Abstract
Background
Plant mediated green synthesis of nanoparticles is an eco-friendly and efficacious approach which finds immense application in the field of medicine. This study aimed to evaluate the cytotoxicity of platinum nanoparticles (ptNPs) synthesized through green technology against normal and different cancer cell lines.
Methods
Platinum nanoparticles were synthesized by green technology and characterized earlier. In this study we examined the cytotoxic effect of platinum nanoparticles (ptNPs) on human lung adenocarcinoma (A549), ovarian teratocarcinoma (PA-1), pancreatic cancer (Mia-Pa-Ca-2) cells and normal peripheral blood mononucleocyte (PBMC) cells and evaluate anticancer potential through induction of apoptosis on PA-1 cells if any. Cytotoxicity was evaluated using MTT assay, trypan blue dye exclusion assay and anticancer potential assessed through clonogenic assay, apoptosis assay, cell cycle analysis.
Results
We found that ptNPs exerted cytotoxic effect on cancer cell lines, whereas no cytotoxic effect was observed at highest dose on normal cells. The results showed that ptNPs had potent anticancer activities against PA-1 cell line via induction of apoptosis and cell cycle arrest.
Conclusion
Overall, these findings have proved that biosynthesized ptNPs could be potent anti-ovarian cancer drugs. Further studies are required to elucidate the molecular mechanism of ptNPs induced anti-tumor effect in vivo.
Keywords: Cytotoxicity, Platinum nanoparticles, Anti-cancer potential, Apoptosis, In vitro
1. Introduction
In recent years, the interest in the synthesis and properties of metal nanoparticles has been increasing because of their unique properties and promising applications as catalysts, ferrofluids, and semiconductors.1, 2 Nanotechnology is the most promising field for generating new applications in medicine. However, only few nano-products are currently in use for medical purposes.3
For many years, platinum-based molecules have received considerable attention because of their electro-catalytic properties.4, 5 For instance, platinum-based therapeutic drugs, notably cisplatin and carboplatin, have been exploited in chemotherapy to kill cancer cells.5 However, these drugs are not selective for cancer cells, because normal cells are also affected, leading to substantial dose-limiting acute and chronic toxicities. Since toxic side effects (particularly nephrotoxicity and gastrointestinal) and frequent development of drug resistance represent the major challenges in the clinical outcome of these patients, it was conceivable to search for cisplatin analogs or other metal complexes able to offer a more acceptable level of toxicity and improved antitumor activity.6, 7
Nanoparticles are making significant contributions to the development of new approaches of drug delivery in cancer and can provide a platform for combined therapeutics with subsequent monitoring of response.8 Increasing evidence suggests that the special physicochemical properties of nanomaterials pose potential risks to human health.9 Therefore it is necessary to understand how cells respond to nanomaterials and through what mechanisms. Green nanotechnology is generating attention of researchers toward eco-friendly biosynthesis of nanoparticles. With a view toward developing nano-therapeutics, we have performed experiments using eco-friendly platinum nanoparticles. Platinum nanoparticles were synthesized by green technology, characterized its zeta potential and size by dynamic light scattering (DLS) as well as scanning electron microscopy (SEM) earlier.10
The present study is the continuation of the earlier work and is carried out to assess the cytotoxicity of ptNPs on normal and three different types of cancer cells. Based on highest cytotoxicity results, anticancer activities of platinum nanoparticles against ovarian terotocarcinoma cells were evaluated.
2. Methods
Cell culture medium reagents were purchased from Himedia laboratories. Fetal bovine serum (FBS) was purchased from Invitrogen (US). An Annexin V-FITC apoptosis detection kit was purchased from BD-Bioscience (Catalogue no. 556547). Cisplatin, used as a positive control was purchased from Cipla (India). Platinum nanoparticles were synthesized through green technology and characterized by particle size, zeta potential and surface morphology.10 The recovered ptNP sample was used for cytotoxicity and anticancer studies.
