Abstract
The lateral line and its associated sensory nerves develop from cephalic epithelial thickenings called neurogenic placodes. In the zebrafish, the transcription factor neurogenin 1 is essential for the generation of the sensory ganglion from the placode, but is dispensable for the migration of the primordium and the initial development of neuromasts. We find that inactivation of the gene encoding neurogenin 1 leads to the development of over twice the normal number of neuromasts along the posterior lateral line of zebrafish larvae. Mutation of the gene encoding another transcription factor, sox10, has a similar effect. After a normal number of proneuromasts is initially deposited by the migrating primordia, interneuromast cells divide and differentiate to form the extra neuromasts. Our results indicate that the development of these intercalary neuromasts occurs principally because of the absence of neural crest-derived peripheral glia, which evidently inhibit the assembly of interneuromast cells into neuromasts.
Keywords: acousticolateralis system, hair cell, neurogenin
The octavolateralis sensory system of an aquatic vertebrate includes both the internal ear and the lateral-line system, the latter serving to detect mechanical disturbances in the surrounding water (1–3). The lateral line comprises a stereotyped array of sensory structures called neuromasts, each containing mechanoreceptive hair cells as well as supporting cells. During the embryonic development of the zebrafish, cephalic ectodermal placodes generate both the neuromasts and their sensory ganglia (4). The posterior lateral-line placode contains ≈120 cells, the caudal three-quarters of which constitute the first primordium that initiates a posteriorward migration ≈20 h postfertilization (hpf) (5, 6). The first primordium completes its journey to the animal's tail at ≈40 hpf, leaving behind seven to nine proneuromasts and a trail of interneuromast cells connecting them (7, 8). Soon thereafter, a second, smaller primordium begins to migrate along the same path, depositing two or three proneuromasts over the rostral part of the trunk (8, 9). Neuromasts of the posterior lateral line mature progressively from head to tail; by the fifth day of development, 9–11 neuromasts contain functional hair cells. The system later gains complexity through the development of additional neuromasts and the growth and elongation of existing clusters (10).
The rostral portion of the posterior lateral-line placode forms a sensory ganglion (6), whose development depends on the activity of the transcription factor neurogenin 1 (11). The somata of neurons in this ganglion remain near the origin of the placode, but extend axons along the path followed by the lateral-line primordia (6, 12, 13). The lateral-line nerve, in turn, serves as a scaffold to guide the migration of a subset of neural crest-derived peripheral glia associated with the lateral line (14). Although sensory axons are dispensable for the migration of the primordia and the initial development of neuromasts, the consequences of an absence of glia on the postembryonic development of the lateral-line organ are not known. Here, we take advantage of mutations that alter the number of glial cells to address this issue.
Materials and Methods
Zebrafish Strains and Husbandry. Zebrafish were maintained in our facility under standard conditions. Wild-type fish were of the Tup Longfin or Tübingen strains. Animals mutant for the gene encoding neurogenin 1, a gift of N. Hopkins (Massachusetts Institute of Technology, Cambridge), carried the hi1059 allele (15). KC66 transgenic animals (16) expressing histone 2B-GFP and mutants bearing the m241 allele of the gene encoding sox10 (17) were obtained from the Zebrafish International Resource Center (Eugene, OR).
Morpholino Oligonucleotides. Morpholinos (11) directed against the mRNA encoding neurogenin 1 (5′-ACGATCTCCATTGTTGATAACCTGG-3′) were purchased from Gene Tools (Corvalis, OR). Diluted to a concentration of 500 μM in 1× Danieau's solution, the morpholinos were injected into one- to four-cell embryos of three different wild-type strains: Tup Longfin, Tübingen, and the histone 2B-GFP transgenics in an AB background strain. Uninjected embryos maintained identically served as controls.
Labeling Procedures and Imaging. Immunohistochemical and vital labeling of hair cells were conducted as described (8). Antisera and monoclonal antibodies were used at the following dilutions: rabbit anti-claudin b, 1/500; rabbit anti-parvalbumin 3, 1/2,000; rabbit anti-phosphohistone H3 (Upstate Biotechnology), 1/1,000; mouse monoclonal antibody 6D2, 1/5; mouse monoclonal antibody HCS1, 1/20; and rat anti-α-tubulin (AbCam), 1/1,000. Fluorescein- and Texas red-labeled donkey anti-mouse, -rat, and -rabbit immunoglobin secondary antibodies (The Jackson Laboratory) were used at a dilution of 1/200. TO-PRO 3 was purchased from Molecular Probes. Specimens were examined with a Zeiss Axioplan II microscope and images were acquired with a Nikon Coolpix-995 digital camera. Confocal images were obtained with a BioRad MRC-1024ES scan head on a Zeiss Axiovert microscope or with a Zeiss Meta510 system.
