Abstract
This study aimed at evaluating the toxicity of some insecticides (abamectin and deltamethrin) on the lethal time (LT50) and midgut of foragers honeybee workers of Apis mellifera jemenatica were studied under laboratory conditions. The bees were provided with water, food, natural protein and sugar solution with insecticide (concentration: 2.50 ppm deltamethrin and 0.1 ppm abamectin). The control group was not treated with any kind of insecticides. The mortality was assessed at 1, 2, 4, 6, 12, 24, 48, and 72 hour (h) after insecticides treatment and period to calculate the value of lethal time (LT50). But the samples the histology study of midgut collected after 24 h were conducted by Scanning Electron Microscope. The results showed the effects of insecticides on the current results show that abamectin has an adverse effect on honeybees, there is a clear impact on the lethal time (LT50) was the abamectin faster in the death of honeybee workers compared to deltamethrin. Where have reached to abamectin (LT50 = 21.026) h, deltamethrin (LT50 = 72.011) h. However, abamectin also effects on cytotoxic midgut cells that may cause digestive disorders in the midgut, epithelial tissue is formed during morphological alterations when digestive cells die. The extends into the internal cavity, and at the top, there is epithelial cell striated border that has many holes and curves, abamectin seems to have crushed the layers of muscle. Through the current results can say abamectin most toxicity on honeybees colony health and vitality, especially foragers honeybee workers.
Keywords: Toxicity, Insecticides, Lethal time (LT50), Midgut, Scanning Electron Microscopy, Apis mellifera jemenatica
1. Introduction
Apis mellifera jemenatica is the main honey bee strain of the Arabian Peninsula, also spread in some parts of Africa (Aljedani, 2009), due to its adaptation under environmental conditions prevailing in Saudi Arabia and to its disease resistance (Alghamdi, 2002). Environmental stressors may interact with each other and potentiate their effect on organisms’ health and survival (Holmstrup et al., 2010, Gonza’lez-Varo et al., 2013). Apis mellifera are constantly exposed to a wide variety of biotic and abiotic stressors. Among these, pathogens and pesticides are important variables that influence honeybee survival. Interactions between stressors in honeybees may be partly responsible for the severe colony losses recorded worldwide for more than ten years (Oldroyd, 2007, Potts et al., 2010, vanEngelsdorp and Meixner, 2010, vanEngelsdorp et al., 2010).
In recent decades, much has been learned about the interaction between bees and the natural toxins that exist in their environment. Thus, the discovery of new active ingredients and new modes of action that can be used to control insect pests is also continual. There have been drastic changes in the toxicology of practical beekeeping, with beekeepers beginning to use pesticides inside the colony in an effort to control pests and pathogens (Johnson, 2015). Many of the pesticides to which A. mellifera are exposed have insecticidal properties and may be harmful to bees (Nasr and Wallner, 2003, Pettis et al., 1991, Pettis et al., 2004), affect A. mellifera’s cardiotoxicity (Papaefthimiou and Theophilidis, 2001), and affect forager bees’ mobility and communicative capacity (Medrzycki et al., 2003).
In a study carried (Husain et al., 2014) out to determine the effect insecticides on three species of A. mellifera and their ability to survive and calculate the value of LT50, there were found differences in the value of LT50 between these species when exposed to different concentrations of insecticides. Aljedani and Almehmadi (2016) was a study conducted against three insecticides was variation in the intensity of the effect of the insecticides on the longevity of the honeybee of different concentrations when used orally. However, among other effects documented in the literature (Gregorc and Ellis, 2011) investigated the effects of pesticide on A. mellifera on cell death and localization in pesticide-treated. Pesticides damage the midgut cells of several species of the Apis genus (Higes et al., 2007, García-Palencia et al., 2010). Insecticides have a major effect on Apis mellefera and should be considered hazardous to pollinators because nectar and pollen can become contaminated (Girolami et al., 2009, Stoner and Eitzer, 2012). Chronic exposure to sublethal doses can be due to an accumulation of insecticides in hive products (Pilling et al., 2013), which induces significant short-term impacts at the colony level, resulting in decreased colony performance and productivity (Sandrock et al., 2014). In insects, the digestive tract is mainly composed of three parts: the foregut, midgut, and hindgut. The midgut is also called the mesenteron, ventriculus, and stomach. It is the main site that secretes granules. In addition, digestion and absorption occur here (Chapman, 1998, Cruz-Landim, 1999).
Insecticides caused more the alterations on midgut cells in a study by Kakmand et al. (2008) aimed to analyze the effects of the acute oral toxicity of insecticides showed that they caused high mortality rates in A. mellifera and disruption in the midgut cells. The midgut epithelium is responsible for the detoxification of ingested xenobiotics (Mao et al., 2011), and some insecticides specifically target the midgut epithelium (Han et al., 2012, Vachon et al., 2012). Damage to the midgut epithelium of honeybees has also been reported as a consequence of acute exposure to the insecticides malathion, deltamethrin, and thiamethoxam (Kakmand et al., 2008). This spatial overlap between immunity and detoxification may facilitate synergistic interactions between pesticides and pathogens to the detriment of honeybee health (Pettis et al., 2012, Johnson et al., 2009). Most studies have focused on mortality or behavioral deficiencies in exposed A. mellifera while neglecting other biological functions and target organs. We aimed to this study the effects of two insecticides representing the most prevalent groups namely; abamectin and deltamethrin on foragers honeybee workers of A. mellifera jemenatica by orally feeding them a sugar solution with an insecticide added to it. We are in this study will analyze the effects on the lethal time (LT50) and on the cells in the midgut.
