Supplemental Digital Content is available in the text
Keywords: advanced gastric cancer, meta-analysis, third-line chemotherapy
Abstract
Backgound:
Little information regarding to the survival advantage of third-line chemotherapy in advanced gastric cancer patients is available. The current study is designed to systematically review and perform meta-analysis on the effect of third-line chemotherapy on progressive or recurrent gastric cancer treatment.
Methods:
After thorough searching of online databases, total 20 articles were included into qualitative systematic review and 6 of them were used to conduct qualitative meta-analysis.
Results:
It was found that the third-line chemotherapy was superior to placebo or best supportive care in terms of prolonging median oval survival (OS) length and progress free survival (PFS) length (Hedges's g for OS = −0.315 ± 0.077, P < .001; and for PFS = −0.382 ± 0.098, P < .001). In addition, the third-line chemotherapy was favored (Hedges's g = 0.848, P < .001) in terms of overall survival rate (Hazard ratio = 0.679, 95% confidence interval: 0.565–0.816, P < .001) or tumor free survival rate (Hazard ratio = 0.561, 95% confidence interval: 0.444–0.709, P < .001).
Conclusion:
The third-line chemotherapy is superior to the best supportive care in advanced gastric cancer patients who had been pretreated with first-line and second-line chemotherapy.
1. Introduction
Although gastric cancer is relatively less common in the United States and other Western countries, where it has the 16th highest incidence rate of all cancers,[1,2] the incidence of gastric cancer is relatively high in Asian, especially in China. In this regard, gastric cancer is the third leading cause of cancer mortality in China.[3,4] Specifically, age-standardized incidence rates of gastric cancer in Chinese population were 41.9 (per 100,000) for male and 19.5 for female in the year 2000, and were 37.1 for the male and 17.4 for the female in 2005. Furthermore, 0.3 million deaths and 0.4 million new cases from gastric cancer ranked the third most common cancer in China in 2005.[5,6]
Although gastric cancer may be cured by surgical resection if it is diagnosed at early-stage, unfortunately, 80% to 90% of patients are diagnosed with advanced-stage disease,[1,2] when surgery and local therapies are no longer effective, and thus, the prognosis for most of gastric cancer patients is poor. The 5-year relative survival rate for patients diagnosed with localized gastric cancer is 64.1%, but the survival rate dramatically declines to only 4.3% for the patients with metastasis of gastric cancer.[7] For the patients with advanced or metastatic gastric cancer or for postoperative recurrence, platinum plus fluoropyimidine was recommended as first-line therapy by the NCCN (National Comprehensive Cancer Network) guidelines.[8] However, many patients experience disease progression or recurrence after treatment with the first-line chemotherapy, and limited therapeutic options were available for those patients with progression or recurrence. Therefore, second-line chemotherapy with irinotecan and taxane has been accepted as salvage treatment options based on the lack of cross-resistance between these 2 reagents.[2]
Phase II or III trials and retrospective analyses have recently provided evidence that second-line or further salvage chemotherapy is more advantage than supportive care.[9–11] Although these clinical trials have been demonstrated that second-line chemotherapy can significantly prolong the survival of patients with progressive or recurrent gastric cancer, compared to that of the patients with supportive care alone,[2,11] there is controversy over the benefit of third-line chemotherapy due to the lack of evidence. In this regard, little information concerning the survival advantage of third-line chemotherapy is available. The current study is, therefore, designed to systematically review and perform meta-analysis on the effect of third-line chemotherapy on progressive or recurrent gastric cancer treatment.
2. Materials and methods
2.1. Data sources
Relevant literature up to August 2016 was searched in the sites of PubMed, Embase, and Web of Science with the following phrases: “third-line chemotherapy” and “gastric cancer,” or “salvage therapy” and “gastric cancer.” The search was limited to English and Chinese, and relevant studies were also identified by hand-searching the references of included articles.
2.2. Inclusion criteria
Studies were included in the current systematic review if: (1) clinical studies on the treatment of gastric cancer with third-line chemotherapy; (2) studies with full text articles.
2.3. Data extraction
Information and data were carefully extracted from all included literature. Data include study name (the first author name), publication year, reagents for the third-line chemotherapy, total number of cases for the third-line chemotherapy and best supportive care (BSC) or placebo treatment, median overall survival (OS) months, median progress free survival (PFS), and adverse effect of third-line chemotherapy.
2.4. Statistical analysis
Median overall survival (OS) month and progress free survival (PFS) month, number of cases and P value were used for the data entry. Hazard ratio (HR) and 95% CIs as relevant effect measures on OS and PFS was estimated if the HR and P value were directly provided by the authors. Odds ratio (OR) and 95% CI as relevant effect measures of the third-line therapy on OS and PFS was estimated if HR and P value were not provided by the authors. The strength of third-line therapeutic effect on advanced gastric cancer was measured by Hedges's g. A fixed effect model was adopted when no heterogeneity was observed among the studies. Otherwise, a random effect model was applied. The heterogeneity between studies was assessed by the Q-test and I2 statistic, and P < .10 and I2 > 50% was considered as heterogeneous between the studies.[12] All meta-analysis was performed using the Comprehensive Meta-analysis software (Version 3, NJ).
