Abstract
The standard automated perimetry (SAP) 24-2 test cannot adequately test the paracentral region because test points are located sparsely in macular area where is crowded with retinal ganglion cells (RGCs), even though paracentral scotoma is clinically related to a risk of losing visual function. More sensitive visual field (VF) tests are needed to assess paracentral VF defects precisely. We investigated the structure–function relationship on the SAP 10-2 test and the frequency doubling technology (FDT) 24-2 test as well as the SAP 24-2 test in glaucoma with parafoveal scotoma (PFS). Glaucoma patients with PFS (134 patients) were included in this cross-sectional study. Sub-analysis was performed with isolated PFS (51 patients). Global and sectoral mean sensitivities (MS) were evaluated using SAP 24-2, 10-2, and FDT 24-2 program. MS was analyzed as dB or unlogged 1/lambert (SAP) or 1/Michelson contrast (FDT). Ganglion cell-inner plexiform layer (GCIPL) thickness was measured using spectral domain optical coherence tomography. Topographic relationships between the structure and the function were analyzed. In the total PFS group, good structure–function correlations were found in all zones with SAP 24-2, 10-2, and FDT 24-2 test. For glaucoma with isolated PFS, average GCIPL thickness was significantly correlated with central cluster MS (dB) using the SAP 10-2 test (r = 0.279, P = .047) and the FDT 24-2 test (r = 0.289, P = .039), but not the SAP 24-2 test (r = 0.264, P = .061). Topographically, the FDT 24-2 test showed significant correlations in all sectors between sectoral MS and corresponding GCIPL thickness. With regard to the SAP 10-2 test, there was significant topographical structure–function correlations for the superotemporal, inferotemporal, and inferonasal sectors. For SAP 24-2, only inferonasal GCIPL thickness was correlated with the corresponding VF sensitivity. Topographical structure–function on the macula was better with the SAP 10-2 test (superotemporal sector) and the FDT 24-2 test (superotemporal sector) than with the SAP 24-2 test in glaucoma with isolated PFS. In conclusion, FDT 24-2 and SAP 10-2 tests performed more favorably than the SAP 24-2 test in the structure–function relationship of glaucoma patients with isolated paracentral scotoma. FDT 24-2 tests can be another good option for detecting and monitoring RGC loss on the macular area while not missing VF defects outside the central 10°.
Keywords: frequency doubling technology, ganglion cell-inner plexiform layer, parafoveal scotoma, standard automated perimetry
1. Introduction
Glaucomatous visual field (VF) loss was found to frequently affect the macular area initially.[1,2] Detection of early glaucomatous damage in the macula is clinically important because central visual disturbance puts patients at greater risk of losing visual function and also interferes with everyday activities such as reading and driving.[3–5]
There are more retinal ganglion cells (RGCs) located in the central and paracentral retina than the peripheral retina.[6] However, only 12 test points fall within the central 10° visual field, where more than 30% of RGCs are located.[7] In standard automated perimetry (SAP) 24-2 program, the stimulus has a diameter of 0.43°and test points are 6° apart both in the central and peripheral retina. Therefore, it is difficult for SAP 24-2 test to adequately test the macular region and reflect structural glaucomatous damage in this area. Early RGC loss often occurs in the central macular region, even in patients with VFs classified as normal.[8] We previously reported that eyes with initial parafoveal scotoma (PFS) on SAP had a more glaucomatous optic nerve head morphology compared to those with initial peripheral scotoma.[9] A discrepancy in structure–function correspondence is more likely to occur in the paracentral retina.
In 10-2 SAP, the test points are 2° apart and more closely spaced than in 24-2 SAP. The 10-2 VF tests are less likely to miss paracentral defects with a more detailed spatial information than 24-2 VF tests.[7,10,11] However, 10-2 VF is limited because it cannot test VF defects outside the central 10°. In glaucoma, VF points showing progression can be located not only in the paracentral region, but also in the peripheral region. Examination of only VF 10-2 program in glaucoma is not sufficient to cover all glaucomatous VF defects, and should be followed by VF 24-2 tests. However, performance of 2 VF tests in the same day would be time consuming and costly. Thus, more sensitive 24-2 VF tests are needed to detect paracentral VF defects.
Matrix frequency doubling technology (FDT) perimetry is claimed to be more useful in detecting onset of early glaucomatous VF defects than SAP,[12,13] even if not all authors agree on this statement.[14,15] Abnormal results on pattern deviation map or rates of pattern standard deviation (PSD) change in FDT are highly predictive of future SAP VF defects.[16–19] FDT with a target of 5° covers a larger area and is less likely to leave the retina untested. There is a possibility that the FDT 24-2 test can be one alternative to detect RGC loss on the macular area adequately while not skipping VF defects outside the paracentral area.
We hypothesized that FDT using the 24-2 program would be helpful in precisely evaluating glaucomatous damage in patients with paracentral VF defects. In this study, we compared the structure–function relationship among SAP 24-2, SAP 10-2, and FDT 24-2 tests in glaucoma with PFS.