2. Materials and methodology
This study was carried out at the Laboratory of Entomology at the King Abdulaziz University. In order to evaluate the effect of two insecticides on the foragers honeybee workers A. mellifera jemenatica. Insecticides used; the adverse effects caused by two types of insecticides, representing the most prevalent groups, available in abundance in the Saudi market, used in pest control, were studied, namely: abamectin pesticide, from the avermectin group and deltamethrin; a compound of pyrethroid group.
2.1. Individuals used in the study
Foragers honeybee workers A. mellifera jemenatica that have been used in the study, which are individuals working outside the hive in collecting nectar and pollen, but are characterized with being fed on the nectar or honey in larger amounts (carbohydrates nutrition). Foragers honeybee workers were chosen to conduct the study according to Oldroyd and Nanork, 2009, Khoury et al., 2011). All individuals that used in this study collected under the normal conditions.
In addition, foragers honeybee workers when you start work outside the hive expect as most individuals are affected by insecticides. In many cases, the foragers workers, which are responsible for collecting nectar and pollen, lose the ability to fly or become disoriented and cannot return to the hive (Oldroyd, 2007).
2.2. Material and food administration
Where two methods were combined for the design of wooden cages for breeding, taking into account that the one face of the wooden box is covered with metal wire mesh, while the opposite face would be of glass, based on what was mentioned by Kakmand et al. (2008), and for holding cage measurements, were (40 × 30 × 30 cm).
The cage was provided, on the top sid with 2 plastic medical syringe (50 ml), one of which with water, and the other syringe with sugar solution of (1:1), that is (50%) (as a source of carbohydrates nutrition). (50% w/v) based on what was conducted by Bortolotti et al., 2003, Medrzycki et al., 2003, Pohorecka, 2004, laced with insecticide solution under test. The cage was also provided with a small plastic pot of 3 cm in diameter, and 1 cm in height, put in the cage bottom to provide natural protein nutrition, (pollen) mixed with sugar in equal parts; where 50 g of pollen and 50 g of powdered sugar as well as a little water are added to get a paste, and then 10 g of the such paste are put in the plastic pot, covered with a transparent perforated plastic sheet to allow feeding of bees and protection of the paste from drying out and preventing the adhesion of such food particles bees during feeding, where food is changed every three days (Hatjina et al., 2013).
2.3. Time period of experiment conductance
The mortality was assessed at 1, 2, 4, 6, 12, 24, 48, and 72 hour (h) after insecticides treatment. Bees were then for holding cage to assess the effects of insecticides until the end of the 72 h period to calculate the value of lethal time (LT50). But the samples the histology study (midgut) collected after 24 h by Thompson, 2001, Kakmand et al., 2008, Costa et al., 2014, Forkpah et al., 2014.
2.4. Study groups division
The research experiences were divided into three groups, namely:
-
•
A non-exposed control group.
-
•
An exposed group to abamectin, with 0.1 ppm concentration.
-
•
An exposed group to deltamethrin, with 2.50 ppm concentration.
2.5. Study procedures
The forager honey bee workers were selected for the study, as they are individuals working outside the hive in the collection of nectar and pollen, where they have been fed orally, where they were provided with water and natural protein nutrition, and sugar solution laced with insecticide in the required concentration. As the insecticides used in the study (abamectin and deltamethrin).
The abamectin pesticide has been used in the following concentration: 0.1 ppm and deltamethrin pesticide was used in the following concentration: 2.50 ppm, while the control group was not treated with any kind of pesticides, and were fed naturally, with water, natural protein nutrition as well as sugar solution, free of any additives, were used. The samples were followed up, as a bee would be considered dead when it remains motionless for ten seconds of the observation period, after moving it gently by a fine brush, (Laurino et al., 2013). The experiment was repeated three times using fifteen insect in each repeated time.
2.6. The study of the lethal time 50 (LT50)
Mortality percentages were calculated for each treatment and corrected using Abott’s equation (Abbott, 1925). The lethal time 50 (LT50) was calculated for each group at p = 0.05, LT50 was also determined by Probit analysis (Finney, 1971) Ldp line (Bakr, 2007).
2.7. The histological study of the midgut by Scanning Electron Microscope (SEM)
The samples of honeybees workers It was were placed in the fixed for 2 h with 2.5% glutaraldehyde. Then specimens were washed in 0.1 M sodium cacodylate buffer (pH 7.2) and post fixed in 1% osmium tetroxide in the same buffer. The midgut was dehydrated in a graded ethanol series. The specimens were coated by gold sputtering for 60 s using Auto Fine Coater (JFC-1600) and viewed with a JSM-6360LV Scanning Electron Microscope at 10 kV.
3. Results
3.1. The lethal time 50 (LT50)
The results showed the effects of insecticides on foragers honeybee workers A. mellifera jemenatica throughout the life of these workers, where they were fed on a sugary solution laced with insecticide under laboratory conditions. The bees were provided with water, food, natural protein and sugar solution with insecticide (concentration: 0.1 ppm abamectin and 2.50 ppm deltamethrin). The control group was not treated with any kind of insecticides.
