Abstract
In the developing cortex, spike timing–dependent long-term depression requires cannabinoid-induced glutamate release from astrocytes. Astrocytes may be integral to the coincidence detection that guides plasticity and map formation.
Normal functionality of the mammalian neocortex is critically dependent on the proper development of the underlying cellular architecture and synaptic connectivity. Thus, characterizing the mechanisms of neocortical development is fundamental to understanding normal neocortical function and neurodevelopmental disorders and is important for guiding regenerative therapies for neurodegenerative diseases. At the most basic level, the development of the neocortex results in functional subdivisions, such as the primary somatosensory and visual cortices, and there is a further topographic organization within such functional subdivisions, whereby neighboring neurons communicate with neighboring sensory or motor locations in the periphery. Early in development, such neocortical topography is initiated by genetic and molecular guidance cues, but fine sculpting of topographic maps is dependent on neural activity and consequent synaptic plasticity1,2. In particular, it is thought that differential induction of long-term potentiation (LTP) and long-term depression (LTD) of synaptic strength in neighboring synapses determines the size and shape of functional topographic units, such as ocular dominance columns in the primary visual cortex and whisker barrels in the primary somatosensory cortex2,3. However, the mechanisms that determine whether a given pattern of neural activity will trigger LTP or LTD at individual synapses are not well understood. In this issue, Min and Nevian4 present an elegant set of experiments that elucidate the central role of astrocyte cannabinoid and glutamate signaling in translating specific patterns of pre- and postsynaptic activity into LTD in the developing primary somatosensory cortex.
Experimental support for the role of LTP and LTD in sculpting neocortical topography is particularly well established at excitatory synapses between layer 4 spiny stellate cells (L4 cells) and layer 2/3 pyramidal cells (L2/3 cells) in the rodent primary somatosensory cortex (barrel cortex). These cells are organized in a topographic representation of the whiskers of the snout, with barrel-shaped clusters of cells responding preferentially to individual whisker stimulation. Although the topographical map maintains a degree of plasticity in adult rodents, there is a critical period of development, during which clipping individual whiskers powerfully alters map topography, with barrels representing lost and spared whiskers shrinking and expanding, respectively5. The exact network interactions are not fully understood, but it is thought that, in part, the induction of LTP and LTD at synapses between L4 cells and L2/3 cells shapes the barrels during development, with LTP and LTD functionally adding L2/3 cells to or removing them from the barrel, respectively1,3,5. Accordingly, much effort has been focused on discovering signaling rules and mechanisms that determine whether LTP and LTD occur at this synapse6–12. These studies have identified spike timing–dependent plasticity as a rule governing whether L4-to-L2/3 transmission undergoes LTP or LTD. Specifically, when a postsynaptic action potential follows a presynaptic action potential in a time window of tens of milliseconds, it induces LTP, whereas the reverse order of action potentials induces LTD9,12.
Mechanistic studies of spike timing–dependent plasticity at the L4-to-L2/3 synapse have determined that timing-dependent LTP is triggered by Ca2+ influx through postsynaptic NMDA receptors similarly to LTP in the hippocampus and other synapses9. In contrast, timing-dependent LTD (tLTD) is more complex, requiring the activation of postsynaptic metabotropic glutamate receptors (mGluRs), postsynaptic voltage-gated Ca2+ channels, presynaptic NMDA receptors and, notably, cannabinoid receptors, which are activated by endocannabinoids that are generated in the postsynaptic cell (consequent to coincident activation of postsynaptic mGluRs and voltage-gated Ca2+ channels)6,9,10,12. Given the prominent presence of cannabinoid receptors on presynaptic glutamatergic terminals, combined with tLTD being expressed presynaptically (through reduced vesicle release probability), it has been proposed that coincident activation of presynaptic NMDA and cannabinoid receptors triggers tLTD9,10,12. However, there is no direct evidence that the cannabinoid receptors that trigger tLTD are actually located on presynaptic terminals, and nothing is known about the source of glutamate that activates presynaptic NMDA receptors.
To directly assess the mechanisms mediating tLTD, Min and Nevian4 used patch-clamp recording and Ca2+ imaging in slices of rodent barrel cortex, monitoring astrocyte Ca2+ during the induction of tLTD at L4-to-L2/3 synapses. They found that, contrary to current hypotheses, the tLTD-inducing cannabinoid receptors were present on astrocytes and that their activation increased astrocyte Ca2+ spiking, which is necessary for the induction of tLTD. First, they showed that stimulation protocols that induce tLTD (but not those that induce tLTP or that do not cause plasticity) markedly increased the frequency and duration of astrocyte Ca2+ spikes. They then showed that blocking postsynaptic synthesis of cannabinoids, blocking cannabinoid receptors or loading astrocytes with Ca2+ chelators prevented the increase in astrocyte Ca2+ spiking and the induction of tLTD. Crucially, bypassing the production of cannabinoids in the postsynaptic neuron and directly activating astrocyte cannabinoid receptors increased astrocyte Ca2+ spiking and induced LTD, and such astrocyte-triggered LTD occluded subsequent induction of tLTD. Thus, cannabinoid receptor–induced increases in astrocyte Ca2+ spiking are both necessary and sufficient to induce LTD at developing L4-to-L2/3 synapses.