2. Methods
This cross-sectional study was approved by the Institutional Review Board of the Catholic University of Korea, Seoul, Korea, and followed the tenets of the Declaration of Helsinki. Patients with glaucoma that met inclusion criteria were consecutively included from all patients examined for glaucoma at the glaucoma clinic of Seoul St. Mary's Hospital between September 2014 and May 2015. Informed consent was obtained from all participants.
Inclusion criteria were best-corrected visual acuity of 20/40 or better, and axial length within 27 mm. Eyes with a normal open angle and the presence of a glaucomatous optic disc, such as diffuse or focal rim thinning, notching, optic disc hemorrhage, or retinal nerve fiber layer defect with a corresponding glaucomatous VF damage including PFS, were included in this study. Patients with uveitis or diseases that might affect the peripapillary or macular areas, or unreliable VF tests were excluded.
All patients underwent complete ophthalmic examinations, including slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, axial length measurement, central corneal thickness measurement, and dilated fundus bimicroscopy. All subjects performed stereoscopic optic disc photography.
2.1. Optical coherence tomography
Spectral-domain optical coherence tomography (OCT) imaging was performed using Cirrus HD-OCT version6.0 (Carl Zeiss Meditec, Inc.). Using a macular cube scan, the ganglion cell-inner plexiform layer (GCIPL) thickness was obtained. The protocol for GCIPL thickness has previously been described in detail.[20,21] Ganglion cell analysis software measures the average, minimum and sectoral (superior, superotemporal, speronasal, inferior, inferotemporal, and inferonasal) GCIPL thickness in a 14.13-mm2 elliptical annulus with vertical inner and outer radii of 0.5 and 2.0 mm, respectively, and horizontal inner and outer radii of 0.6 and 2.4 mm, respectively. Poor-quality images with signal strength less than 6 were discarded.
2.2. Visual field testing
All subjects underwent SAP using the 24-2 and 10-2 SITA standard programs with a Humphrey field analyzer II 750i (Carl Zeiss Meditec, Dublin, CA). Goldmann size III targets with diameters of 0.43° were presented. FDT perimetry was performed using the 24-2 program with 5° stimuli, spatial frequency of 0.5 cycles/deg, and temporal frequency of 18 Hz with the FDT Humphrey Matrix (Carl Zeiss Meditec).
Mean deviation (MD) and PSD were analyzed. Twelve central 10° VF points in the 24-2 test and 68 VF points in the 10-2 test were used for analysis. One central point performed by only FDT and not SAP was not included in the comparison of VF sensitivity. In glaucoma, the fovea typically maintains most of its function until the end-stage of disease, even though fovea can be mildly affected early in glaucoma.[22] Because other diseases influence foveal function, it would not by itself be a sensitive or specific test for glaucoma. Therefore, the foveal sensitivity was not evaluated with SAP. In SAP, VF sensitivity was evaluated using the logarithmic dB [10 × log(1/Lambert)] scale and a nonlogarithmic 1/L scale in 12 points with the 24-2 program and 68 points with the 10-2 program. The non-logarithmic 1/L value was calculated by conversion of decibel values to the non-logarithmic form as with the equation above. In FDT matrix perimetry, the non-logarithmic scale was calculated by converting decibel figures to the antilog scale using the equation VF sensitivity [(dB, logarithmic scale) = 20 × log (1/Michelson contrast)].[23,24] VF sensitivity was also analyzed using both the logarithmic and anti-logarithmic scales for FDT. Reliable tests were defined as <15% fixation losses, false positives, or false negatives.
2.3. Total and isolated PFS group
A glaucomatous VF defect was defined as a cluster of 3 or more points with P value <5%, one of which had a P value of < 1% on the pattern deviation plot. One glaucoma specialist (KIJ) determined PFS based on pattern deviation probability plots in the SITA 24-2 test. PFS was defined as a single glaucomatous VF defect within 12 points of a central 10° radius in 1 hemifield. (Fig. 1). Among total glaucoma patients with PFS, subanalysis was performed for patients with isolated PFS with MD > –10 dB. If glaucoma patients with the PFS had VF defects in both the central 10° and peripheral nasal fields or other area than central or in both superior and inferior hemifield, they were assigned to the total PFS group, not in the isolated PFS group.
Figure 1.

(A) On pattern deviation plot, parafoveal scotoma indicated abnormal points within 12 points of the central 10° radius (dashed line). (B) Structure–function correspondence map according to Garway-Heath et al[18]: Visual field sectors (B1, B2) and ganglion cell-inner plexiform layer thickness (B3). (C) Topographical structure–function relationship in glaucoma with isolated parafoveal scotoma. Values in each sector are Pearson's correlation coefficient. ∗Statistically significant values (P < .05) are shown in bold. †Statistically significant difference with P < .05 between SAP 24-2 and SAP 10-2 or FDT. FDT = frequency doubling technology.