For live imaging, animals mutant for the gene encoding sox10 were incubated in fluorescein-labeled ceramide (Molecular Probes) for 12 h at 28.5°C in the dark, then rinsed and kept in system water until analysis. Each larva was mounted on a glass coverslip, covered with 1% low-melting-point agarose in a 610 μM solution of the anesthetic 3-aminobenzoic acid ethyl ester, and imaged with a confocal system mounted on an inverted microscope.
Statistical results are provided as means ± standard deviations for the indicated number of observations.
Results
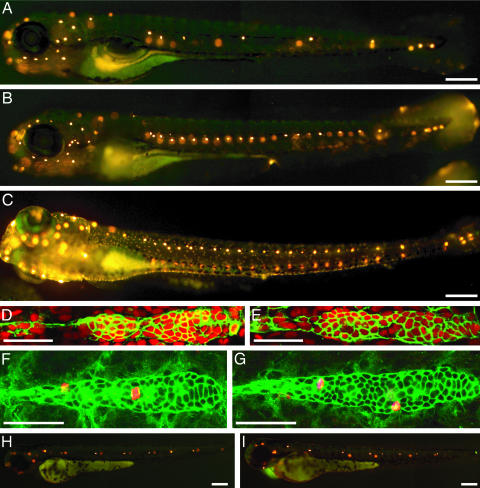
Mutant Fish Develop Supernumerary Neuromasts. The posterior lateral-line placode gives rise to the first migratory primordium and a sensory ganglion. Although blocking the production of the basic helix-loop-helix transcription factor neurogenin 1 with morpholinos prevents the formation of the sensory ganglion, this procedure does not affect the initial development of neuromasts (11). However, it is not known how a lack of innervation affects the postembryonic development of neuromasts. We observed that a mutation in the neurogenin 1 (ngn1) gene leads to the formation of supernumerary neuromasts along the posterior lateral line of zebrafish larvae (Fig. 1 A and B). The average number of neuromasts along one side of an ngn1 mutant at 6 days postfertilization (dpf) is 23.2 ± 1.7 (n = 6), over twice the complement of 10.7 ± 1.1 (n = 10) characteristic of wild-type animals. We reproduced this phenotype by injecting morpholinos directed against the ngn1 transcript (Fig. 1C).
Fig. 1.
ngn1 mutant zebrafish develop extra neuromasts postembryonically. (A) When treated with the fluorescent compound 4-Di-2-ASP at 6 dpf, a wild-type larva displays only seven to nine neuromasts with functional hair cells on one side of its trunk. The neuromasts on the animal's opposite side appear as diffuse spots of fluorescence. (B) An ngn1 mutant at 6 dpf possesses more than twice as many neuromasts in its posterior lateral line. The number and pattern of neuromasts on the head is essentially normal, as is the animal's pigmentation. (C) At 6 dpf, an animal injected with a morpholino directed against the ngn1 transcript displays a pattern of supernumerary neuromasts comparable to that of an ngn1 mutant. Tilting of the larva separates the images of the neuromasts on the animal's two sides. (D) In a wild-type larva, an antibody against claudin b (green) labels the first primordium and the trail of cells left behind it; the nuclear stain TO-PRO 3 (red) marks all cells. (E) The primordium of an ngn1 mutant does not contain significantly more cells. (F) In a wild-type animal at 24 hpf, antiserum against α-tubulin strongly labels primordial cells (green), whereas that against phosphohistone H3 marks cells in mitosis (red). (G) The primordium is not more active mitotically in a homozygous ngn1 mutant larva. (H) When treated with 4-Di-2-ASP at 48 hpf, a wild-type animal shows only five or six neuromasts with functional hair cells on each side of its trunk. (I) At the same stage, an ngn1 mutant bears an equivalent number of mature neuromasts. In these and all subsequent illustrations, the animal's anterior is oriented to the left and its dorsum is situated to the top. (Scale bars, 50 μm for D–G and 100 μm for A–C, H, and I.)