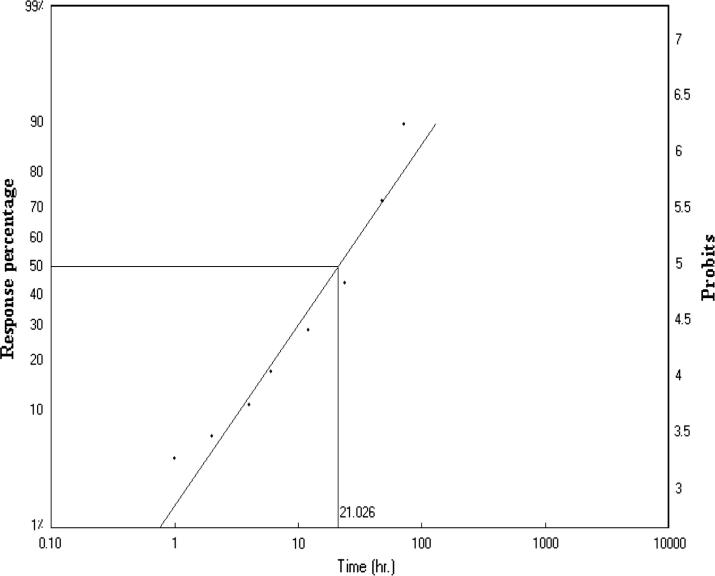
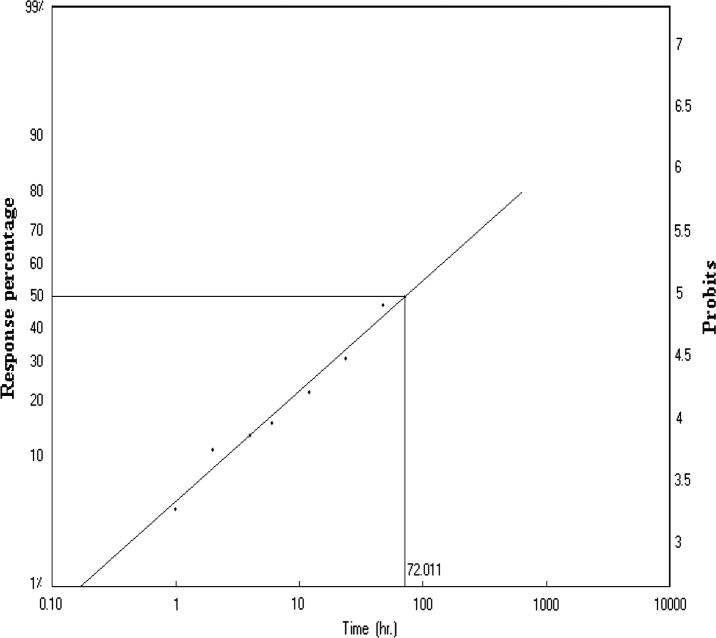
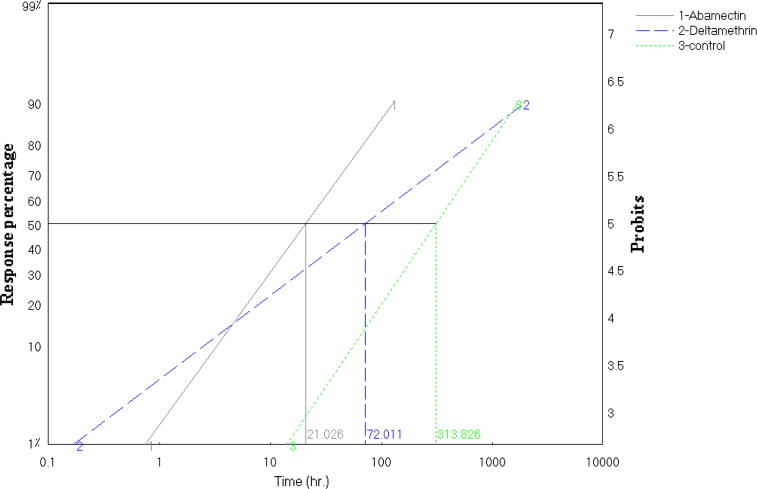
This study results of lethal time (LT50) that has been conducted to find that two insecticides used abamectin and deltamethrin. Firstly, abamectin shortest time needed to death half the number individuals it takes to death 50% to 21.026 h (LT50 = 21.026), and 130.954 h of death 90% of individuals (LT90 = 130.954) Fig.1. Secondly, deltamethrin death 50% of individuals through 72.011 h (LT50 = 72.011), and 2010.599 h of death 90% of individuals (LT90 = 2010.599) Fig.2. Finally, that can conclude that insecticide abamectin be faster in the death of foragers honeybee workers compared to deltamethrin. While in the control was value of (LT50 = 313.826) and (LT90 = 1724.129). Fig.3, Table 1.
Figure 1.
Lethal time (LT50) after exposure to abamectin after 72 h of foragers honeybee workers.
Figure 2.
Lethal time (LT50) after exposure deltamethrin after 72 h of foragers honeybee workers.
Figure 3.
Comparing of lethal time (LT50) after exposure of insecticides after 72 h of foragers honeybee workers.
Table 1.
Comparing of lethal time (LT50) after exposure of insecticides after 72 h of foragers honeybee workers.
| No | Line name | LT50 | LT90 | Lower limit | Upper limit | Index | RR | Slope | Slope± |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abamectin | 21.026 | 130.954 | 15.572 | 29.966 | 100 | 1 | 1.613 | 0.104 |
| 2 | Deltamethrin | 72.011 | 2010.599 | 49.984 | 118.837 | 29.198 | 3.425 | 0.886 | 0.09 |
| 3 | Control | 313.826 | 1724.129 | 166.389 | 1478.904 | 6.7 | 14.926 | 1.732 | 0.385 |
Index compared with Abamectin. Resistance Ratio (RR) compared with Abamectin.