2.4. Structure–function relationship
Central and sectoral mean sensitivity(MS) were evaluated on threshold printouts in VF tests. Central MS is calculated as the mean of VF sensitivities in the central 12 points in 24-2 VFs and 68 points in 10-2 VFs. The sectoral MS in the central 12 points of 24-2 VFs was determined in superotemporal, inferotemporal, superonasal, and inferonasal sectors according to the structure–function correspondence map suggested by Garway-Heath et al[25] (Fig. 1). Sixty-eight VF test points on 10-2 SAP were assigned to 4 sectors adapting the Garway–Heath map designed for 24-2 SAP.
The relationship between central cluster MS and average GCIPL thickness was evaluated. Superotemporal and inferotemporal GCIPL thickness was used for the superotemporal and inferotemporal topographical structure–function relationship, respectively. For superonasal and inferonasal sectors, the sum of superior and superonasal GCIPL thickness and that of inferior and inferonasal GCIPL thickness was employed, respectively.
2.5. Statistical analysis
Differences between the different VF tests were evaluated by a paired t test. Correlations between VF parameters and GCIPL variables were assessed based on Pearson's correlation coefficients. To compare the correlation between VF tests, a Hotelling–Williams test was used. In all analyses, P < .05 indicated statistical significance. SPSS software (ver. 17.0; SPSS Inc., Chicago, IL) was used for statistical analyses.
3. Results
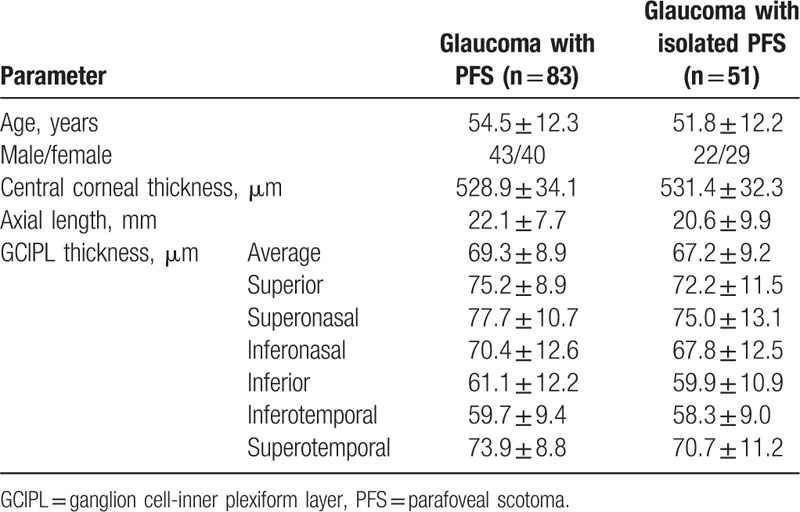
Data from 83 glaucoma patients with PFS were analyzed. Among them, 51 patients with glaucoma had isolated PFS. Table 1 shows the demographics of patients with PFS. Average GCIPL thickness was 67.2 ± 9.2 μm in the total PFS group and 69.3 ± 8.9 μm in the isolated PFS group.
Table 1.
Characteristics of glaucoma patients with the isolated or total parafoveal scotoma (PFS) group.
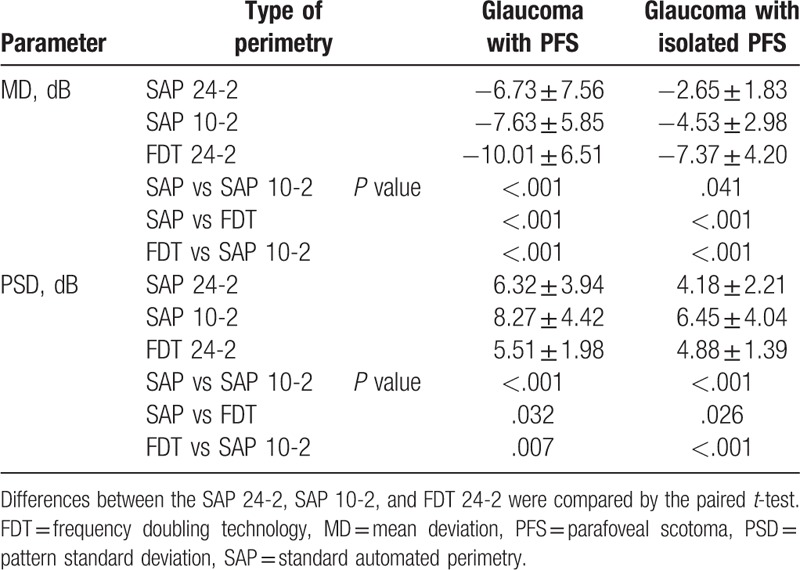
In glaucoma patients with isolated PFS, FDT had the lowest MD value (–7.37 ± 4.20 dB), followed by the SAP 10-2 test (–4.53 ± 2.98 dB), and SAP 24-2 test (–2.65 ± 1.83 dB) (SAP vs FDT, FDT vs SAP 10-2, SAP vs SAP 10-2, all P < .05; Table 2). PSD was the highest for SAP 10-2 (6.45 ± 4.04 dB), followed by FDT (4.88 ± 1.39 dB) and SAP 24-2 test (4.18 ± 2.21 dB) (SAP vs FDT, FDT vs SAP 10-2, SAP vs SAP 10-2, all P < .05).