One possible explanation for the presence of supernumerary neuromasts is that the lack of neurogenin 1 function increases the number of primordial cells before migration begins, for example by biasing the development of the entire placode into nonneuronal cell fates. To address this point, we doubly labeled wild-type and ngn1 mutant embryos at 24 hpf with an antibody to claudin b to identify the primordium (8) and with TO-PRO 3 to mark cellular nuclei. We found only a modest increase in the number of primordial cells in the mutants: 115 ± 12 (n = 10) for ngn1 larvae compared to 106 ± 11 (n = 10) for wild-type animals (Fig. 1 D and E). The observed increment in cell number cannot by itself account for a complete duplication of the neuromasts in ngn1 mutants.
Cells in the first primordium of the posterior lateral line characteristically undergo mitosis during migration; even after depositing approximately six neuromasts with ≈30 cells apiece, the primordium still contains 70–90 cells when it reaches the tail of the fish, where it produces two or three terminal neuromasts (18). A second possibility to explain the elevated number of neuromasts in ngn1 mutants is that their primordia are mitotically more active. We tested this possibility in two complementary ways. First, we used an antibody against the phosphorylated form of histone H3 to score the number of primordial cells entering mitosis in wild-type and ngn1 mutant animals (Fig. 1 F and G). Second, we injected ngn1 morpholinos into the eggs of animals expressing a histone 2B-GFP transgene that allows the observation of mitotic events in primordial cells in vivo (12, 16) (data not shown). Neither experiment revealed an augmented mitotic rate within the migrating primordium of fish lacking neurogenin 1.
We also analyzed animals at 48 hpf and found no difference in the size or behavior of the second primordium between wild-type and ngn1 mutant animals (data not shown). Furthermore, larvae of both types bore an equivalent number of neuromasts at this stage (Fig. 1 H and I). Therefore, we conclude that the extra neuromasts in ngn1 mutants do not originate during the initial development of the lateral line, and in particular from larger or mitotically more active primordia.
Additional Neuromasts Develop from Interneuromast Cells. The observation that wild-type and ngn1 mutant animals are indistinguishable until 48 hpf indicates that additional neuromasts develop after the entire initial set of neuromasts has been deposited. Therefore, we used antibodies to claudin b and to HCS1, a hair cell-specific marker (Fig. 2) (19), to analyze ngn1 mutant animals at 6-h intervals commencing at 48 hpf. Around 52 hpf, the interneuromast cells left behind by the migrating first primordium began to coalesce in small groups between primary neuromasts (Fig. 3 A and B). These interneuromast cells divided mitotically (Fig. 3C) and, by 56 hpf, formed clusters resembling small proneuromasts (Fig. 3D). These clusters had already constituted several new neuromasts by 72 hpf (Fig. 3E). At 120 hpf, ngn1 mutants bore twice the number of mature neuromasts found in wild-type animals (Fig. 3F). These results indicate that proliferating interneuromast cells are the source of the additional, intercalary neuromasts in ngn1 mutant zebrafish.
Fig. 2.
The HCS1 antiserum marks mature hair cells in neuromasts. (A) In a triply labeled wild-type larva, α-tubulin is present throughout the neuromast, whereas parvalbumin 3 and HCS1 occur in mature hair cells. (B) In a wild-type larva at 48 hpf, a neuromast deposited by the first primordium already bears mature hair cells labeled for HCS1 (red). The migrating second primordium is negative for this marker. Both structures are labeled by immunoreactivity against claudin b (green). (Scale bars, 10 μm.)
Fig. 3.
Interneuromast cells form extra neuromasts in ngn1 mutant larvae. (A) In a wild-type larva at 52 hpf, an antiserum against claudin b (green) reveals cells derived from the posterior lateral-line placode. HCS1 labeling (red) marks mature hair cells; the arrowhead designates the migrating second primordium. (B) At the same stage, a homozygous ngn1 larva displays claudin b-positive cell clusters (asterisks) between the mature neuromasts. The arrowhead marks the second primordium. (C) Labeling of an ngn1 mutant embryo at 52 hpf for phosphohistone H3 (red) shows that interneuromast cells remain mitotically active. Labeling for α-tubulin (green) delineates all cells. (D) Labeling for claudin b demonstrates that the interneuromast cells of an ngn1 mutant begin to coalesce at 52 hpf. (E) By 72 hpf, these cell clusters resolve progressively into new neuromasts. (F)An ngn1 mutant larva labeled for HCS1 (red) and TO-PRO 3 (blue) confirms the presence of mature hair cells within the additional neuromasts at 120 hpf. (Scale bars, 10 μm for C–E and 50 μm for A, B, and F.)
