Abstract
Rationale:
PSA responses have been associated with a survival benefit in patients treated with enzalutamide in retrospective analyses.
Patient concerns:
However the prognostic value of PSA declines in highly pretreated patients receiving enzalutamide remains to be defined.
Diagnoses and interventations:
Medical records of patients with documented mCRPC treated with enzalutamide between September 2011 and August 2016 were reviewed at multiple participating centers and assessed for overall survival (OS), PSA variations, and other variables of interest. Univariable and multivariable analyses were conducted.
Outcomes:
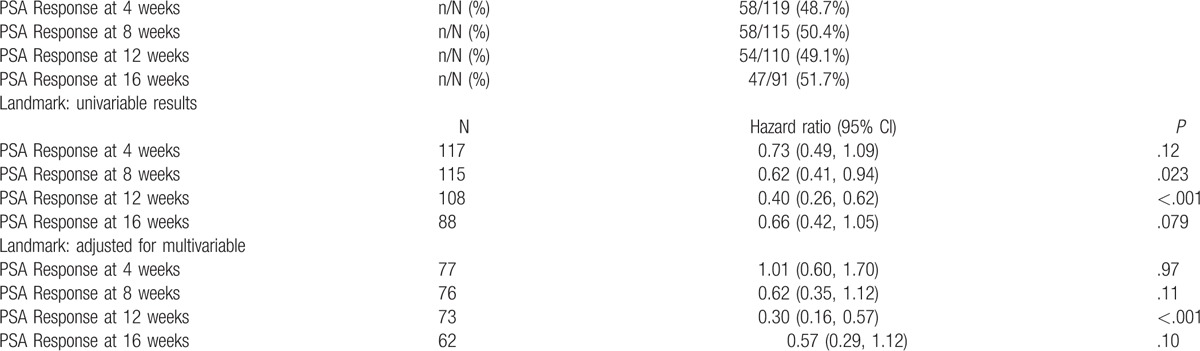
A total of 129 patients received enzalutamide. PSA response rates (>50% PSA declines) were 58/119 (48.7%), 58/115 (50.4%), 54/110 (49.1%), and 47/91 (51.7%) at weeks 4, 8, 12, and 16, respectively. Having a PSA response was a statistically significant prognostic factor of improved OS at 8 and 12 weeks in univariable analysis, whereas it was significant at 12 weeks in the multivariable analysis. Patients treated with enzalutamide had a median OS of 7.8 months.
Lessons:
Our study supports the prognostic value of PSA declines in heavily treated patients receiving enzalutamide.
Keywords: castration-resistant setting, enzalutamide, prostate cancer, PSA
1. Introduction
Prostate cancer is the most prevalent malignancy in men, with an estimated 1,111,700 incident cases diagnosed in 2012 worldwide.[1] After benefitting from androgen ablation treatment, virtually all patients with recurrent/advanced disease develop castration-resistant prostate cancer (CRPC), which progresses despite androgen deprivation treatment.[2] In most patients with CRPC, the androgen receptor (AR) pathway continues to drive tumor growth,[3] which is consistent with the effectiveness of novel antiandrogen treatments abiraterone and enzalutamide, both in the predocetaxel and in the postdocetaxel setting.[4–7] Despite both of these agents inhibit AR signaling, they have a different mechanism of action. Although abiraterone acetate inhibits the residual adrenal and intratumoral androgen synthesis by blocking the CYP17A activity,[8] enzalutamide directly inhibits the AR with no agonist activity.[9]
In patients with metastatic CRPC, PSA declines have been consistently associated with improved outcomes associated with systemic therapy, including docetaxel,[10] cabazitaxel,[11] and abiraterone.[12] Recently, a retrospective analysis of the AFFIRM trial[13] showed that PSA declines in men treated with enzalutamide after prior docetaxel were consistently associated with progression-free survival (PFS), overall survival (OS), and improvement of pain. In this retrospective review study, we aim to investigate the prognostic role of PSA declines recorded during the first 16 weeks of treatment by the use of multivariate analysis to account for potential confounding factors.