Table 2.
Mean deviation and pattern standard deviation of SAP and FDT in glaucoma patients with the isolated or total parafoveal scotoma (PFS) group.
3.1. Overall structure–function relationships
Table 3 shows the overall structure–function relationship between the central cluster sensitivity of the visual field, measured by dB and 1/L or 1/Michelson contrast scales, and average GCIPL thickness. In the total PFS group, all VF tests showed significant correlations between average GCIPL thickness and central cluster MS expressed in either form (dB or 1/L; all P < .05). In the isolated PFS group, average GCIPL thickness was not significantly correlated with central cluster MS for the SAP 24-2 test (r = 0.264, P = .061; Fig. 2). For the SAP 10-2 test and FDT, average GCIPL showed a correlation with central cluster MS expressed as dB (SAP 10-2 test, r = 0.279, P = .047; FDT, r = 0.289, P = .039). In the unlogged VF sensitivity scales, there was no significant overall structure–function correlation for all types of VF tests (all P > .05).
Table 3.
The structure–function relationship between central cluster sensitivity of visual field and average ganglion cell-inner plexiform layer thickness.
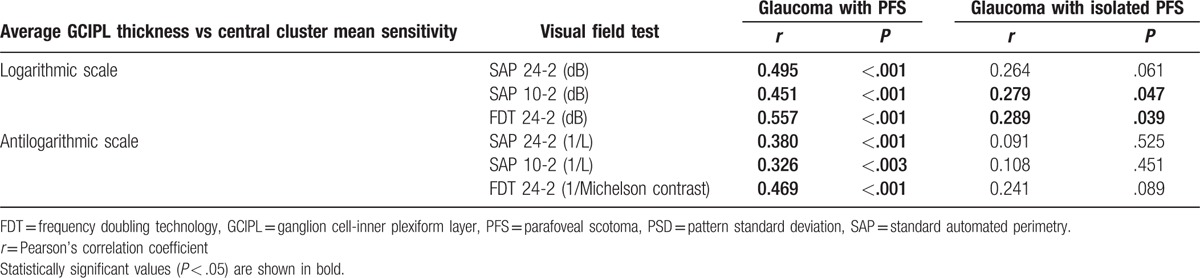
Figure 2.
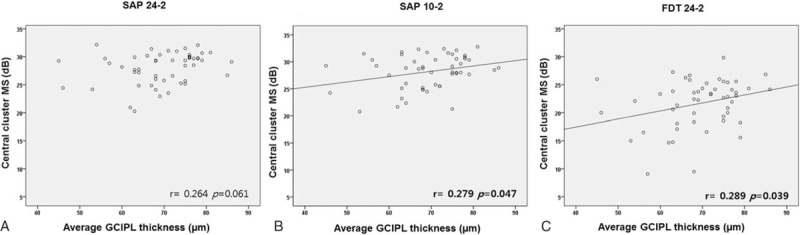
Scatterplots showing correspondence between macular mean sensitivity (MS) (dB) of the visual field and average ganglion cell-inner plexiform layer thickness in patients with isolated parafoveal scotoma. Global MS was measured by SAP 24-2 (A), SAP 10-2 (B), and frequency doubling technology 24-2 (C). MS = mean sensitivity, SAP = standard automated perimetry.
3.2. Topographical structure–function relationships
In the total PFS group, there were significant structure–function correlations for all sectors in all VF tests. There was no significant difference in topographical structure–function correlations between VF tests for all sectors (Table 4).
Table 4.
The structure–function relationship between regional visual field sensitivity measured with SAP or SAP10-2 or FDT and the corresponding GCIPL thickness.

In glaucoma patients with isolated PFS, SAP 24-2 program had significant correlation between sectoral GCIPL thickness and corresponding VF sensitivity expressed as dB only at the inferonasal sector (r = 0.456, P = .001), but not at the superotemporal (r = 0.152, P = .285), inferotemporal (r = 0.227, P = .109), and superonasal sectors (r = 0.252, P = .074; Fig. 3). With regard to the SAP 10-2 program, there were significant topographical structure–function correlations for the superotemporal, inferotemporal, and inferonasal sectors with the highest correlation found in the inferonasal sector (r = 0.264–0.481). For FDT, topographical correlations between sectoral GCIPL thickness and corresponding VF MS (dB) were statistically significant for all sectors, showing the highest correlation in superotemporal sectors (r = 0.298–0.593). Similar results were found for the topographical structure–function relationships when VF sensitivity was indicated 1/L. Topographical structure–function associations in the SAP 10-2 test were stronger for the superotemporal sector than those in the SAP 24-2 test (dB scale, P = .036; 1/L scale, P = .019). The association between sectoral GCIPL and corresponding VF sensitivity was greater for the superotemporal sector in FDT than in the SAP 24-2 test (dB, P = .002; 1/L, P = .002). For topographical structure–function relationships, there was no significant difference between the SAP 10-2 test and FDT.