Lack of Glia Antecedes the Development of Extra Neuromasts. Our previous results suggest that the development of extra neuromasts in ngn1 mutant fish stems from the absence of sensory innervation to the posterior lateral line. However, in an independent line of investigation, we discovered that colourless (cls) mutant zebrafish also form supernumerary neuromasts along the posterior lateral line. The time course of development of additional neuromasts in cls larvae matches that of ngn1 animals (Fig. 4 A–F and Movie 1, which is published as supporting information on the PNAS web site). The cls gene encodes sox10, a transcription factor associated with the development of nonectomesenchymal derivatives of the neural crest (17, 20, 21). The absence of sox10 leads to deficient pigmentation and defective development of peripheral glia in the zebrafish, but does not affect the development of sensory axons innervating the lateral line (14, 17). We used the monoclonal antibody 6D2, which recognizes a carbohydrate epitope of the piscine P0-like myelin glycoproteins IP1 and IP2 (22, 23), to confirm that glial cells are absent along the horizontal myoseptum in both cls (Fig. 4 G and H) and ngn1 mutant larvae (Fig. 4 I and J). Together, these observations indicate that the absence of glia, rather than that of sensory axons, is the most likely cause of supernumerary neuromasts in these mutant zebrafish.
Fig. 4.
Additional neuromasts also develop in cls mutant larvae. (A) A bright-field micrograph shows a wild-type larva at 48 hpf. (B) A homozygous cls larva displays a similar pace of development. (C) Exposure of the wild-type animal to the fluorescent compound 4-Di-2-ASP reveals the pattern of functional neuromasts. (D) The mutant larva bears an equivalent number of neuromasts at this stage. (E) A bright-field image of a cls mutant larva at 6 dpf demonstrates the absence of pigmentation on the animal's head and along its body. (F) Treated with 4-Di-2-ASP, the same animal displays 25 neuromasts on the left side of its trunk. However, the number of neuromasts on the head resembles that of a wild-type larva. (G) The marker 6D2 delineates the distribution of glia in the posterior lateral line of a wild-type larva at 72 hpf. (H) At the same stage, a cls mutant lacks peripheral glia along its trunk. (I) 6D2-positive cells occur along the nerve contacting a neuromast in a wild-type larva. (J) No cells of the posterior lateral-line nerve label with the 6D2 antibody in an ngn1 mutant fish. (Scale bars, 100 μm for A–H and 20 μm for I and J.)
Discussion
The number of neuromasts is highly regulated in wild-type zebrafish larvae (18, 24). However, in ngn1 and cls mutants, more than twice the normal complement of neuromasts forms in the posterior lateral line. Because the posterior lateral-line ganglion fails to develop normally in ngn1 mutants, a possible explanation for the presence of extra neuromasts is that the entire posterior lateral-line placode constitutes a primordium that is consequently endowed with more cells before its migration commences. However, our results indicate that the primordia in ngn1 mutant fish are not significantly more populated than those in wild-type animals. Moreover, primordial cells in ngn1 mutant embryos proliferate at a normal rate. Finally, wild-type and mutant animals are indistinguishable at 48 hpf, indicating that additional neuromasts do not originate during the migration of the first and second primordia. Therefore, the formation of additional neuromasts cannot be explained by the presence of a larger pool of neuromast precursor cells.
We confirmed this inference by analysis of cls mutant zebrafish, which form sensory ganglia and primordia of a normal size, but also develop twice the normal number of neuromasts in the posterior lateral line. We used a combination of specific molecular markers and live imaging to establish that ngn1 and cls mutants begin to form extrasensory clusters after the primary set of neuromasts has been deposited, at ≈52 hpf. Additional neuromasts originate through the coalescence of mitotically active interneuromast cells in ngn1 and cls larvae. This finding suggests that the absence of a subset of neural crest-derived cells, rather than of sensory innervation, underlies the development of supernumerary neuromasts in these mutants.
The production of supernumerary neuromasts in the two mutant lines under investigation represents only a partial failure of the mechanisms that normally determine the distribution of these sensory organs. It is noteworthy that, despite their excess number, the resultant neuromasts are spaced in a highly regular array. Notwithstanding a deficit in the inhibitory influence that neural-crest derivatives ordinarily exert on the differentiation of interneuromast cells, an intact signaling system evidently continues to regulate the multiplication or coalescence of these cells.