2.1. Cell culture and exposure of drug
A human cancer cells, A549, PA-1, Mia-Pa-Ca-2 were obtained from National Center for Cell Science (NCCS), sub-cultured and then used to determine cell cytotoxicity after exposure to the drug. The cells were cultured in minimum essential medium (MEM), Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% FBS at 5% CO2 and 37 °C. At 85% confluence, the cells were harvested using 0.25% trypsin and seeded in 25 cm2 flasks, 96 well plates, 6-well plates, according to the experiment being performed. The cells were allowed to 70% attach to the surface prior to treatment. A stock solution of ptNPs 10 mg/ml was made in vehicle and diluted to appropriate concentrations for treatment. Suspensions were vortexes and aspirated 10 times before treatment. Cells treated with vehicle control were taken as control.
2.2. MTT cell proliferation assay
The MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay measures the cell proliferation rate and conversely, the reduction in cell viability when metabolic events lead to apoptosis or necrosis. The yellow compound MTT is reduced by mitochondrial dehydrogenases to the water insoluble blue formazan compound, depending on the viability of the cells.11
A549, PA-1, Mia-Pa-Ca-2 cells (2 × 104 cells/ml) were seeded in 96-well plates and exposed to different concentrations (50, 100 and 200 μg/ml) of ptNPs and 10 μg/ml of cisplatin for a period of 48 hours. After the treatment period, the cells were allowed to react with MTT for a period of 3–4 hours in dark at 37 °C. At the end of the incubation period, dark purple formazan crystals were formed. These crystals were solubilized with an organic solvent (e.g. isopropanol) and absorbance at 595 nm was measured spectrophotometrically. The experiment was repeated at least three times. Cisplatin was used as positive control for this experiment. To determine the cell viability, we calculated percent viability as % viability = [(Optical density {OD} of treated cell − OD of blank)/(OD of vehicle control − OD of blank) × 100].
2.3. Preparation of PBMC and assessment of cytotoxicity using trypan blue assay
Peripheral blood mononuclear cells (PBMC) were isolated from healthy human volunteer by Ficoll-Paque (Histopaque 1077, Himedia laboratories) density gradient centrifugation as per standard procedure.12 PBMC (2 × 105 cells/well) were cultured in complete RPMI-1640 media as usual and incubated with ptNPs (200 μg/ml), cisplatin (10 μg/ml) to evaluate cytotoxicity for 48 hours using trypan blue exclusion assay. This methods yields approximately more than 95% viable PBMC.
2.4. Clonogenic survival assay
Clonogenic assay or colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. The colony is defined to consist of at least 50 cells. The assay essentially tests every cell in the population for its ability to undergo “unlimited” division.13 Clonogenic assay is the method of choice to determine cell reproductive death after treatment with ptNPs. After harvesting with 0.05% trypsin, 200 (depending on the treatment) cells were plated 24 hours before treatment in MEM at 37 °C. Cultured cells were treated with doses 50 to 200 μg/ml of ptNPs and cisplatin (10 μg/ml). After the treatment, cells were incubated in 5% CO2 atmosphere at 37 °C for 14 days to allow colony formation. Colonies were fixed with methanol and stained with 1% crystal violet. Colonies of more than 50 cells counted and the plating efficiency (PE) was calculated.
2.5. Observation of morphological changes with Acridine orange/Ethidium bromide (AO/EB) staining
Cells were seeded at a concentration of 2 × 105 cell/ml in 6-well tissue culture plates. Following incubation, the medium was removed and replaced with phosphate-buffer saline (PBS) and supplemented with ptNPs (100 and 200 μg/ml). After the treatment period, monolayer cells were stained with AO/EB stain (1 mg/ml).14 After staining, the cells were visualized immediately under the fluorescence microscope (Axiovert, Carl Zeiss) at 20× magnification.