How does cannabinoid-induced astrocyte Ca2+ spiking trigger tLTD? Most synapses are ensheathed by astrocytic processes and, in a variety of preparations, astrocytes can modify synaptic transmission by means of Ca2+-dependent exocytosis of glutamate. Given that tLTD requires increases in astrocytic Ca2+ and activation of presynaptic NMDA receptors, Min and Nevian4 hypothesized that astrocytic exocytosis may be the source of glutamate that triggers tLTD. In clear support of their hypothesis, blocking either astrocytic vesicle exocytosis or glutamate accumulation in astrocytic vesicles prevented tLTD. Taken together, these data suggest that astrocytes are important components in the induction of tLTD (Fig. 1a). Contrary to prevailing thinking, these data indicate that, during the induction of tLTD, postsynaptically generated cannabinoids directly activate astrocytic cannabinoid receptors, and the consequent increase in astrocyte Ca2+ spiking causes vesicular release of glutamate, which triggers the induction of presynaptically expressed tLTD.
Figure 1.
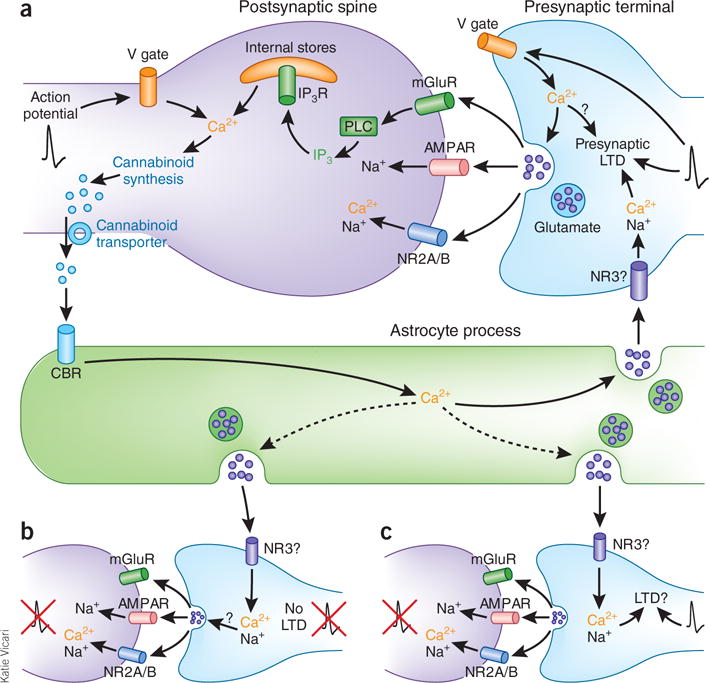
Astrocyte cannabinoid and glutamate signaling triggers tLTD at developing somatosensory cortical synapses. (a) The signaling cascade at developing L4-to-L2/3 synapses. Action potentials (black traces) at presynaptic terminals cause vesicular glutamate release, which generates the postsynaptic current mediated by AMPA-type (AMPAR) and NMDA-type (NR2A/B subunits) glutamate receptors and activates metabotropic glutamate receptors (mGluRs). The activation of mGluRs increases phospholipase C (PLC) production of inositol-1,4,5-trisphosphate (IP3), which binds to its receptor (IP3R) to release Ca2+ from intracellular stores. When a postsynaptic action potential precedes a presynaptic one by 25 ms, Ca2+ influx through postsynaptic voltage-gated Ca2+ channels (V gate) combines with Ca2+ released from intracellular stores to stimulate cannabinoid synthesis. Newly synthesized cannabinoids are transported out of the postsynaptic spine, where they activate astrocytic cannabinoid receptors (CBRs). Activation of astrocytic CBRs increases astrocyte Ca2+, which triggers vesicular release of glutamate onto presynaptic NMDA receptors (possibly the NR3 type). The activation of presynaptic NMDA receptors combined with presynaptic action potentials induces presynaptically expressed tLTD. (b,c) Although an activated astrocyte will likely release glutamate onto presynaptic NMDA receptors at other synapses it contacts, LTD will not occur at fully quiescent synapses (b) but might occur at synapses receiving presynaptic action potentials even in the absence of postsynaptic firing (c).