Figure 3.
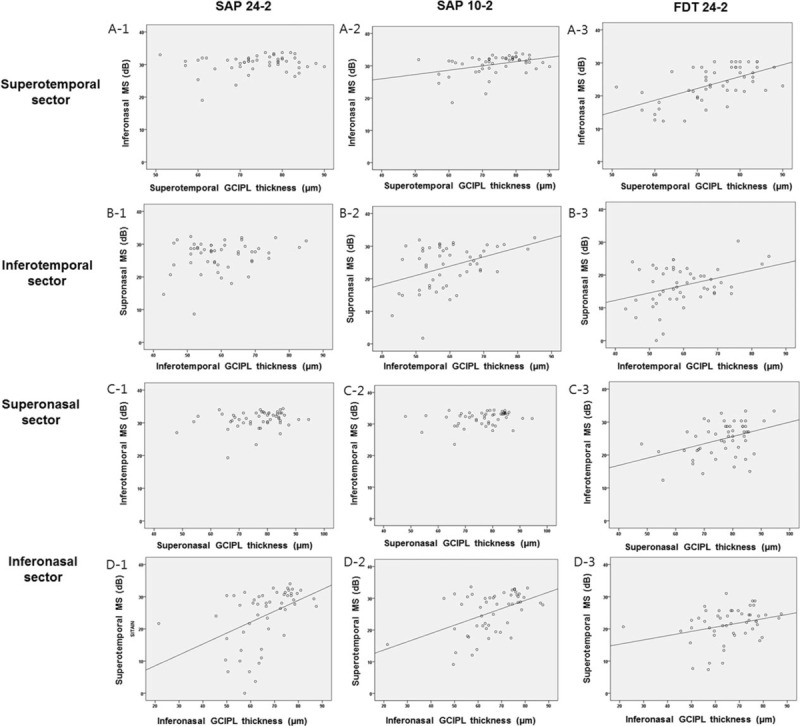
Scatter plots expressing topographical structure–function relationship in patients with isolated parafoveal scotoma on the superotemporal (A-1,2,3), inferotemporal (B-1,2,3), superonasal (C-1,2,3), and inferonasal (C-1,2,3) sectors.
4. Discussion
In glaucoma patients with isolated paracentral scotoma, we found that average GCIPL thickness is significantly correlated with central cluster MS using the SAP 10-2 test and the FDT 24-2 test, but not the SAP 24-2 test. Topographically, the SAP 10-2 test and the FDT 24-2 test showed significant correlations in most sectors between sectoral MS and corresponding GCIPL thickness. The topographical structure–function relationship on the macula was better with the SAP 10-2 test and the FDT 24-2 test than SAP 24-2 test in glaucoma with isolated PFS.
The total PFS group expressed good structure–function correlations in all zones with 24-2 SAP. This finding corresponds to previous studies showing a good topographical structure–function relationship between VF sensitivity in the SAP 24-2 program and GCIPL thickness in glaucoma patients with varying degrees of VF defects.[21,26]
In early stage glaucoma patients with isolated PFS, the topographic structure–function relationship was generally poor, with the SAP 24-2 test showing a significant correlation only in the inferonasal sector. To the best of our knowledge, there has been no previous study investigating structure–function relationships using the SAP 24-2 test and GCIPL thickness in early glaucoma patients with only PFS. As RGCs are located more centrally, their density increases and their receptive field radius declines.[6,27] Half of RGCs are placed within 4.5 mm (16°) of the foveal center, a region within 10% of the total retina.[6] Glaucoma with initial PFS seemed to involve greater loss of structural reserve of RGCs compared to glaucoma with initial peripheral scotoma having similar functional damage.[9] In VF 24-2 tests with VF points 6° apart, only 12 test points fall within the central 10° visual fields. With poor sampling, the SAP 24-2 program can miss structural damage caused by glaucoma and lack detailed spatial information of the central zone.
Using the SAP 10-2 program, the correspondence between average GCIPL thickness and central cluster MS was confirmed in glaucoma patients with total or isolated PFS. Sectoral GCIPL thickness was topographically well correlated with MS on SAP 10-2 test in the corresponding sector. This result is comparable to those of previous studies reporting a good structure–function relationship between GCIPL thickness and SAP 10-2 VF sensitivities in glaucoma with early to advanced VF damage.[26,28–30]
A direct comparison of the structure–function correspondence between SAP 24-2 and SAP 10-2 test has not been determined yet. Kim et al[26] reported that the topographical correlation coefficients of GCIPL thickness and corresponding VF MS were 0.469–0.801 for the SAP 24-2 test and 0.406–0.750 for the SAP 10-2 test in glaucoma patients with various types of glaucoma VF defects. Although structure–function correlations were not statistically compared between the SAP 24-2 test and the SAP 10-2 test, they had similar correlation coefficients. The results of a previous study[26] correspond to our study, showing comparable performance of the SAP 10-2 test over the SAP 24-2 test in total glaucoma patients with PFS.
