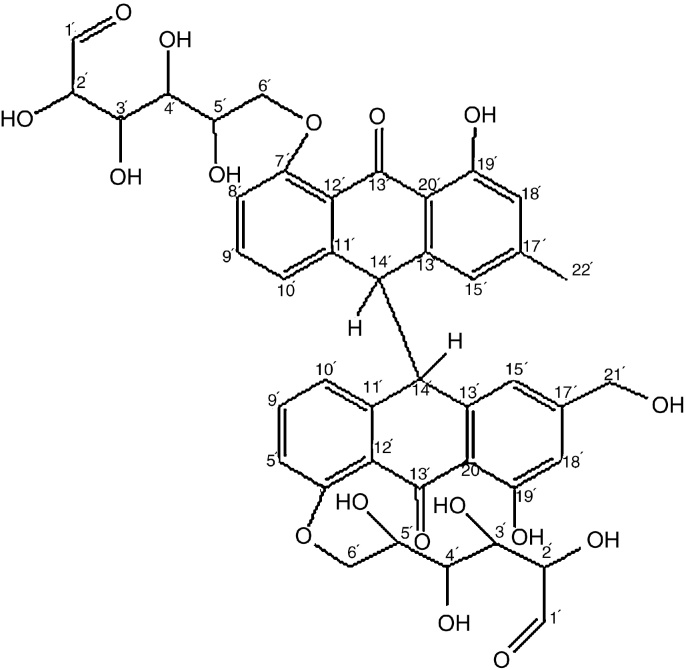
The mass spectrum showed the molecular ion peak at m/z 834 which corresponds to the formula weight of the isolated compound. From the interpretation of the spectral analysis, the tentative structure of this compound is represented in Fig. 4 and named as dianthrone A.
Fig. 4.
Structure of dianthrone A (Fraction C2c).
4.5. Characterization of C2f fraction of C. auriculata
The spectral data of the isolated compound was in full agreement with the proposed structure. In general, IR revealed the presence of −OH, >C O, and —C C by the formation of strong absorption bands at 3421 cm−1, 1620 cm−1, and 1558 cm−1, respectively.
In the 1H NMR spectra, the signals of the respective protons of the isolated compound were verified on the basis of their chemical shifts, multiplicities, and coupling constants. The spectra showed the aromatic protons at 6.2–7.2 ppm, methyl (-CH3) at 2.4 ppm, methylene proton at 5.3; 4.9 ppm, C-H protons at 3.8–4.7 ppm, aldehyde protons at 8.3 ppm, and hydroxy proton at 2.0 ppm. The 13C NMR spectrum showed the >C O signals at 158.9 ppm, the hydroxyl carbon at 39.9–46.22 ppm, and the aromatic carbon at 113.2–128.07 ppm.
The mass spectrum showed the molecular ion peak at m/z 818 which corresponds to the formula weight of the isolated compound. From the interpretation of the spectral analysis, the tentative structure of this compound is given below (Fig. 5) and named as dianthrone B.
Fig. 5.
Structure of dianthrone B (Fraction C2f).
Earlier reports showed the presence of tannins,25 polysaccharides,26 hepatotoxic alkaloids, and flavonoids27 in C. auriculata. The presence of other compounds as luteolin, quercetin, kaempferol, and kaempferol-3-O-rutinoside had been identified.5 In the present work, additional compounds dianthrone A and dianthrone B were identified.
Our study is in line with previous studies revealing the effect of folklore treatment against diabetes by potentiating the secretion of insulin. Enzymes and hormones play a key role in maintaining the homeostasis during metabolism. Any alteration in these enzymes and hormones therefore results in diabetic complications and the present study justifies the reports and mechanisms of previous studies. Hence, the present study reveals that C. auriculata and its fractions not only reduce the hyperglycemic effect after treatment, but also decrease the risk of associated complications with diabetes.
4.6. Conclusion
The methanol flower extract of C. auriculata has a pronounced effect in controlling the hyperglycemic condition and also has the ability to combat the complications associated with diabetes mellitus. The fractions exhibited remarkable potential to be ideal candidates in the management of diabetes. Further exploration on the identified compounds and the mechanism of action in reducing the complications of diabetes has to be revealed to develop a drug of choice for the treatment of various ailments.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are thankful to the management of MMES and the Principal of C. Abdul Hakeem College, Melvisharam, Vellore, for providing infrastructure and facilities to carry out this research work successfully.