3.2. The histological of the midgut by Scanning Electron Microscope (SEM)
3.2.1. A non-exposed (control)
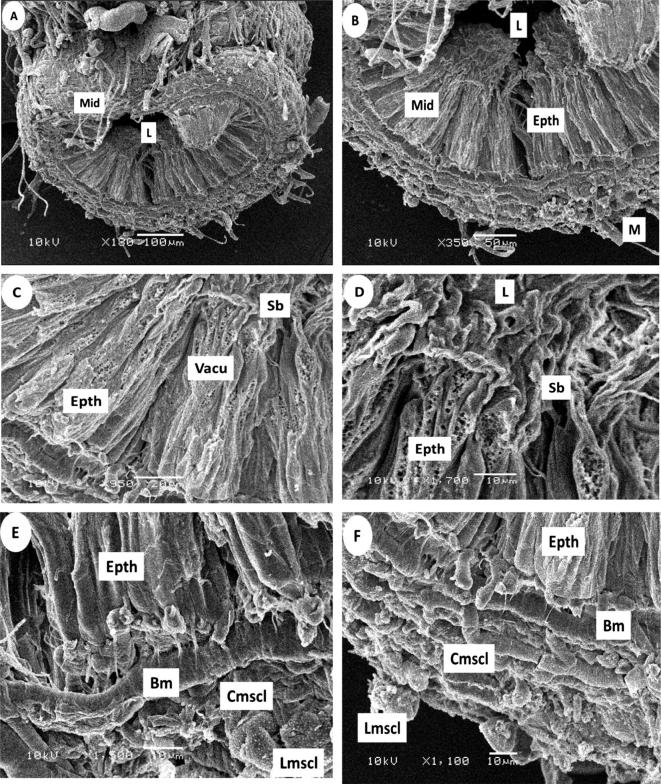
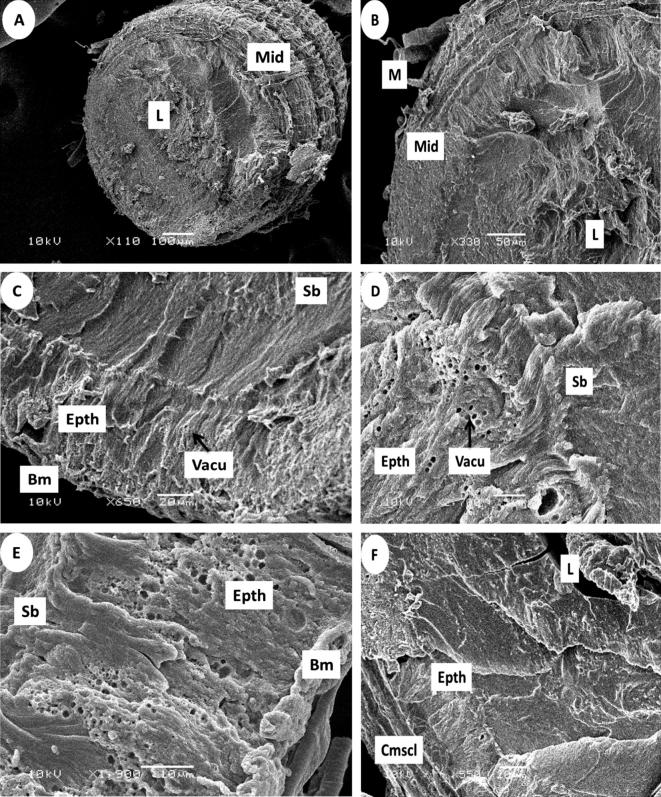
It was found through the results obtained in the present study. The midgut (stomach) (mid gut (mid)) area of the abdominal cavity in honeybee weavers is a member of the following proventriculus flexes into a body cavity. The stomach wall has several sections that extend into the turns of the cavity (lumen (L)). The midgut consists of the roaming honeybees articulated that feed on the solution (non-diabetes exhibition for insecticides (control) of epithelial cells (Epth). Epithelial cells are mostly made up of columnar digestive cells that appear large during cell proliferation, which is characterized by small gaps or cytoplasmic holes (vacuoles (vacu)) on the outer wall. In the midgut in A. mellifera under normal conditions, epithelial tissue is formed during morphological alterations when digestive cells die. The extends into the internal cavity, and at the top, there are epithelial cell cilia (striated border (Sb) that have many holes and curves. The cells were on the basement membrane (basement membrane (Bm)), which is the base and anchor for all cells. The midgut is surrounded from the outside by two layers of muscle (muscles (M)); circular muscles inside (circular muscles (Cmscl)) and longitudinal muscles outside (longitudinal muscles (Lmscl) Fig.4.
Figure 4.
Cross section of the midgut after 24 h of Control in the foragers honeybee workers by Scanning electron photographs (SEM):(A,B) General view of midgut (Mid), Lumen (L), muscles (M), (C,D) Showing the epithelium cells (Epth), vacuoles (Vacu). striated border (Sb), (E,F) basement membrane (Bm), circular muscles (Cmscl), longitudinal muscles (Lmscl).
3.2.2. An exposed to abamectin and deltamethrin
The results of the current study examining the effect of insecticides on bees shows that deltamethrin caused the midgut lumen (lumen (L)) to become less spacious, but the inside scoop of the luminal average with exposure to abamectin was more affected, opaque, and almost solid.