2.6. Annexin V and propidium iodide (PI) staining for apoptosis assay
Apoptosis was assessed via flow cytometric analysis of control and ptNPs treated cells that were stained with FITC-Annexin V and PI using the Annexin V-FITC apoptosis detection kit according to the manufacturer’s protocol (BD Bioscience). PA-1 cells were seeded onto 6 well plates and allowed to adhere. After cells become 70% of confluent, cell were treated with 200 μg/ml of ptNPs for 48 hours at 37° C and 5% CO2. Subsequently, the cells were collected, washed in PBS and resuspended in 500 μl of 1X Annexin-binding buffer. Cells were then incubated at room temperature with Annexin V-FITC and PI stain in the absence of light. Following the 10 minute incubation, samples were immediately analyzed via flow cytometry. Annexin V staining was detected as green fluorescence and PI as red fluorescence.
2.7. Cell-cycle analysis
Cell cycle perturbations were assessed using flow cytometry to measure the proportion of cells in different phases. Cell cycle perturbations induced by ptNPs were analyzed using propidium iodide DNA staining.14 Approximately 2 × 105 cells per well were plated in six-well plates and allowed to attach. After cells becomes 70% confluent, treated with 100 and 200 μg/ml ptNPs for 48 hours and then collected and fixed in ice-cold 70% ethanol for 4 hours and stored at 4 °C until PI staining. Ethanol-suspended cells were then centrifuged at 1000 rpm for 5 min and washed twice in PBS to remove residual ethanol. Pellets were suspended in 1 ml of PI/RNase A reagent and incubated at 37 °C for 30 min. Cell cycle profiles were obtained using a BD FACScan Cell flow Cytometer (Becton Dickinson USA). Debris and aggregates were gated out during data acquisition and 5000-10,000 events were collected from each sample. Data were analyzed with the Cell Quest Pro software.
2.8. Statistical analysis
Statistical comparisons were made using Student’s t-test. Results were expressed as means ± standard errors (SEs). P-values of less than 0.05 were considered significant.
3. Results
3.1. Cytotoxicity of ptNPs on cancer cells
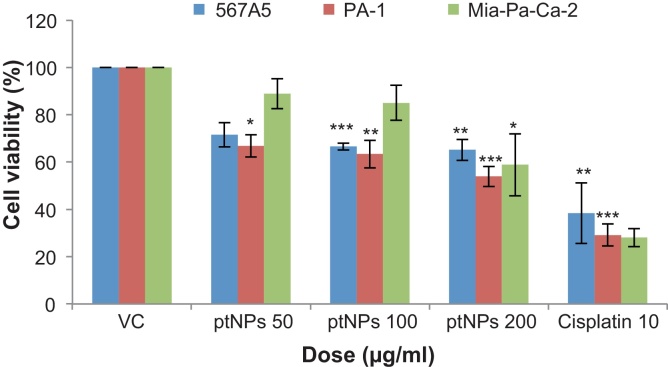
Different cancer cell lines were used to screen for the in vitro cytotoxic activity of ptNPs. A549, PA-1, Mia-Pa-Ca-2 cells were incubated with different concentration of ptNPs for 48 hours. Cell viability was determined by MTT assay. We observed that ptNPs suppressed growth of cancer cells (Fig. 1) and growth inhibition were 28.52-34.85% in A549 cells, 33.16-46.06% in PA-1 cells and 11.12-41.18% in Mia-Pa-Ca-2 cells after treatment of ptNPs (50–200 μg/ml). The results shown in Fig. 1 indicated that ptNPs (200 μg/ml) caused a significant decrease in cell viability of A549 (**P < 0.01), PA-1 (***P < 0.001), Mia-Pa-Ca-2 (*P < 0.05) respectively as compared to control. PtNPs showed the highest growth inhibitory effects on PA-1 cells. As presented in Fig. 1, some cytotoxic effect was already observed at 50 μg/ml dose of ptNPs whereas maximal effect was obtained at a concentration of 200 μg/ml in PA-1. Based on these data, the present study focused on PA-1 cells for subsequent tests.
Fig 1.