In the isolated PSF group, however, structure–function correspondence was better with SAP 10-2 than SAP 24-2 program. This discrepancy is likely caused by a different composition of the study populations in total and isolated PFS groups. The isolated PFS group had only early-stage (MD ≥ –6 dB) glaucoma (mean MD = –2.65 ± 1.83 dB in SAP 24-2 test), whereas the total PFS group had moderate (–12 dB ≤MD < –6 dB) to advanced stage (MD < –12 dB) glaucoma as well as early stage glaucoma (mean MD = –6.73 ± 7.56 dB in the SAP 24-2 test).[31] In the SAP 10-2 program, test points are closely spaced (2° apart) in the central retina with substantial RGC density. This arrangement seems to result in good structure–function correlation even in early stage glaucoma with PFS, similar to what previous studies reported regarding earlier detection of glaucomatous macular damage with the SAP 10-2 test.[1,11]
For the FDT 24-2 test, GCIPL thickness correlated well with the corresponding macular VF sensitivity globally and topographically, in both the total and isolated PFS groups. There have been no previous reports about correspondence between FDT MS and GCIPL thickness in glaucoma. One report found that macular ganglion cell complex thickness, including the retinal nerve fiber layer, RGC layer, and inner plexiform layer, was correlated with FDT sensitivity in SAP normal or abnormal halves, but not with SAP sensitivity in SAP normal halves.[32] In that study, Hayashi et al[32] suggested that SAP normal hemifields in glaucoma patients may do not exclude preperimetric glaucoma. Early glaucoma with isolated PFS expressed significant topographical correlation between GCIPL thickness and the corresponding MS in the FDT 24-2 test, but not in the SAP 24-2 test. That result corresponds to those of Hayashi et al,[32] which demonstrated a good structure–function relationship for FDT in early glaucoma.
We attribute the favorable performance of FDT 24-2 test to the following reasons. First, the target size is 5° for FDT and 0.43° for SAP when test points are spaced 6° apart for both kind of tests. With larger targets, FDT samples more retinal area. Second, glaucomatous functional damage is reliably observed with low variability with FDT, which has larger targets and decreased stimulus range.[33,34] Third, VF sensitivity was higher for the frequency-doubling stimulus than for the size III SAP stimulus for both magnocellular and parvocellular RGCs.[35] There are more RGCs located in the central and paracentral retina than in the peripheral retina. It is difficult for conventional VF test to adequately test the macular region. Therefore, sensitive VF tests are greatly needed to evaluate this macular area. We speculated that good sensitivity for RGCs could be related to favorable structure–function correspondence with FDT in paracentral retina. Further efforts are needed to disclose the exact underlying mechanism for a good structure–function relationship in FDT.
Figure 4 shows 2 representative cases with PFS. On the SAP 24-2 test, case A has more severe VF damage than case B. However, case A had thicker average GCIPL thickness (76 μm) than that of case B (63 μm). On the FDT 24-2 test, case A with thicker GCIPL thickness had higher central MS than that in case B. In these cases, structural glaucomatous loss on GCIPL thickness was correlated better with the FDT 24-2 test than the SAP 24-2 test.
Figure 4.
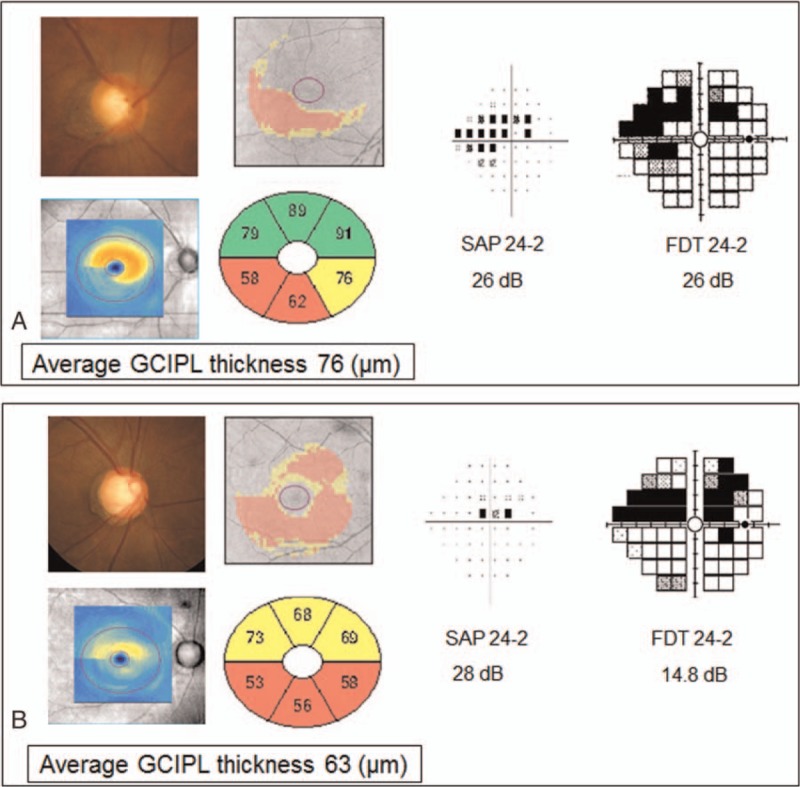
Representative cases with parafoveal scotoma (PFS). Case A (76 μm) had thicker average GCIPL thickness than that of case B (63 μm). Visual field damage was more severe in case A than in case B on the SAP 24-2 test, and in case B than in case A on the FDT 24-2 test. The FDT 24-2 test better reflected structural glaucomatous loss on GCIPL thickness than the SAP 24-2 test in these cases. FDT = frequency doubling technology, GCIPL = ganglion cell-inner plexiform layer, PFS = parafoveal scotoma, SAP = standard automated perimetry.