2.5. Ethical approval and patient consent
This study does not require ethical approval and patient consent because the study was a systematic review of previous studies and does not involve patients.
3. Results
3.1. Study features
The process of selecting literature and final selection was outlined as in Fig. 1. After careful reading “abstract” of publications, total 71 full-text articles were retrieved. The retrieved full-text articles were then independently assessed by 2 investigators (YZ and X-QZ). As shown in Table 1, total 6 articles were included in the meta-analysis (quantitative synthesis)[13–18] and 20 articles were included in the systematic review (qualitative synthesis). Among the 20 articles for systematic review and meta-analysis, 8 articles were from South Korea,[9,14,15,19–23] 6 articles from Japan,[24–29] 3 articles from China,[16,17,30] 2 articles from Italy,[13,31] and 1 from Germany.[18]
Figure 1.
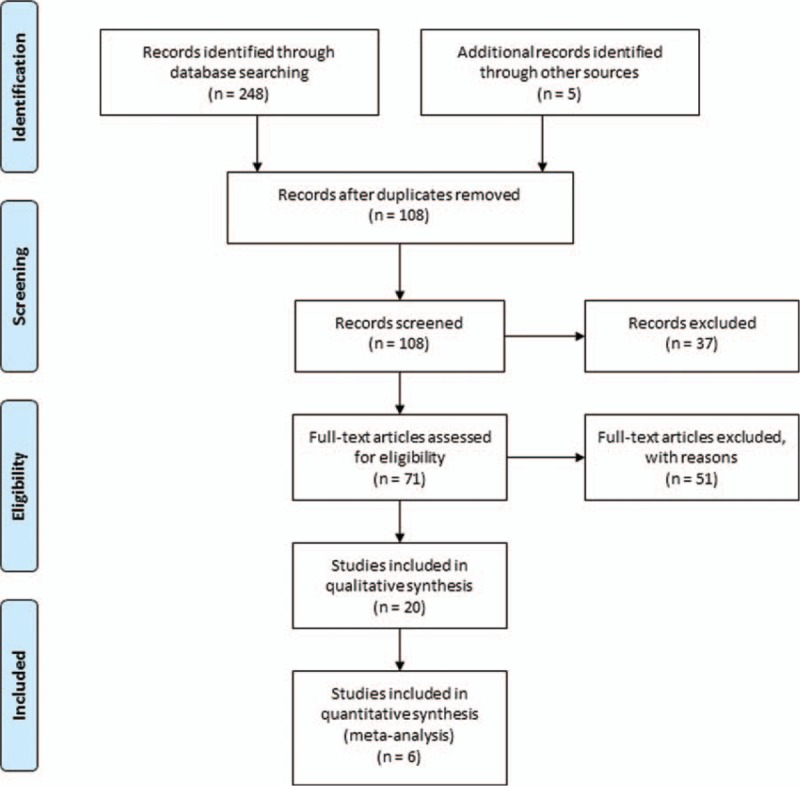
Flow diagram of literature search and eligible publication selection.
Table 1.
Articles enrolled into the current systematic review and meta-analysis.
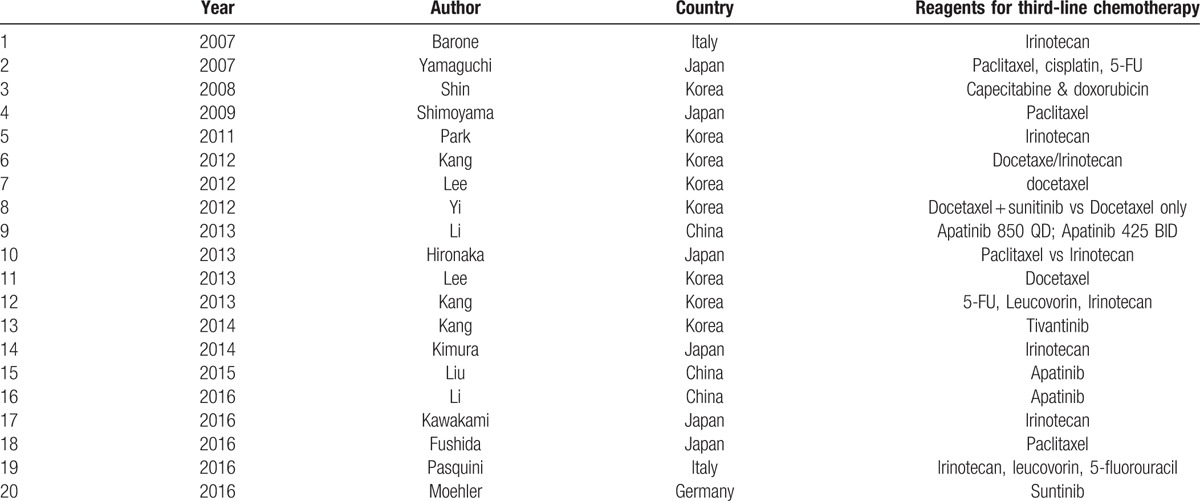
Most commonly used agent for third-line chemotherapy was Paclitaxel followed by Irinotecan or tyrosine kinase inhibitors (apatinib or sunitinib, Table 1).