Effect of ptNPs on viability of lung adenocarcinoma (A549), ovarian teratocarcinoma (PA-1), pancreatic cancer (Mia-Pa-Ca-2) cells. A. Cells were treated with vehicle, different concentrations of ptNPs and positive control (10 μg/ml cisplatin) for 48 hours. Cell viability was analyzed using the MTT assay. Data represented as mean ± SE of three independent experiments made in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle control (VC) group.
3.2. Cytotoxicity of ptNPs on normal cells
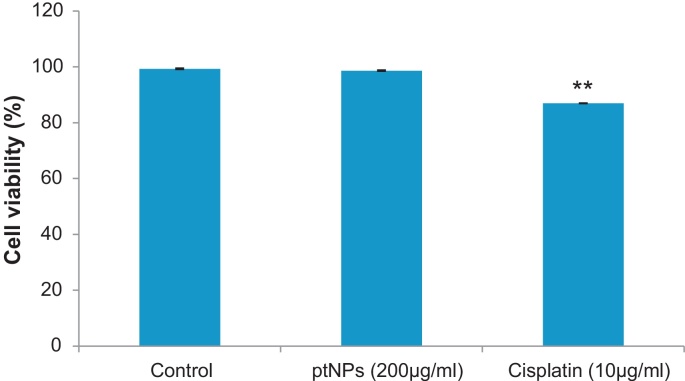
To examine the cytotoxic effect of the ptNPs against normal PBMC cells, trypan blue dye exclusion assay was performed. PBMC cells were treated with highest dose (200 μg/ml) of the ptNPs for 48 hours. No significant cytotoxic effect was observed in the normal PBMC at the highest concentration (200 μg/ml) (Fig. 2) of ptNPs that significantly affected the cancer cells Fig. 1, suggesting that the effect of the ptNPs was selective for cancer cells.
Fig. 2.
Cytotoxic effect of ptNPs (200 μg/ml) and cisplatin (10 μg/ml) on normal PBMC cells. Data represented as mean ± SE of three independent experiments. **P < 0.01 versus control group.
3.3. Clonogenic survival assay
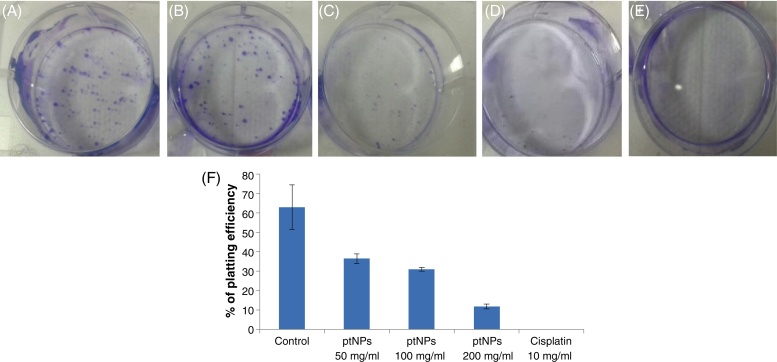
The clonogenic assay showed the effect of ptNPs on the colony-forming capacity of exponentially growing PA-1 cells. We performed a clonogenic assay for confirming the growth inhibition results of ptNPs. Platinum nanoparticles treatment enhances cell death and also inhibits colony formation capability in the PA-1 cell population in a concentration dependent manner. After treatments with different concentrations (50, 100, 200 μg/ml), the plating efficiency of PA-1 cells declines, as evidenced by the reduction in the number of colonies formed (Fig. 3B-D). Exposure of ptNPs (50, 100 and 200 μg/ml) shows a decline in colony survival and plating efficiency was found to be 36, 31 and 11 respectively (Fig. 3F). This result indicates that ptNPs at 200 μg/ml significantly (p < 0.05) inhibit the colony formation capabilities of PA-1 cells.
Fig. 3.