One of limitations is that the calculation of stimulus contrast for each perimetry is not identical. Stimulus contrast was calculated as ΔL/L in SAP and as Lmax-Lmin/Lmax+Lmin in Matrix FDT. Nonetheless, the comparison between currently available perimetries can be clinically meaningful. Reproducibility of VFs was not checked in this study, and it could be another limitation. In this study, the definition of “parafoveal scotoma” can be debatable because the central 10° includes perifoveal region as well as the parafoveal region. We focused on the structure–function relationship in the central 10° visual field, where more than 30% of RGCs are located and only 12 VF test points fall. Therefore, VF defects in the perifoveal region were broadly regarded as PFS in our study.
A growing body of evidence has shown that the SAP 10-2 program is clinically useful for detecting early glaucomatous damage or progression on the macular area.[1,7,10,11] However, 10-2 VF cannot cover VF defects outside the central 10°. In this study, we found that the FDT 24-2 test was comparable to the SAP 10-2 test and better than the SAP 24-2 test in structure–function relationships for early glaucoma patients with isolated paracentral scotoma. We can consider FDT 24-2 tests as another good option for detecting and monitoring RGC loss on the macular area while not missing VF defects outside the central 10°.
Footnotes
Abbreviations: FDT = frequency doubling technology, GCIPL = ganglion cell-inner plexiform layer, MS = mean sensitivity, PFS = parafoveal scotoma, RGC = retinal ganglion cell, SAP = standard automated perimetry, VF = visual field.
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1940).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Schiefer U, Papageorgiou E, Sample PA, et al. Spatial pattern of glaucomatous visual field loss obtained with regionally condensed stimulus arrangements. Invest Ophthalmol Vis Sci 2010;51:5685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res 2013;32:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kolker AE. Visual prognosis in advanced glaucoma: a comparison of medical and surgical therapy for retention of vision in 101 eyes with advanced glaucoma. Trans Am Ophthalmol Soc 1977;75:539–55. [PMC free article] [PubMed] [Google Scholar]
- [4].Coeckelbergh TR, Brouwer WH, Cornelissen FW, et al. The effect of visual field defects on driving performance: a driving simulator study. Arch Ophthalmol 2002;120:1509–16. [DOI] [PubMed] [Google Scholar]
- [5].Fujita K, Yasuda N, Oda K, et al. [Reading performance in patients with central visual field disturbance due to glaucoma]. Nippon Ganka Gakkai Zasshi 2006;110:914–8. [PubMed] [Google Scholar]
- [6].Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol 1990;300:5–25. [DOI] [PubMed] [Google Scholar]
- [7].Park SC, Kung Y, Su D, et al. Parafoveal scotoma progression in glaucoma: Humphrey 10-2 versus 24-2 visual field analysis. Ophthalmology 2013;120:1546–50. [DOI] [PubMed] [Google Scholar]
- [8].Hood DC, Raza AS, de Moraes CG, et al. The nature of macular damage in glaucoma as revealed by averaging optical coherence tomography data. Transl Vis Sci Technol 2012;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jung KI, Park HY, Park CK. Characteristics of optic disc morphology in glaucoma patients with parafoveal scotoma compared to peripheral scotoma. Invest Ophthalmol Vis Sci 2012;53:4813–20. [DOI] [PubMed] [Google Scholar]
- [10].Hangai M, Ikeda HO, Akagi T, et al. Paracentral scotoma in glaucoma detected by 10-2 but not by 24-2 perimetry. Jpn J Ophthalmol 2014;58:188–96. [DOI] [PubMed] [Google Scholar]
- [11].Traynis I, De Moraes CG, Raza AS, et al. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol 2014;132:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Landers J, Goldberg I, Graham S. A comparison of short wavelength automated perimetry with frequency doubling perimetry for the early detection of visual field loss in ocular hypertension. Clin Experiment Ophthalmol 2000;28:248–52. [DOI] [PubMed] [Google Scholar]
- [13].Sample PA, Bosworth CF, Blumenthal EZ, et al. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci 2000;41:1783–90. [PubMed] [Google Scholar]