We found that exposure to deltamethrin caused the epithelial cells to become contiguous with each other and that the holes or gaps due to curves on their striated border almost or completely disappeared. Exposure to abamectin clearly affects the epithelial layer, as the cells merge with each other.
In the midgut epithelial tissue is formed during morphological alterations when digestive cells die. The extends into the internal cavity, and at the top, there are epithelial cell cilia (striated border (Sb) that have many holes and curves, under exposure to deltamethrin, the striated border was characterized by softness with no holes.
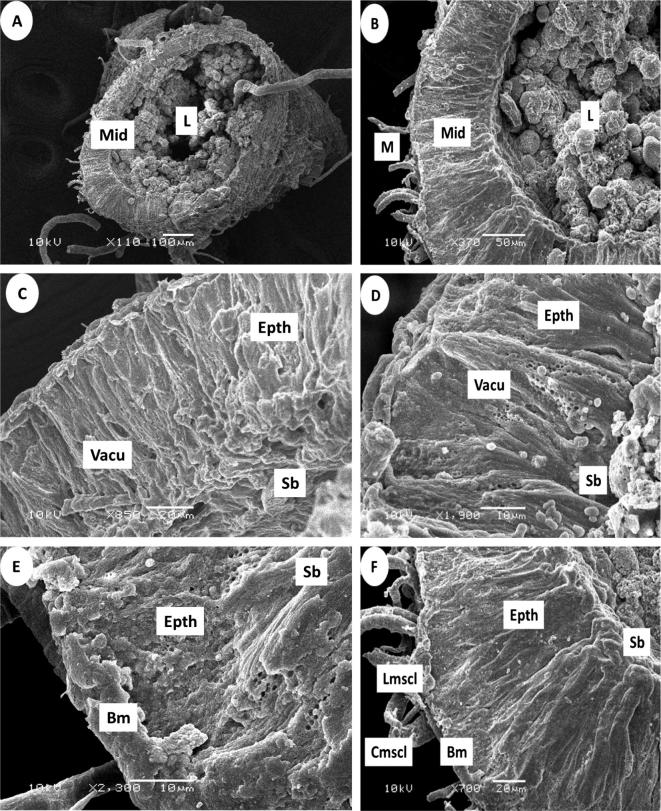
The cells were on the basement membrane (basement membrane (Bm)), which is the base and anchor for all cells. The midgut is surrounded from the outside by two layers of muscle (muscles (M)) and two circular muscles: inside (circular muscles (Cmscl)) and longitudinal muscles (longitudinal muscles (Lmscl). This was evident in the natural state; deltamethrin appears to have caused some changes and caused separation from the basal membrane. Abamectin seems to have crushed the layers of muscle. Fig.5 the midgut exposure of abamectin) and Fig.6 the midgut exposure of deltamethrin.
Figure 5.
Cross section of the midgut after 24 h exposure of abamectin in the foragers honey bee workers by Scanning electron photographs (SEM): (A,B) General view of midgut (Mid), Lumen (L), muscles (M), (C,D) Showing the epithelium cells (Epth), vacuoles (Vacu). striated border (Sb), (E,F) basement membrane (Bm), circular muscles (Cmscl).
Figure 6.
Cross section of the midgut after 24 h. exposure of deltamethrin in the foragers honey bee workers by Scanning electron photographs (SEM): (A,B) General view of midgut (Mid), Lumen (L), muscles (M), (C,D) Showing the epithelium cells (Epth), vacuoles (Vacu). striated border (Sb), (E,F) basement membrane (Bm), circular muscles (Cmscl), longitudinal muscles (Lmscl).
The current results show that abamectin has an adverse effect on honeybees, especially foragers honeybee workers; there is a clear impact on the lethal time (LT50) and effects on midgut cells that may cause digestive disorders in the midgut, slowing its efficiency and thus affecting honeybee colonies' health and vitality.
4. Discussion
The foragers bees A. mellifera jemenatica effects of insecticides (abamectin and deltamethrin) on throughout the life. It was found through the results obtained in the present study. When we analyzed the effects of the lethal time (LT50) was the abamectin faster in the death of foragers honeybee workers compared to deltamethrin. Where have reached to abamectin (LT50 = 21.026) hour, deltamethrin (LT50 = 72.011) hour. While in the control was value of (LT50 = 313.826) hour. Additional studies by Carvalho et al. (2009) found also independent of the form of contamination, thiamethoxam was extremely toxic to bees, causing the death of more than 80% of the specimens after 3 days. And confirmed the high toxicity of this compound and showed that regardless of the exposure mode (spray, intake, or residue on the surface of the culture), thiamethoxam (37.5 ng a.i./l L) is extremely toxic to bees. This chemical had a LT50 on average of 3.57 h Husain et al. (2014) was a study conducted against three species of honey bees, to check long-term survival of honeybees when exposed to different insecticides. The LT50 of spinosad was increased up to 18 h with decreasing concentrations at 125 ppm against A. mellifera, LT50 of spinosad was increased up to 15 h with decreasing concentrations at 125 ppm against Apis florea as well as LT50 of spinosad and Emamectin benzoate were increased up to 20 h with decreasing concentrations at 125 ppm against Apis dorsata., LT50 of all controlled species was 91–103 h.