In fishes and amphibians, neuromasts may have a dual origin from placodes and the neural crest (25). Although the fate of neural-crest cells in the lateral line remains uncertain, these cells could associate with the interneuromast cells and prevent the later from forming more neuromasts. One likely possibility is that these cells differentiate as peripheral glia that traverse the path formed by the lateral-line nerve and accumulate along the posterior lateral-line nerve adjacent to the interneuromast cells. The initial neuromasts form in the absence of these glia, because the initial patterning of the lateral line is established within the moving primordium, ahead of the neural crest-derived glia (26).
At later stages of development, zebrafish normally produce numerous neuromasts in addition to those deposited by the primordia. If interneuromast cells are the source of these new neuromasts, the proliferation observed in the mutant animals represents an acceleration of the normal developmental schedule triggered by the absence of neural crest-derived cells. The phenomenon reported here may thus represent an example of heterochrony, a change in the developmental timing of a particular structure relative to that of the remainder of a developing organism. Occurring in a sensory system, such a heterochronic change could contribute to a mutant animal's ability to colonize new ecological niches, avoid predators, or capture novel prey. By allowing the animal to outperform its predecessors, these traits could contribute to speciation. Although variations in developmental timing are evident during the formation of the lateral lines in various fish species, the cellular mechanisms underlying these changes remain unknown (9, 27). We suspect that changes in the ability of neural crest cells to migrate, multiply, and survive influence the timing of development of neuromasts, thus underlying heterochrony at the organismal level (27, 28).
Various Danio species display unique patterns of pigmentation that stem from changes in the development, migration, or survival of neural-crest derivatives such as melanocytes. By the same token, it may be valuable to analyze the development of neuromasts across the genus. The identification of the genes mutated in zebrafish with defects in the development and behavior of the neural crest and in the number of neuromasts should allow experimental exploration of the genetic basis of heterochrony (29, 30).
Supplementary Material
Acknowledgments
We thank N. Hopkins (Massachusetts Institute of Technology, Cambridge) and the Zebrafish International Resource Center for zebrafish lines, J. Corwin (University of Virginia School of Medicine, Charlottesville) and G. Jeserich (University of Osnabrück, Osnabrück, Germany) for antisera, D. Elreda and A. North (The Rockefeller University Imaging Center) for advice on microscopy, and A. Afolalu and P. Espitia for expert maintenance of the zebrafish colony. We also acknowledge the kindness of T. Piotrowski and D. Raible for communicating their results before publication. A. Ghysen, R. G. Northcutt, T. Whitfield, and the members of our research group provided valuable comments on the manuscript. This research was supported by National Institutes of Health Grant DC00241. During this investigation, H.L.-S. was a fellow of the Wellcome Trust and subsequently a Howard Hughes Medical Institute fellow of the Life Sciences Research Foundation. A.J.H. is an Investigator of Howard Hughes Medical Institute.
Abbreviations: hpf, hours postfertilization; dpf, days postfertilization.
References
- 1.Lowenstein, O. (1967) in Lateral Line Detectors, ed. Cahn, P. (Indiana Univ. Press, Bloomington), pp. 3-12.
- 2.Dijkgraaf, S. (1989) in The Mechanosensory Lateral Line: Neurobiology and Evolution, eds. Coombs, S., Görner, P. & Münz, H. (Springer, New York), pp. 7-14.