- [14].Patel A, Wollstein G, Ishikawa H, et al. Comparison of visual field defects using matrix perimetry and standard achromatic perimetry. Ophthalmology 2007;114:480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Redmond T, O’Leary N, Hutchison DM, et al. Visual field progression with frequency-doubling matrix perimetry and standard automated perimetry in patients with glaucoma and in healthy controls. JAMA Ophthalmol 2013;131:1565–72. [DOI] [PubMed] [Google Scholar]
- [16].Landers JA, Goldberg I, Graham SL. Detection of early visual field loss in glaucoma using frequency-doubling perimetry and short-wavelength automated perimetry. Arch Ophthalmol 2003;121:1705–10. [DOI] [PubMed] [Google Scholar]
- [17].Reus NJ, Lemij HG. The relationship between standard automated perimetry and GDx VCC measurements. Invest Ophthalmol Vis Sci 2004;45:840–5. [DOI] [PubMed] [Google Scholar]
- [18].Fan X, Wu LL, Ma ZZ, et al. Usefulness of frequency-doubling technology for perimetrically normal eyes of open-angle glaucoma patients with unilateral field loss. Ophthalmology 2010;117:1530–7. e1531–2. [DOI] [PubMed] [Google Scholar]
- [19].Meira-Freitas D, Tatham AJ, Lisboa R, et al. Predicting progression of glaucoma from rates of frequency doubling technology perimetry change. Ophthalmology 2014;121:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mwanza JC, Oakley JD, Budenz DL, et al. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci 2011;52:8323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shin HY, Park HY, Jung KI, et al. Comparative study of macular ganglion cell-inner plexiform layer and peripapillary retinal nerve fiber layer measurement: structure-function analysis. Invest Ophthalmol Vis Sci 2013;54:7344–53. [DOI] [PubMed] [Google Scholar]
- [22].Anctil JL, Anderson DR. Early foveal involvement and generalized depression of the visual field in glaucoma. Arch Ophthalmol 1984;102:363–70. [DOI] [PubMed] [Google Scholar]
- [23].Anderson AJ, Johnson CA, Fingeret M, et al. Characteristics of the normative database for the Humphrey matrix perimeter. Invest Ophthalmol Vis Sci 2005;46:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pinto LM, Costa EF, Melo LA, Jr, et al. Structure-function correlations in glaucoma using matrix and standard automated perimetry versus time-domain and spectral-domain OCT devices. Invest Ophthalmol Vis Sci 2014;55:3074–80. [DOI] [PubMed] [Google Scholar]
- [25].Garway-Heath DF, Holder GE, Fitzke FW, et al. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci 2002;43:2213–20. [PubMed] [Google Scholar]
- [26].Kim S, Lee JY, Kim SO, et al. Macular structure–function relationship at various spatial locations in glaucoma. Br J Ophthalmol 2015;99:1412–8. [DOI] [PubMed] [Google Scholar]
- [27].Lee BB. Paths to colour in the retina. Clin Exp Optom 2004;87:239–48. [DOI] [PubMed] [Google Scholar]
- [28].Raza AS, Cho J, de Moraes CG, et al. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol 2011;129:1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ohkubo S, Higashide T, Udagawa S, et al. Focal relationship between structure and function within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci 2014;55:5269–77. [DOI] [PubMed] [Google Scholar]
- [30].Rao HL, Qasim M, Hussain RS, et al. Structure-function relationship in glaucoma using ganglion cell-inner plexiform layer thickness measurements. Invest Ophthalmol Vis Sci 2015;56:3883–8. [DOI] [PubMed] [Google Scholar]
- [31].Anderson D, Patella V. Automated Static Perimetry. 2nd ed.St. Louis: Mosby; 1999. [Google Scholar]
- [32].Hayashi K, Araie M, Konno S, et al. Correlation of macular ganglion cell complex thickness with frequency-doubling technology perimetry in open-angle glaucoma with hemifield defects. J Glaucoma 2015;25:426–32. [DOI] [PubMed] [Google Scholar]
- [33].Wall M, Woodward KR, Doyle CK, et al. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Invest Ophthalmol Vis Sci 2009;50:974–9. [DOI] [PubMed] [Google Scholar]
- [34].Swanson WH, Horner DG, Dul MW, et al. Choice of stimulus range and size can reduce test-retest variability in glaucomatous visual field defects. Transl Vis Sci Technol 2014;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Swanson WH, Sun H, Lee BB, et al. Responses of primate retinal ganglion cells to perimetric stimuli. Invest Ophthalmol Vis Sci 2011;52:764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