2. Patients and methods
2.1. Inclusion criteria
Medical records of patients with documented mCRPC treated with enzalutamide between September 2011 and August 2016 were reviewed at multiple participating centers (Azienda Ospedaliera Universitaria Federico II, Naples, Italy; Azienda Ospedaliera di Rilievo Nazionale ‘Antonio Cardarelli,’ Naples, Italy; Istituto Nazionale Tumori ‘Fondazione G. Pascale,’ Naples, Italy; Azienda Ospedaliero “G. Rummo,” Benevento, Italy; Azienda Sanitaria Locale Napoli 3, Naples, Italy; Presidio Ospedaliero di Sapri, Sapri (Salerno), Italy; Ospedale di Vallo della Lucania, Vallo Della Lucania (Salerno), Italy; Casa Sollievo della Sofferenza, San Giovanni Rotondo (Foggia), Italy; the University of Alabama at Birmingham (UAB) Cancer Center, Birmingham, AL.
2.2. Retrieved data
Demographic data of eligible patients were retrieved along with information regarding known and previously recognized baseline prognostic factors (performance status [PS], hemoglobin [Hb], PSA, albumin, pain, neutrophil count, lymphocyte count, LDH, alkaline phosphatase, Gleason score, visceral metastasis status), previous local and systemic treatments and treatment with abiraterone/enzalutamide, statin use, early PSA declines at 4,8,12, and 16 weeks. All patients who received at least one dose of abiraterone were included in the abiraterone dataset, and all patients who received at least one dose of enzalutamide were included in the enzalutamide dataset. Patients receiving both abiraterone and enzalutamide could be included in analyses of both datasets. Approval of retrospective observational studies by the ethics committee is not required according to the existing law and regulations.
2.3. Data analysis