The midgut of A. mellifera presents an important interface between the insect and its environment that is responsible for the absorption of nutrients in A. mellifera. The midgut wall has several sections that extend into the turns of the cavity (lumen). The midgut consists of the roaming honeybees articulated that feed on the solution (non-diabetes exhibition for insecticides (control) of epithelial cells. The midgut epithelium is the only tissue of adult A. mellifera that exhibits widespread cell proliferation (Forkpah et al., 2014). Thiboldeaux et al., 1998, Sorour, 2001 stating that the midgut is the most important site for the terminal digestion and absorption of nutrients and is the main way pesticide molecules present in food are absorbed. The intestine can reveal morphological alterations induced by environmental contaminants ingested by A. mellifera and that may lead to the death of the midgut epithelium (Gregorc et al., 2007). Additionally, it may reduce A. mellifera’s activity, longevity, and nursing behavior (Schneider et al., 2012). Forkpah et al. (2014) indicate that some insecticides frequently used in apiculture and known to accumulate in A. mellifera hives may have hitherto unknown physiological effects. The nutritional status and susceptibility to pathogens of A. mellifera could be compromised by the impact of insecticides on the maintenance of the midgut epithelium. A growing body of evidence contributes to more comprehensive testing being considered safe for A. mellifera and other non-target species. The results of the current study examining the effect of insecticides on bees shows that deltamethrin caused the midgut lumen to become less spacious, but the inside scoop of the luminal average with exposure to abamectin was more affected, opaque, and almost solid.
Epithelial cells are mostly made up of columnar digestive cells that appear large during cell proliferation, which is characterized by small gaps or cytoplasmic holes (vacuoles) on the outer wall. We found that exposure to deltamethrin caused the epithelial cells to become contiguous with each other and that the holes or gaps due to curves on their striated border almost or completely disappeared. Exposure to abamectin clearly affects the epithelial layer, as the cells merge with each other.
In the midgut in A. mellifera under normal conditions, epithelial tissue is formed during morphological alterations when digestive cells die (Cruz-Landim and Cavalcante, 2003, Martins et al., 2006). The midguts of the Africanized honeybee from different control groups for all periods analyzed that showed typical characteristics of this structure: preserved digestive cells usually with nuclei of spherical shape, organelles with no alterations, vacuoles were also observed in the cytoplasm. The control groups that were treated with acetone also showed these same characteristics. The ultrastructural analysis of midguts from honeybees exposed to a concentration corresponding to the CL50/10 of thiamethoxam showed that the effect of this chemical was most evident in honeybees that were exposed for 1 day. Digestive cells of bees exposed to the insecticide for 3 days had nuclei with a more regular shape (Catae et al., 2014).
The peritrophic membrane could be easily observed in larvae and presented a double origin: epithelium of the proventriculus and the transition cells between the ventriculus and proventriculus. Although this was not observed in the present investigation, it may have been overlooked. No peritrophic membrane was observed in adults (Caetano et al., 1990). The extends into the internal cavity, and at the top, there are epithelial cell cilia (striated border) that have many holes and curves to increase surface absorption and the exchange of nutrients and enzymes. Under exposure to deltamethrin, the striated border was characterized by softness with no holes. The midgut is internally lined by a single layer of columnar epithelium. There were cubic epithelial cells shaped like baskets that formed cellular nest at their basal surface (regenerative cells). The apical surface of the columnar epithelial cells had microvilli (Çakici and Ergen, 2012).
The cells were on the basement membrane, which is the base and anchor for all cells. The midgut is surrounded from the outside by two layers of muscle; inside circular muscles and outside longitudinal muscles. This was evident in the natural state; deltamethrin appears to have caused some changes and caused separation from the basal membrane. Abamectin seems to have crushed the layers of muscle. Some species of Lepidoptera, which have two muscle layers (external circular and inner longitudinal muscles) (Levy et al., 2004). Conversely that Coleoptera in which the inner muscle layer is circular and the external layer is longitudinal (Silva-Olivares et al., 2003). The presence of a single layer of circular muscle has also been reported in ants (Villaro et al., 1999).
5. Concluding remarks
From these study, we was concluded that the insecticides proved to be lethal for A. mellifera jemenatica of honeybee. The results showed that abamectin had high LT50 than deltamethrin. Also, it was the latest more harmful effects on midgut cells. Insecticides are used to harm honeybees were exposed to a great variety of other potentially toxic chemicals.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbott W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- Alghamdi A.A. The effect of pollen supplementary feeding on some activities of honeybee colonies during summer season in Riyadh, Saudi Arabia, Saudi. J. Biol. Sci. 2002;9(2):85–94. [Google Scholar]
- Aljedani D.M. King Abduaziz University; Jeddah: 2009. Anatomical and Histological Structure of Alimentary canal And Reproductive Systems in Queen and Worker of Local Honeybee Apis mellifera jemenatica (Hymenoptera: Apidae) (Master’s Thesis) 256 pp. [Google Scholar]
- Aljedani D.M., Almehmadi R.M. Effects of some insecticides on longevity of the foragers honey bee worker of local honey bee race Apis mellifera jemenatica. Electron. Physician. 2016;8(1):1843–1849. doi: 10.19082/1843b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakr, E. 2007. Ldp Line. (http://embark. tripod.com /Idpline/index.htm).