Effect of platinum nanoparticles on colony forming capacity or clonogenic survival of exponentially growing PA-1 cells studied by a clonogenic assay. PA-1 cells were treated with ptNPs (50, 100, 200 μg/ml) and cisplatin (10 μg/ml), allowed to form colonies in fresh medium for 14 days. A = Control; B = ptNPs (50 μg/ml); C = ptNPs (100 μg/ml); D = ptNPs (200 μg/ml); E = Cisplatin (10 μg/ml). F: Representative histogram showing percentage of platting efficiency in PA-1 cells. Data are expressed as mean ± SE (n = 3). *P < 0.05, ***P < 0.001 versus control group.
3.4. Morphological changes using AO/EB staining
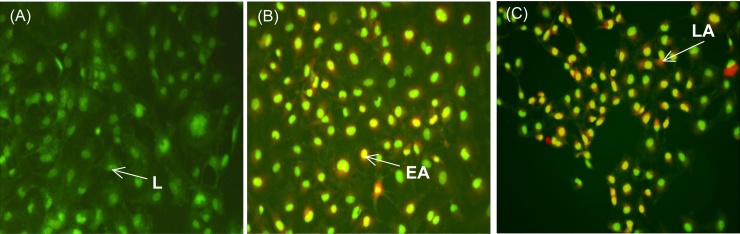
Apoptosis was also confirmed by examining the nuclear morphology by AO/EB staining. As shown in Fig. 4A, control PA-1 cells were stained with uniform green fluorescence and no apoptotic features were observed. Following treatment of PA-1 cells with ptNPs for 48 hours, obvious morphological changes and apoptotic cells with chromatin condensation were observed (Fig. 4B-C). The results suggest that ptNPs induced PA-1 cell apoptosis. Cells stained green represent viable cells, whereas yellow staining represents early apoptotic cells and reddish or orange staining represents late apoptotic cells.
Fig. 4.
PA-1 cells were stained by AO/EB and observed under fluorescence microscope. PA-1 cells were treated with (A) vehicle control, (B) 100 μg/ml and (C) 200 μg/ml of ptNPs for 48 hours. L indicates live cells, EA indicates early apoptotic cells and LA indicates late apoptotic cells.
3.5. Effect of ptNPs on apoptosis in PA-1
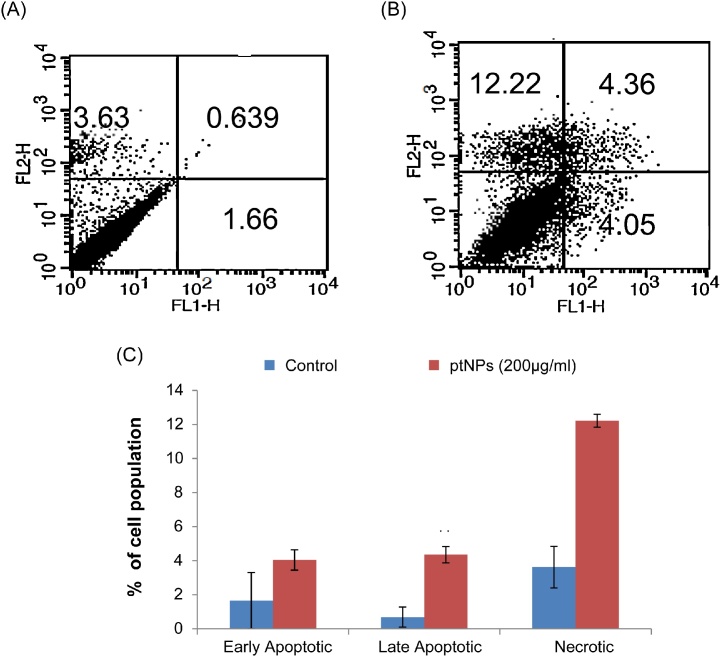
Apoptosis, autophagy and necrosis are the major types of cell death.15 To determine the percentage of apoptotic and necrotic cells, the cells were treated with 200 μg/ml ptNPs for 48 hours and stained with Annexin V and PI using flow cytometry. Here, we found that that ptNPs is capable of inducing apoptosis in PA-1 cells. The flow cytometry analysis results showed that the rate of apoptosis was 8% in the cells treated with 200 μg/ml of ptNPs after 48 hours (Fig. 5).