- 3.Montgomery, J., Baker, C. & Carton, G. (1997) Nature 389, 960-963. [Google Scholar]
- 4.Ghysen, A. & Dambly-Chaudière, C. (2004) Curr. Opin. Neurobiol. 14, 67-73. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe, W. K., Kimmel, C. B. & Schabtach, E. (1985) J. Comp. Neurol. 233, 377-389. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe, W. K. (1985) J. Comp. Neurol. 238, 218-224. [DOI] [PubMed] [Google Scholar]
- 7.David, N. B., Sapède, D., Saint-Etienne, L., Thisse, C., Thisse, B., Dambly-Chaudière, C., Rosa, F. M. & Ghysen, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16297-16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Schier, H., Starr, C. J., Kappler, J. A., Kollmar, R. & Hudspeth, A. J. (2004) Dev. Cell. 7, 401-412. [DOI] [PubMed] [Google Scholar]
- 9.Sapède, D., Gompel, N., Dambly-Chaudière, C. & Ghysen, A. (2002) Development (Cambridge, U.K.) 129, 605-615. [DOI] [PubMed] [Google Scholar]
- 10.Ledent, V. (2002) Development (Cambridge, U.K.) 129, 597-604. [DOI] [PubMed] [Google Scholar]
- 11.Andermann, P., Ungos, J. & Raible, D. W. (2002) Dev. Biol. 251, 45-58. [DOI] [PubMed] [Google Scholar]
- 12.Gilmour, D., Knaut, H., Maischein, H.M., Nüsslein-Volhard, C. (2004) Nat. Neurosci. 7, 491-492. [DOI] [PubMed] [Google Scholar]
- 13.Li, Q., Shirabe, K. & Kuwada, J. Y. (2004) Dev. Biol. 269, 123-136. [DOI] [PubMed] [Google Scholar]
- 14.Gilmour, D. T., Maischein, H. M. & Nüsslein-Volhard, C. (2002) Neuron 34, 577-588. [DOI] [PubMed] [Google Scholar]
- 15.Golling, G., Amsterdam, A., Sun, Z., Antonelli, M., Maldonado, E., Chen, W., Burgess, S., Haldi, M., Artzt, K., Farrington, S., et al. (2002) Nat. Genet. 31, 135-140. [DOI] [PubMed] [Google Scholar]
- 16.Pauls, S., Geldmacher-Voss, B. & Campos-Ortega, J. A. (2001) Dev. Genes Evol. 211, 603-610. [DOI] [PubMed] [Google Scholar]
- 17.Kelsh, R. N. & Eisen, J. S. (2000) Development (Cambridge, U.K.) 127, 515-525. [DOI] [PubMed] [Google Scholar]
- 18.Gompel, N., Cubedo, N., Thisse, C., Thisse, B., Dambly-Chaudière, C. & Ghysen, A. (2001) Mech. Dev. 105, 69-77. [DOI] [PubMed] [Google Scholar]
- 19.Gale, J. E., Meyers, J. R. & Corwin, J. T. (2000) J. Assoc. Res. Otolaryngol. 1, 172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutton, K., Dutton, J. R., Pauliny, A. & Kelsh, R. N. (2001) Genesis 30, 188-189. [DOI] [PubMed] [Google Scholar]
- 21.Dutton, K. A., Pauliny, A., Lopes, S. S., Elworthy, S., Carney, T. J., Rauch, J., Geisler, R., Haffter, P. & Kelsh, R. N. (2001) Development (Cambridge, U.K.) 128, 4113-4125. [DOI] [PubMed] [Google Scholar]
- 22.Jeserich, G. & Rauen, T. (1990) Glia 3, 65-74. [DOI] [PubMed] [Google Scholar]
- 23.Bastmeyer, M., Jeserich, G. & Stuermer, C. A. (1994) Glia 11, 300-314. [DOI] [PubMed] [Google Scholar]
- 24.Pichon, F. & Ghysen, A. (2004) Evol. Dev. 6, 187-193. [DOI] [PubMed] [Google Scholar]
- 25.Collazo, A., Fraser, S. E. & Mabee, P. M. (1994) Science 264, 426-430. [DOI] [PubMed] [Google Scholar]
- 26.Itoh, M. & Chitnis, A. B. (2001) Mech. Dev. 102, 263-266. [DOI] [PubMed] [Google Scholar]
- 27.Webb, J. F. (1989) in The Mechanosensory Lateral Line: Neurobiology and Evolution, eds. Coombs, S., Görner, P. & Münz, H. (Springer, New York), pp. 79-97.
- 28.Parichy, D. M. (2001) in Beyond Heterochrony: The Evolution of Development, ed. Zelditch, M. L. (Wiley, New York), pp. 229-269.
- 29.Kelsh, R. N., Brand, M., Jiang, Y. J., Heisenberg, C. P., Lin, S., Haffter, P., Odenthal, J., Mullins, M. C., van Eeden, F. J., Furutani-Seiki, M., et al. (1996) Development (Cambridge, U.K.) 123, 369-389. [DOI] [PubMed] [Google Scholar]
- 30.Whitfield, T. T., Granato, M., van Eeden, F. J., Schach, U., Brand, M., Furutani-Seiki, M., Haffter, P., Hammerschmidt, M., Heisenberg, C. P., Jiang, Y. J., et al. (1996) Development (Cambridge, U.K.) 123, 241-254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.