Summary statistics were used to describe the patient population and outcomes. In each subset, survival was computed from the start of enzalutamide to the time of death. Univariable Cox proportional hazards regression was used to evaluate PSA declines and other variables as potentially prognostic for OS. Multivariable modeling was conducted by using the full model method, that is, including all potential factors in a multivariable model, regardless of whether they were statistically significant or not. The association of PSA response with survival was then assessed after adjusting for factors included in the multivariable model. Logarithmic transformation of factors was performed for statistical normalization purposes as necessary. All tests were 2-sided and a P-value of .05 or less was considered statistically significant. No statistical adjustments were made for multiple testing. OS was defined as the time between treatment initiation and either the date of death or of last follow-up for surviving patients.
3. Results
3.1. Cohorts’ characteristics
A total of 129 patients starting enzalutamide between September 2011 and August 2016 were included in the enzalutamide cohort, as shown in Table 1. Eight patients remained on enzalutamide treatment at the time of analysis, with a median duration of enzalutamide treatment for the remaining patients of 18.6 (range = 0-94.6) weeks.
Table 1.
Baseline characteristics: patients treated with enzalutamide.
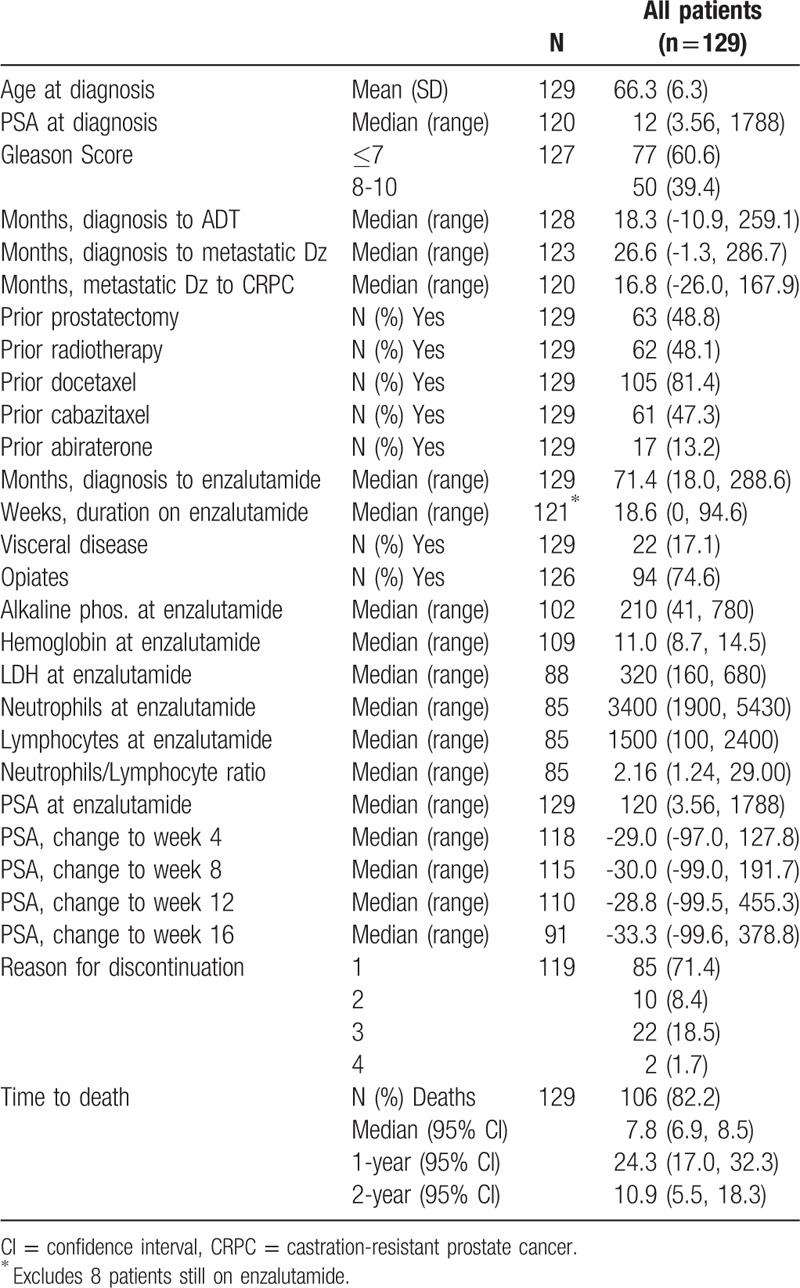
Patients in the enzalutamide cohort had an advanced disease at the time of treatment initiation, as shown consistently by multiple variables (Table 2), including time from diagnosis to initiation of enzalutamide therapy (71.4 months), prior cabazitaxel treatment (47.3%), use of opiates (74.6%), alkaline phosphatase levels (median, 210 IU/L), and LDH levels (median, 320 IU/L). As a result, median OS was 7.8 months in the cohort.
Table 2.
Prognostic factors of overall survival: patients treated with enzalutamide.
3.2. Variation of PSA levels
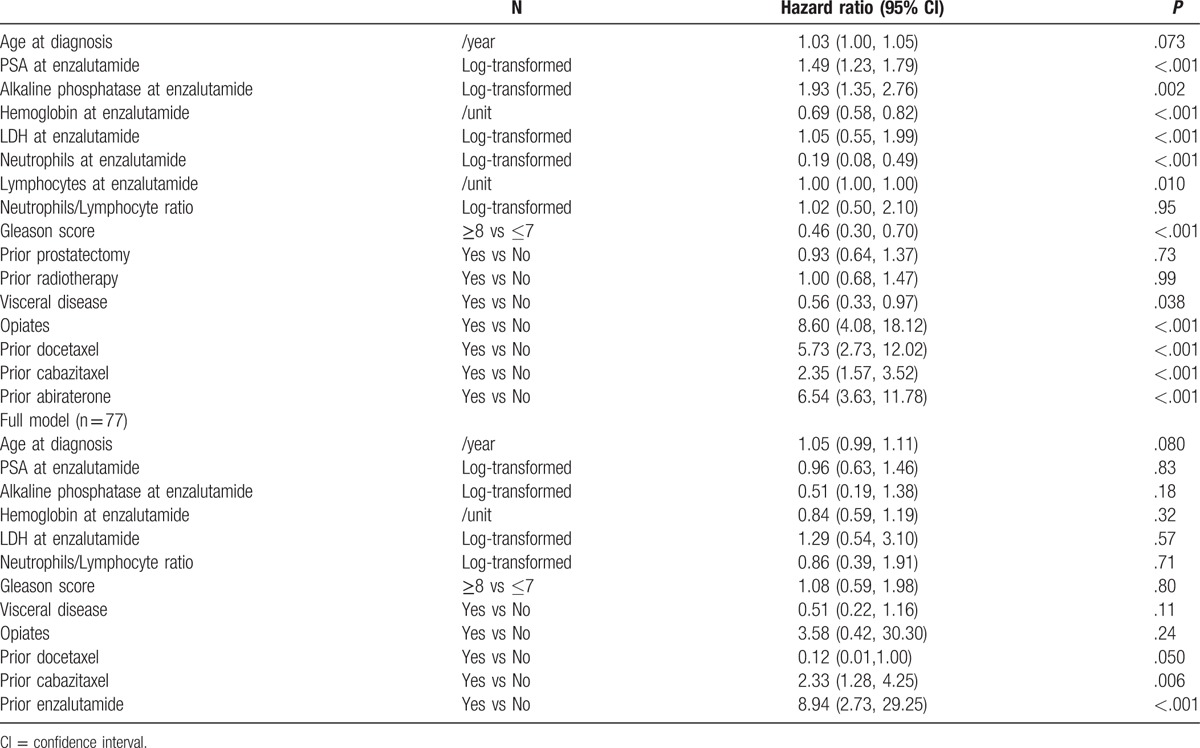