- Bortolotti L., Montanari R., Marcelino J., Medrzycki P., Maini S., Porrini C. Effects of sub-lethal imidacloprid doses on the homing rate and foraging activity of honey bees. Bull. Insectol. 2003;56(1):63–67. [Google Scholar]
- Caetano F.H., Tomotake M.E.M., Pimentel M.A.L., Mathias M.I.C. Morfologia interna de operárias de Dolichoderus attelaboides (Fabricius, 1775) (Formicidae: Dolichoderinae). I. Trato digestivo e sistema excretor anexo. Naturalia. 1990;15:57–65. [Google Scholar]
- Çakici Ӧ., Ergen G. Histologic description of midgut in Melanogryllus desertus (Pallas, 1771) (Orthoptera: Gryllidae) Biharean Biologist. 2012;6(2):108–111. ©Biharean Biologist, Oradea, Romania. Article No.: 121116. http://biozoojournals.3x.ro/bihbiol/index.html. [Google Scholar]
- Carvalho S.M., Carvalho G.A., Carvalho C.F., Bueno Filho J.S.S., Babtista A.P.M. Toxicidade de acaricidas/inseticidas empregados na citricultura para a abelha africanizada Apis mellifera L., 1758 (Hymenoptera: Apidae) Arq. Inst. Biol. 2009;76:595–603. [Google Scholar]
- Catae A.F., Roat T.C., Oliveira R.A., Nocelli R.E., Malaspina O. Cytotoxic effects of thiamethoxam in the midgut and malpighian tubules of Africanized Apis mellifera (Hymenoptera: Apidae) Microsc. Res. Tech. 2014;77:274–281. doi: 10.1002/jemt.22339. [DOI] [PubMed] [Google Scholar]
- Chapman R.F. fourth ed. Cambridge University Press; U.K.: 1998. Alimentary canal, digestion and absorption. The Insects, Structure and Function; pp. 38–58. [Google Scholar]
- Costa E.M., Araujo E.L., Maia A.V.P., Silva F.E.L., Bezerra C.E.S., Silva J.G. Toxicity of insecticides used in the Brazilian melon crop to the honey bee Apis mellifera under laboratory conditions. Apidologie. 2014;45:34–44. [Google Scholar]
- Cruz-Landim C. Ultrastructural features of the regenerative cells of the bees (Hymenoptera: Apidae) midguts. Sociobiology. 1999;34(3):597–603. [Google Scholar]
- Cruz-Landim C., Cavalcante V.M. Ultraestructural and cytochemical aspects of metamorphosis in the midgut of Apis mellifera L. (Hymenoptera: Apidae: Apinae) Zool. Sci. 2003;20:1099–1107. doi: 10.2108/zsj.20.1099. [DOI] [PubMed] [Google Scholar]
- Finney D.J. third ed. Cambridge University Press; London: 1971. Probit Analysis; p. 333. [Google Scholar]
- Forkpah C., Dixon L.R., Fahrbach S.E., Rueppell O. Xenobiotic effects on intestinal stem cell proliferation in adult honey bee (Apis mellifera L) workers. PLoS ONE. 2014;9(3):91180. doi: 10.1371/journal.pone.0091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Palencia P., Martín-Hern_andez R., Gonz_alez-Porto A.V., Marin P., Meana A., Higes M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera) J. Apic. Res. 2010;49:278–283. [Google Scholar]
- Girolami V., Mazzon L., Squartini A., Mori N., Marzaro M., Di Bernardo A., Greatti M., Giorio C., Tapparo A. Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. J. Econ. Entomol. 2009;102:1808–1815. doi: 10.1603/029.102.0511. [DOI] [PubMed] [Google Scholar]
- Gonza’lez-Varo J.P., Biesmeijer J.C., Bommarco R., Potts S.G., Schweiger O. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 2013;28:524–530. doi: 10.1016/j.tree.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Gregorc A., Ellis J.D. Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pestic. Biochem. Physiol. 2011;99:200–207. [Google Scholar]
- Gregorc A., Smodis, Skerl M.I. Toxicological and immunohistochemical testing of honeybees after oxalic acid and rotenone treatments. Apidologie. 2007;38(3):296–305. [Google Scholar]
- Han P., Niu C.Y., Biondi A., Desneux N. Does transgenic Cry1Ac+CpTI cotton pollen affect hypopharyngeal gland development and midgut proteolytic enzyme activity in the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotoxicology. 2012;21:2214–2221. doi: 10.1007/s10646-012-0976-2. [DOI] [PubMed] [Google Scholar]
- Hatjina F., Papaethimiou C., Charistos L., Dogaroglu T., Bouga M., Emmanoull C., Arnold G. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie. 2013 INRA, DIB and Springer-Verlag France. [Google Scholar]
- Higes M., Garcia-Palencia P., Martín-Hern_andez R., Meana A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia) J. Invertebr. Pathol. 2007;94:211–217. doi: 10.1016/j.jip.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Holmstrup M., Bindesbøl A.-M., Oostingh G.J., Duschl A., Scheil V. Interactions between effects of environmental chemicals and natural stressors: a review. Sci. Total Environ. 2010;408:3746–3762. doi: 10.1016/j.scitotenv.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Husain D., Qasim M., Saleem M., Akhter M., Khan K.A. Bioassay of insecticides against three honey bee species in laboratory conditions. Cercetări Agronomice în Moldova. 2014;XLVII. 2(158):69–79. [Google Scholar]
- Johnson R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015;60:415–434. doi: 10.1146/annurev-ento-011613-162005. www.annualreviews.org. [DOI] [PubMed] [Google Scholar]
- Johnson R.M., Evans J.D., Robinson G.E., Berenbaum M.R. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera) Proc. Natl. Acad. Sci. U.S.A. 2009;106:14790–14795. doi: 10.1073/pnas.0906970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakmand F.A., Mahmoud T.T., Amin A.M. The role of three insecticides in disturbance the midgut tissue in honeybee Apis mellifera L. workers. Kurdistan 1st Conference on Biological Sciences. J. Dohuk Univ. 2008;11(1):144–151. [Google Scholar]