Fig. 5.
Apoptosis induced by ptNPs in PA-1 cells. PA-1 cells were treated with (A) vehicle control and (B) 200 μg/ml of ptNPs for 48 hours. Then cells were stained with FITC-conjugated Annexin V and PI for flow cytometric analysis. The flow cytometry profile represents Annexin V-FITC staining in x axis and PI in y axis. (C) Results showing the percentage of early apoptotic cells, late apoptotic cells and necrotic cells. Data are expressed as Mean ± SE (n = 3). **P < 0.01 versus control group.
3.6. Cell-cycle analysis
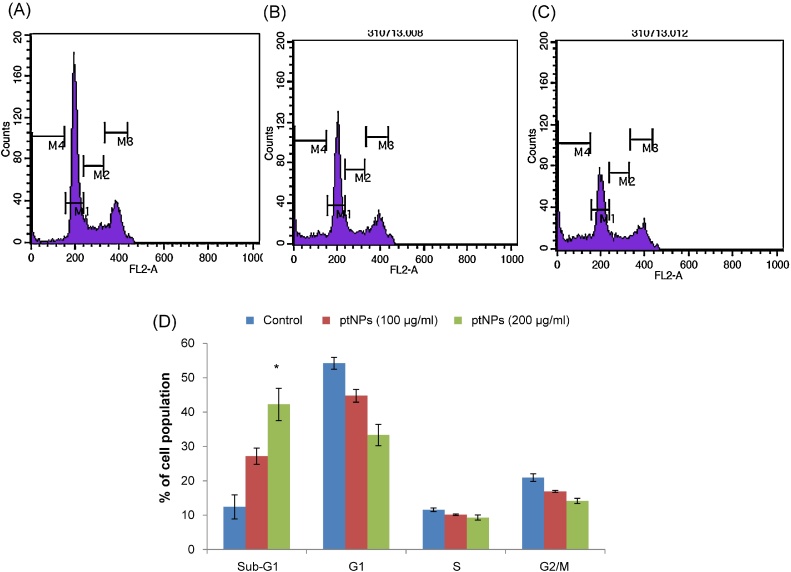
Cell flow cytometry was used to determine the effect of ptNPs on the cell cycle progression. A significant increase in the percentage of cells in sub-G1 phase was found after treatment with ptNPs, compared with control cells (Fig. 6). As shown in Fig. 6, when cells were exposed to ptNPs (200 μg/ml) for 48 hours, the percentage of sub-G1 population showed a marked increase of 42% (p < 0.05) compared with the control (12%). G0/G1 phase were decrease along with the increase of sub-G1 phase compared with that of control cells. All these data indicate that inhibition of cell growth causes increased cells entering in sub-G1 phase, subsequently induces cell apoptosis.
Fig. 6.
Flow cytometry analysis of cell cycle phase distribution in PA-1 cells. Histogram representing propidium iodide staining of control (A) and ptNPs (100 and 200 μg/ml) (B-C) treated PA-1 cells for 48 hours. D: Bar diagram showing the cell distribution in the subG1, G0/G1, S and G2/M phases for PA-1 cells treated with vehicle control and ptNPs (100 and 200 μg/ml). Data are expressed as mean ±SE (n = 3). *P < 0.05 versus control group.