In the study cohort, PSA response rates (>50% PSA declines) were 58/119 (48.7%), 58/115 (50.4%), 54/110 (49.1%), and 47/91 (51.7%) at weeks 4, 8, 12, and 16, respectively. Having a PSA response was a statistically significant prognostic factor of improved OS at 8 and 12 weeks at univariable analysis, whereas it was significant at 12 weeks at multivariable analysis. Trends towards significance were observed after 8 and 16 weeks at multivariable analysis (see Table 3). Consistent results were reported for 50% PSA declines and 25% increases (data not shown).
Table 3.
PSA response information: patients treated with enzalutamide.
4. Discussion
In the retrospective study involving 1199 men enrolled in the AFFIRM trial reported by Armstrong et al,[13] patients were grouped by maximal PSA decline during the first 3 months’ treatment, with each PSA decline category being assessed for OS surrogacy using Prentice criteria. This analysis showed that patients with PSA declines of any, ≥30%, ≥50%, and ≥90% with enzalutamide had a much longer OS, PSA, PFS, radiographic progression-free survival (rPFS) (P < .001), and better pain response (P < .026) versus PSA increase/no decline. In particular, the median duration of PSA PFS for men with PSA increase/no decline and confirmed PSA declines of ≥30% and ≥50% was 4.6 months (95% confidence interval [CI], 4.6–4.6), 11.1 months (95% CI, 11.0–13.8), and 11.1 months (95% CI, 11.1–14.0), respectively. The median duration of PSA PFS and rPFS had not been reached for men with confirmed PSA declines of ≥90% and ≤0.2 ng/mL. The median rPFS duration for men with PSA increase/no decline and confirmed PSA decline of ≥30% and ≥50% were 5.5 months (95% CI, 5.3–5.6), 16.1 months (95% CI, 13.8–16.6), and 16.5 months (95% CI, 13.8–18.1). Confirmed PSA declines of ≥30%, ≥50%, and ≥90% within 90 days of enzalutamide treatment were associated with a greater proportion of men with pain response (52.3%, 50.9%, and 63.2%, respectively) compared with increase/no decline in PSA levels (32.0%; P < .026 for all). Importantly, although patients who did not show any decrease or showed an increase of PSA values had a median survival of approximately 12 months, patients who had a greater than 30% presented a 12-month survival rate >80%.