References
- 1.ADA Diagnosis and classification of diabetes mellitus. American Diabetes Association. Diabetes Care. 2009;2009(32):62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddouks M., Maghrani M., Michel J.B. Hypoglycemic effect of Triticumrepens P: Beauv in normal and diabetic rats. J Ethnopharmacol. 2005;102:228–232. doi: 10.1016/j.jep.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Mhaskar K.S., Blatter E., Caius J.F., editors. Vol. IV. Indian Books Centre; Delhi, India: 2000. pp. 1212–1214. (Indian Medicinal Plants). [Google Scholar]
- 4.Thabrew M.I., Munasinghe T.M., Senarath S., Yapa R.M. Effects of Cassia auriculata and Cardospermum halicacabum teas on the steady, state blood levels of theophylline in rats. Drug Metabol Drug Interact. 2004;20:263–272. doi: 10.1515/dmdi.2004.20.4.263. [DOI] [PubMed] [Google Scholar]
- 5.Juan-Badaturuge M., Habtemariam S., Thomas M.J.K. Antioxidant compounds from a south Asian beverage and medicinal plant, Cassia auriculata. Food Chem. 2011;125:221–225. [Google Scholar]
- 6.OECD (Organization for Economic Co-operation Development) OECD; Paris: 2001. Guidance document on acute oral toxicity testing. Environment Directorate; pp. 1–24. [Google Scholar]
- 7.Nagappa A.N., Thakurdesai P.A., Venkat Rao N., Jiwan Singh N. Antidiabetic activity of Terminalia catappa Linn fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 8.Pari L., Uma Maheswari J. Antihyperglycaemic activity of Musa sapientum flowers: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. 2000;14:136–138. doi: 10.1002/(sici)1099-1573(200003)14:2<136::aid-ptr607>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Friedwald J., Levy Y.R., Friedrickson S.D. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Brandstrup N., Kirk J.E., Bruni C. Determination of hexokinase in tissues. J Gerontol. 1957;12:166–171. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 11.Baginsky E.S., Foa P.P., Zak B. In: 2nd ed. Bergymeyer H.U., editor. Vol. 2. Academic Press; New York: 1974. Glucose-6-phosphatase; pp. 788–792. (Methods of enzymatic analysis). [Google Scholar]
- 12.Gancedo J.M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non-fermenting yeasts. Arch Microbiol. 1971;76:132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- 13.Morales M.A., Jabbagy A.J., Tenenzi H.P. Mutations affecting accumulation of glycogen. Neurospora News Lett. 1973;20:24–25. [Google Scholar]
- 14.Fiske C.H., Subbarow J. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 15.Shirwaikar A., Rajendran K., Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb: in streptozotocin nicotinamide induced type II diabetes mellitus. J Ethnopharmacol. 2006;107:285–290. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Abdel M.A., El-Feki M., Salah E. Effect of Nigella sativa, fish oil and gliclazide on alloxan diabetic rats, 1-biochemical and histopathological studies. J Egyptian German Soc Zool. 1997;23:237–265. [Google Scholar]
- 17.Ghosh S., Suryawanshi S.A. Effect of Vinca rosea extracts in treatment of alloxan rats. Indian J Expt Biol. 2001;39:748–759. [PubMed] [Google Scholar]
- 18.Krentz A.J. Lipoprotien abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5:19–27. doi: 10.1046/j.1462-8902.2003.0310.x. [DOI] [PubMed] [Google Scholar]
- 19.Saxena R., Madhu V., Shukla R., Prabhu K.M., Gambhir J.K. Postprandial hypertriglyceridemia and oxidative stress in patients of type 2 diabetes mellitus with macrovascular complications. Clinica Chimica Acta. 2005;359:101–108. doi: 10.1016/j.cccn.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Bopanna K.N., Kannan J., Gangil Sushma, Blarama R., Rathod S.P. Antidiabetic and antilipidemic effects of Neem seed kernel powder on alloxan diabetic rabbits. Indian J Pharmacol. 1997;29:162–167. [Google Scholar]
- 21.Sharma S.B., Nasir A., Prabhu K.M., Murthy P.S. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol. 2006;104:367–373. doi: 10.1016/j.jep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Gupta B.L., Nehal M., Baquer N.Z. Effect of experimental diabetes on the activities of hexokinase, glucose-6-phosphate dehydrogenase and catecholamines in rat erythrocytes of different ages. Indian J Exp Biol. 1997;35:792–795. [PubMed] [Google Scholar]
- 23.Berg J.M., Tymoczko J.L., Stryer L. In: Biochemistry. Berg J.M., Tymoczko J.L., Stryer L., editors. WH Freeman and Company; New York: 2001. Glycolysis and gluconeogenesis; pp. 425–464. [Google Scholar]
- 24.Garvey W.T. Glucose transport and NIDDM. Diabetes Care. 1992;15 doi: 10.2337/diacare.15.3.396. 396–417. [DOI] [PubMed] [Google Scholar]
- 25.Nageswara Rao G., Mahesh Kumar P., Dhandapani V.S., Rama Krishna T., Hayashi Toshimitsu. Constituents of Cassia auriculata. Fitoterapia. 2000;71:82–83. doi: 10.1016/s0367-326x(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 26.Rai K.N., Dasaundhi R.A. New glycoside from the roots of Cassia auriculata Linn. J Bangladesh Acad Sci. 1999;14:57–61. [Google Scholar]
- 27.Therasa Y.M., Bhanu K.M., Naudamma Y. Studies on the tannins of avaram (Cassia auriculata) bark. Aust J Chem. 1968;21:1633–1637. [Google Scholar]