4. Discussion
Dependence of human life on nanotechnology emerged naturally from ayurveda, a 5000-year-old system of Indian medicine. Though the modern science has started exploring the term “Nano” in 21st century, ayurvedic medicinal systems used noble metals such as gold, silver etc., in nano form as bhasmas for various medical applications.16 Since nanoparticles (NPs) are more biocompatible than the conventional therapeutics, they play an important role in improving their bioavailability as well as compatibility for therapeutical applications in diseases like cancer.17 There is a growing list of reports indicating that NPs might be medically and environmentally toxic, as their high surface-to-volume ratio makes the particles of some metals very reactive or catalytic.18 There is also evidence that NPs pass through cell membranes and interact with cellular structures, and thus have a direct impact on cell functioning and consequently on cell viability. Therefore, in this study, we have determined the cytotoxicity of ptNPs using cancer cell lines and normal human peripheral blood mononucluocyte (PBMC) cells for understanding toxicity under in vitro conditions. We carried out screening of ptNPs in different cancer cells of lung, ovarian, pancreatic, breast, colon, renal, leukemia types. Interestingly, the cytotoxicity of ptNPs toward mammalian cells depends on the cell type. Our in vitro studies showed that ptNPs induced cell death in ovarian, lung and pancreatic cancer cell lines (data of breast, colon, renal, leukemia cancer cell lines were not shown). In contrast, no significant cytotoxic effect was observed in the normal human PBMC cells (Fig. 2) at the highest concentration (200 μg/ml) of the nanoparticles that significantly affected the lung, ovarian and pancreatic cancer cells (Fig. 1), suggesting that the effect of the platinum nanoparticles was cytotoxic for the selected cancer cell lines. As ptNPs exhibiting greater cytotoxicity on PA-1 cells, PA-1 cell line was selected to find out mode of action of platinum nanoparticles and evaluated its anticancer potential.
The present study revealed that potential cytotoxic effect of ptNPs in PA-1 cells after short (48 hours) and long term (14 days) exposure. Clonogenic assay, used to evaluate the effects induced after prolonged exposure (14 days). Our finding shows that ptNPs dose dependently inhibited the PA-1 colony formation as compared to the control cells (Fig. 3). This indicates that ptNPs decreases the potential of individual cells to form a colony and thereby acts as an anti-cancer drug. This finding well corroborated with our cell proliferation studies.
The major drawbacks of many effective cancer chemotherapeutic agents are systemic toxicity and drug resistance.19 Cisplatin is the first approved platinum drug that has been used for more than three decades in standard chemotherapy regimens. However, the use of cisplatin is restricted because of its severe side effects, including nephrotoxicity, neurotoxicity, ototoxicity and myelo-suppression, as well as the intrinsic and acquired resistance developed by various cancers. Thus, the new therapeutic agents should be more active while producing fewer side effects. They also should act through a mechanism different from that of cytotoxic agents already used. A variety of nanomaterials are being evaluated in clinical trials as drug carrier and for imaging of specific targets, with aim of increasing the efficiency of drug bioavailability, reducing side effects and prevention damage to other tissues.20, 21 At present there has been a considerable interest in the biological synthesis of nanoparticles because of its simple, safe and eco-friendly principles and it does not require elaborate process. The biosafety and biocompatibility of any biomaterial are vital concerns that should be addressed before such materials are applied to biological systems. We investigated the acute toxic effect of ptNPs in different doses and no toxic effect was found upto 5000 mg/kg bw after oral administration to mice (data not published). No cytotoxicity was also found after treatment of different doses of ptNPs (25-100 μg/ml) against normal PBMC.10 Our present PBMC results also showed that no cytotoxicity was observed at highest 200 μg/ml dose. The activity of biosynthesized ptNPs in human cancer cells in vitro and in vivo provides the rationale for clinical use of orally administered agent in patients with solid tumor as most of the chemotherapeutic drug like cisplatin administered intravenously and have side effects. In this regard, platinum nano particles synthesized by green technology with anticancer activity and no toxicity to normal tissues has been suggested as possible candidates for their capability to improve the efficacy of anticancer drugs.