It has generally been thought that the importance of the precise order and timing of pre- and postsynaptic spiking for the induction of tLTD reflects postsynaptic spatiotemporal integration of Ca2+ influx through voltage-gated channels and mGluR-triggered release of Ca2+ from intracellular stores. The postsynaptic integrator was presumed to be the endo cannabinoid synthetic machinery, with retrograde transport of cannabinoids to the presynaptic terminal signaling the detection of appropriate coincidence between pre- and postsynaptic spiking9,12. However, the requirement for activation of presynaptic NMDA receptors added another potential level of coincidence detection, the nature of which has been unclear owing to the lack of information about the source of glutamate6,9. The discovery by Min and Nevian4 of astrocytes as the primary vehicle of retrograde signaling from postsynaptic induction to presynaptic expression of tLTD clarifies the nature of pre- and postsynaptic coincidence detection, but it raises several intriguing issues and questions. The discovery that tLTD depends on activation of astrocytic cannabinoid receptors and sequential, consequent activation of presynaptic NMDA receptors indicates that the respective receptors do not contribute to coincidence detection per se. Instead, they represent two stages of a sequential retrograde signal informing the presynaptic neuron that the postsynaptic neuron has detected an appropriate coincidence of pre- and postsynaptic action potentials. However, if coincidence is detected solely by the postsynaptic neuron and astrocytic Ca2+-induced glutamate release then triggers tLTD, tLTD would presumably be induced at all synapses contacted by the astrocyte, not just the synapse that detected coincident pre- and postsynaptic activity (Fig. 1). Such hetero synaptic LTD would obviously lessen the precision with which tLTD could sculpt barrel map topography.
To determine whether an activated astrocyte could induce LTD independently of pre- or postsynaptic activity, Min and Nevian4 directly activated astrocytic Ca2+ signaling by applying depolarizing pulses to the astrocyte and monitored the effect on synaptic transmission at neighboring L4-to-L2/3 synapses. Surprisingly, they found that direct stimulation of astrocytes elicits LTD, but only if it is combined with stimulation of presynaptic afferents. Notably, this form of LTD is prevented by blocking NMDA receptors and occludes subsequent induction of tLTD, indicating that direct stimulation engages the same mechanisms as tLTD, but only if combined with presynaptic stimulation. Thus, induction of tLTD is dependent on a second level of coincidence detection at the presynaptic terminal: activation of presynaptic NMDA receptors must coincide with presynaptic action potential firing. This second level of coincidence detection is predicted to prevent LTD from occurring at totally quiescent synapses that are contacted by an activated astrocyte (Fig. 1b), but leaves open the possibility that heterosynaptic LTD would occur at contacted synapses exhibiting presynaptic action potentials, but without coincident postsynaptic firing (Fig. 1c). This possibility is particularly intriguing because the role of presynaptic NMDA receptors in triggering tLTD is lost during development, with adult tLTD requiring only activation of postsynaptic NMDA receptors11. If astrocyte activation of presynaptic NMDA receptors does enable broader heterosynaptic tLTD in the developing barrel cortex, then the loss of this mechanism during development may result in more synapse-specific tLTD in adults. Future studies should determine whether heterosynaptic tLTD is more prevalent during development and how such changes affect the functional outcome of tLTD.
In addition to determining the functional effect of the identified presynaptic coincidence requirement, it will be important to determine how coincident activation of presynaptic NMDA receptors and action potential firing are detected and translated into presynaptically expressed tLTD. Ca2+ influx through NMDA receptor channels is a classic inducer of plasticity, but what is the downstream effector mechanism and what is the function of presynaptic action potentials? Notably, if developing presynaptic NMDA receptors contain the Mg2+-insensitive NR3A subunit, then the traditional requirement of membrane depolarization to alleviate the Mg2+ blockade of Ca2+ influx through activated NMDA receptor channels may not function13.
Min and Nevian’s findings4 highlight yet another important action of astrocytes in the tripartite synapse. Identifying their central function in mediating tLTD in developing somatosensory cortex should substantially facilitate efforts to determine the specific roles and mechanisms of tLTD in establishing cortical topographic maps, a fundamental process in neocortical development and function.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Inan M, Crair MC. Neuroscientist. 2007;13:49–61. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- 2.Hensch TK. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 3.Foeller E, Feldman DE. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Min R, Nevian T. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 5.Feldman DE, Brecht M. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Moreno A, Paulsen O. Nat Neurosci. 2008;11:744–745. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Moreno A, et al. J Neurosci. 2011;31:8564–8569. doi: 10.1523/JNEUROSCI.0274-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee A, et al. Cereb Cortex. 2009;19:2959–2969. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender VA, Bender KJ, Brasier DJ, Feldman DE. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström PJ, Turrigiano GG, Nelson SB. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 11.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevian T, Sakmann B. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen RS, et al. Nat Neurosci. 2011;14:338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]