In the patient population of our retrospective study, only approximately one fourth of patients were alive after 12 months, but we also found an association of early PSA declines with survival outcomes in patients receiving abiraterone and enzalutamide. As opposed to the patient population of the AFFIRM trial, our retrospective study has the merit to investigate a highly pretreated population of CRPC patients, with 47.3% of patients having received cabazitaxel and 13.2% of patients having received abiraterone. Of note, although a statistically significant or a trend were observed for 30% PSA declines at 8,12, and 16 weeks at multivariable analysis, early 30% PSA decline after 4 weeks was not associated with survival in patients receiving enzalutamide. This result is consistent with those obtained by Conteduca et al[14] who did not find an association of PSA declines at 4 weeks and OS in a cohort of patients receiving enzalutamide. Furthermore, we noted that the 30% PSA response rate was similar at 4,8, 12, and 16 weeks in the enzalutamide cohort, which is also consistent with the results obtained by Conteduca et al, and suggests that enzalutamide patients who do not show early PSA declines may not be likely to have a PSA decline at all. Importantly, of the dozen of variables that were explored at multivariate analysis, including age, baseline PSA, alkaline phosphatase, Hb, and LDH levels, and other clinical variables such as the Gleason score, presence of visceral disease or use of opiates, only PSA declines at 12 were significantly associated with OS.
Despite the limitations typical of retrospective studies, including the lack of sample size computation and missing/inaccurate data, we believe that the association reported here is of great importance to confirm the finding reported in the AFFIRM trial in a real-word setting of routine clinical practice. The lack of PSA declines during the first 3 months may prompt early radiological evaluation or re-assessment for Androgen-Receptor Splice Variant 7, which is known to confer resistance to novel hormonal treatments,[15] or even change of the therapeutic strategy.
Novel prospective trials are required in order to assess the optimal treatment in CRPC patients receiving systemic therapy who do not achieve a PSA decline within the first 3 months of treatment.
Footnotes
Abbreviations: AR = androgen receptor, CI = confidence interval, CRPC = castration-resistant prostate cancer, Hb = hemoglobin, OS = overall survival, PFS = progression-free survival, PS = performance status, rPFS = radiographic progression-free survival.
GS is the consultant for Bayer, Sanofi, Pfizer, Novartis, Eisai, Janssen, Amgen, Astrazeneca, Merck, Genentech, Argos, and Agensys.
This work was partially supported by LILT (Lega Italiana alla Lotta ai Tumori), Sez, Napoli, Italy.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Di Lorenzo G, Buonerba C, Autorino R, et al. Castration-resistant prostate cancer: current and emerging treatment strategies. Drugs 2010;70:983–1000. [DOI] [PubMed] [Google Scholar]
- [3].Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol 2012;30:644–6. [DOI] [PubMed] [Google Scholar]
- [4].Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- [6].Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grist E, Attard G. The development of abiraterone acetate for castration-resistant prostate cancer. Urol Oncol 2015;33:289–94. [DOI] [PubMed] [Google Scholar]
- [9].Bhattacharya S, Hirmand M, Phung D, et al. Development of enzalutamide for metastatic castration-resistant prostate cancer. Ann N Y Acad Sci 2015;1358:13–27. [DOI] [PubMed] [Google Scholar]
- [10].Armstrong AJ, Tannock IF, de Wit R, et al. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer 2010;46:517–25. [DOI] [PubMed] [Google Scholar]
- [11].Halabi S, Armstrong AJ, Sartor O, et al. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J Clin Oncol 2013;31:3944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu XS, Ryan CJ, Stuyckens K, et al. Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res 2015;21:3170–7. [DOI] [PubMed] [Google Scholar]
- [13].Armstrong AJ, Saad F, Phung, et al. Clinical outcomes and survival surrogacy studies of prostate-specific antigen declines following enzalutamide in men with metastatic castration-resistant prostate cancer previously treated with docetaxel. Cancer 2017;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Conteduca V, et al. Association between early PSA increase and clinical outcome in patients treated with enzalutamide for metastatic castration resistant prostate cancer. Mol Diagn Ther 2016;20:255–63. [DOI] [PubMed] [Google Scholar]
- [15].Buonerba C, Di Lorenzo G, Sonpavde G. Contemporary molecular tests for prognosis and treatment guidance for castration-resistant prostate cancer. Expert Rev Mol Diagn 2016;16:1113–20. [DOI] [PubMed] [Google Scholar]