In conclusion, biosynthesized ptNPs exhibited cytotoxic activity against ovarian, lung and pancreatic cancer cells without showing toxicity against normal peripheral blood mononucleocyte cells. Results also demonstrated that ptNPs induce apoptosis and cell cycle arrest in PA-1. Based on our observation, we suggest that ptNPs may represent an emerging novel therapeutic agent for the treatment of human ovarian cancer without showing cytotoxicity towards normal cells. Further studies are required to support our observations of the anti-tumor potential of these platinum nanoparticles in vivo.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by grants from Department of Biotechnology(DBT), Government of India (BT/BIPP0446/13/11). We also would like to acknowledge Dr. Ulhas Wagh for technical advice during the experiments. We thank Dr. Padma Shastri of the National Centre for Cell Science (NCCS) for revising the manuscript.
Contributor Information
Yogesh Bendale, Email: dr.bendale@gmail.com.
Vineeta Bendale, Email: vineeta.bendale@gmail.com.
Saili Paul, Email: saili_paul@rediffmail.com.
References
- 1.Henglein A. Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem Rev. 1989;89:1861. [Google Scholar]
- 2.Oggawa S., Hayashi Y., Kobayashi N., Tokizakiand T., Nakamura A. Novel preparation method of metal particles dispersed in polymer films and their third-order optical nonlinearities. Jpn J Appl Phys. 1994;33:331. [Google Scholar]
- 3.Chen X., Schluesener H.J. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;4:176. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Stephens I.E.L., Bondarenko A.S., Grønbjerg U., Rossmeisl J., Chorkendorff I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ Sci. 2012;5:6744–6762. [Google Scholar]
- 5.Kostova I. Platinum complexes as anticancer agents. Recent Pat Anti-Cancer Drug Discov. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 6.Respondek J., Engel J. Organometallics in medicine. Drugs Future. 1996;21:391–408. [Google Scholar]
- 7.Cagnoli M., Alama A., Barbieri F., Novelli F., Bruzzo C., Sparatore F. Synthesis and biological activity of gold and tin compounds in ovarian cancer cells. Anticancer Drugs. 1998;9:603–610. doi: 10.1097/00001813-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Liong M., Lu J., Kovochich M., Xia T., Ruehm S.G., Nel A.E. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nel A., Xia T., Madler L., Li N. Toxic potential of materials at the nano level. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 10.Bendale Y., Bendale V., Paul S., Bhattacharyya S.S. Green synthesis, characterization and anticancer potential of platinum nonoparticles. Zhong Xi Yi Jie He Xue Bao. 2012;10:681–689. doi: 10.3736/jcim20120613. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M.D., Ghosh R., Patra A., Hazra B. Synthesis and antiproliferative activity of some novel derivatives of diospyrin, a plant-derived naphthoquinonoid. Bioorg Med Chem. 2007;15:3672–3677. doi: 10.1016/j.bmc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Kaushik N.K., Kim H.S., Chae Y.J., Lee Y.N., Kwon G.C., Choi E.H. Synthesis and Anticancer Activity of Di(3-thienyl)methanol and Di(3-thienyl)methane. Molecules. 2012;17:11456–11468. doi: 10.3390/molecules171011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava D., Joshi G., Somasundaram K., Mulherkar R. Mode of cell death associated with adenovirus-mediated suicide gene therapy in HNSCC tumor model. Anticancer Res. 2011;31:3851–3857. [PubMed] [Google Scholar]
- 15.Leist M., Jaattela M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar P.K., Chaudhary A.K. Ayurvedic bhasma: most ancient application of nanomedicine. J Sci Ind Res. 2010;69:901–905. [Google Scholar]
- 17.Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Oberdorster G., Oberdorster E., Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelloff G.J. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 20.Jee J., Na J.H., Lee S., Kim S.H., Choi K., Yeo Y. Cancer targeting strategies in nanomedicine: design and application of chitosan nanoparticles. Current Opin Solid State Mater Sci. 2012;16:333–342. [Google Scholar]
- 21.Rosenholm J.M., Sahlgren C., Linden M. Towards multifunctional, targeted drug delivery system using mesoporous silica nanoparticles-opportunities and challenges. Nanoscale. 2010;2:1870–1883. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]