- Khoury D.S., Myerscough M.R., Barron A.B. A quantitative model of honey bee colony population dynamics. PLoS ONE. 2011;6(4):18491. doi: 10.1371/journal.pone.0018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurino D., Manino A., Patetta A., Porporato M. Toxicity of neonicotinoid insecticides on different honey bee genotypes. Bull. Insectology. 2013;66(1):119–126. [Google Scholar]
- Levy S.M., Falleiros A.M.F., Moscardi F., Gregorio G.A., Toledo L.A. Morphological study of the hindgut in larvae of Anticarsia gemmatalis Hubner (Lepidoptera: Noctuidae) Neotrop. Entomol. 2004;33:427–431. [Google Scholar]
- Mao W.F., Schuler M.A., Berenbaum M.R. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera) Proc. Natl. Acad. Sci. U.S.A. 2011;108:12657–12662. doi: 10.1073/pnas.1109535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G.F., Neves C.A., Campos L.A.O., Serrão J.E. The regenerative cells during the metamorphosis in the midgut of bees. Micron. 2006;37:161–168. doi: 10.1016/j.micron.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Medrzycki P., Montanari R., Bortolotti L., Sabatini A.G., Maini S., Porrini C. Effects of imidacloprid administered in sublethal doses on honey bees’ (Apis mellifera L.) behaviour. Laboratory tests. Bull. Insectol. 2003;56(1):59–62. [Google Scholar]
- Nasr M., Wallner K. Residues in honey and wax: implications and safety, Proc, of the North American Apicultural Research Symposium. Am. Bee J. 2003;143:322. [Google Scholar]
- Oldroyd B.P. What’s killing American honey bees? PLoS Biol. 2007;5:168. doi: 10.1371/journal.pbio.0050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd B.P., Nanork P. Conservation of Asian honey bees. Apidologie. 2009;40:296–312. [Google Scholar]
- Papaefthimiou C., Theophilidis G. The cardiotoxic action of the pyrethroid insecticide deltamethrin, the azole fungicide prochloraz, and their synergy on the semi-isolated heart of the bee Apis mellifera macedonica Pestic. Biochem. Phys. 2001;69:77–91. [Google Scholar]
- Pettis J.S., Wilson W.T., Shimanuki H., Teel P.D. Fluvalinate treatment of queen and worker honey bees (Apis mellifera L.) and effects on subsequent mortality, queen acceptance and supersedure. Apidologie. 1991;22:1–7. [Google Scholar]
- Pettis J.S., Collins A.M., Wilbanks R., Feldlaufer M.F. Effects of coumaphos on queen rearing in the honey bee, Apis mellifera. Apidologie. 2004;35:605–610. [Google Scholar]
- Pettis J.S., vanEngelsdorp D., Johnson J., Dively G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften. 2012;99:153–158. doi: 10.1007/s00114-011-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling E., Campbell P., Coulson M., Ruddle N., Tornier I. A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecka K. Effect of standardized plant herb extracts on general condition of the honey bee (Apis mellifera L.) Bull. Vet. Inst. Pulawy. 2004;48:415–419. [Google Scholar]
- Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Sandrock C., Tanadini M., Tanadini L.G., Fauser-Misslin A., Potts S.G. Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS ONE. 2014;9:103592. doi: 10.1371/journal.pone.0103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Eisenhardt D., Rademacher E. Sublethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): changes in behaviour and longevity. Apidologie. 2012;43(2):218–225. [Google Scholar]
- Silva-Olivares A., Diaz E., Shibayama M., Tsutsumi V., Cisneros R., Zuniga G. Ultrastructural study of the midgut and hindgut in eight species of genus Dendroctonus Erichson (Coleoptera: Scolytidae) Ann. Entomol. Soc. Am. 2003;96:883–900. [Google Scholar]
- Sorour J. Ultrastructural variations in Lethocerus niloticum (Insecta: Hemiptera) caused by pollution in Lake Mariut, Alexandria, Egypt. Ecotoxicol. Environ. Saf. 2001;48:268–274. doi: 10.1006/eesa.2000.2003. [DOI] [PubMed] [Google Scholar]
- Stoner K.A., Eitzer B.D. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo) PLoS ONE. 2012;7:39114. doi: 10.1371/journal.pone.0039114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiboldeaux R.L., Lindroth R.L., Tracy J.W. Effects of juglone (5-hydroxy-1,4-naphthoquinone) on midgut morphology and glutathione status in Saturniid moth larvae. Comp Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;120(3):481–487. doi: 10.1016/s0742-8413(98)10070-1. [DOI] [PubMed] [Google Scholar]
- Thompson H.M. Assessing the exposure and toxicity of pesticides to bumblebees (Bombus sp.) Apidologie. 2001;32:305–321. [Google Scholar]
- Vachon V., Laprade R., Schwartz J.L. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J. Invertebr. Pathol. 2012;111:1–12. doi: 10.1016/j.jip.2012.05.001. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D., Meixner M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010;103(1):S80–S95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D., Speybroeck N., Evans J.D., Nguyen B.K., Mullin C. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 2010;103:1517–1523. doi: 10.1603/ec09429. [DOI] [PubMed] [Google Scholar]
- Villaro A.C., Garayoa M., Lezaun M.J., Sesma P. Light and electron microscopic study of the hindgut of the ant Formica nigricans (Hymenoptera): I. Structure of the ileum. J. Morphol. 1999;242:189–204. doi: 10.1002/(SICI)1097-4687(199912)242:3<189::AID-JMOR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]