3.2. Efficacy of third-line chemotherapy
Of the 20 articles selected for systematic review, 6 were studied with randomized placebo control or best supportive care control, 14 studies reported efficacy of third-line chemotherapy without comparison to control. Lee and colleagues[22] retrospectively evaluated the efficacy and toxicity of single reagent, docetaxel (75 mg/m2 on day 1 every 3 weeks), in 33 cases of advanced gastric cancer patients who was not responsible to oxaliplatin with leucovorin and 5-fluorouracil, or to irinotecan with leucovorin and 5-fluorouracil. They reported that an overall response was 15%, median time to progression was 2.1 months (95% CI, 1.63–2.58), and median overall survival time was 4.7 months (95% CI, 3.2–6.2).
Results of the quantitative meta-analysis of the 6 RCTs showed that average of the median overall survival (OS) length and progress free survival (PFS) length were significantly longer in the patients treated with third-line chemotherapy compared to the patients received best supportive care (Hedges's g for OS = 0.315 ± 0.077, P < .001; I2 = 45.6%, P = .67, Fig. 2; Hedges's g for PFS = 0.382 ± 0.098, P < .001; I2 = 87.2%, P = .003, Fig. 3), and that third-line therapy was favored in terms of overall survival rate (Hazard ratio = 0.679, 95% confidence interval: 0.565–0.816, P < .001; I2 = 0.12, P = .424, Fig. 4) or tumor free survival rate (Hazard ratio = 0.561, 95% confidence interval: 0.444–0.709, P < .001; I2 = 82.9, P = .004, Fig. 5).
Figure 2.
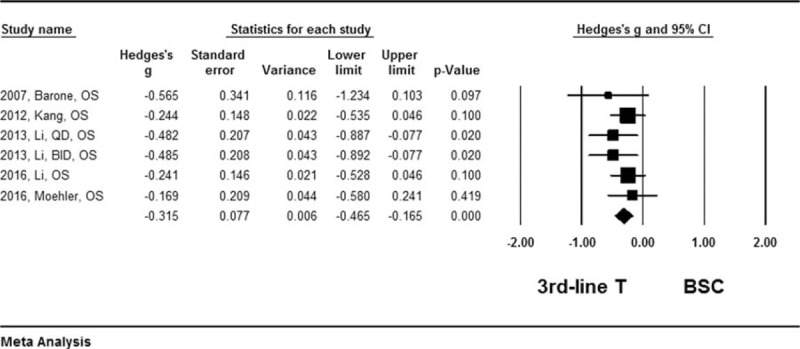
Forest plot for median survival length of overall survival. A fixed effect model was used due to non-significant heterogeneity of publications (I2 = 45.6%, P = .067). The effect size was assessed by Hedges's g and 95% CI, and the median survival length was in favors of third-line chemotherapy (Hedges's g = −0.315 ± 0.077, P < .001). 3rd-line T = third-line chemotherapy, BSC = best supportive care, CI = confidence interval.
Figure 3.
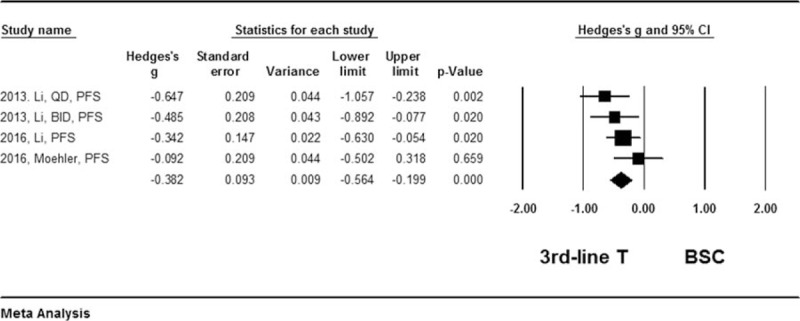
Forest plot for median survival length of progress free survival. A random effect model was used due to non-significant heterogeneity of publications (I2 = 87.2%, P = .003). The effect size was assessed by Hedges's g and 95% CI, and the median survival length was in favor of third-line chemotherapy (Hedges's g = −0.382 ± 0.093, P < .001). 3rd-line T = third-line chemotherapy, BSC = best supportive care, CI = confidence interval.
Figure 4.
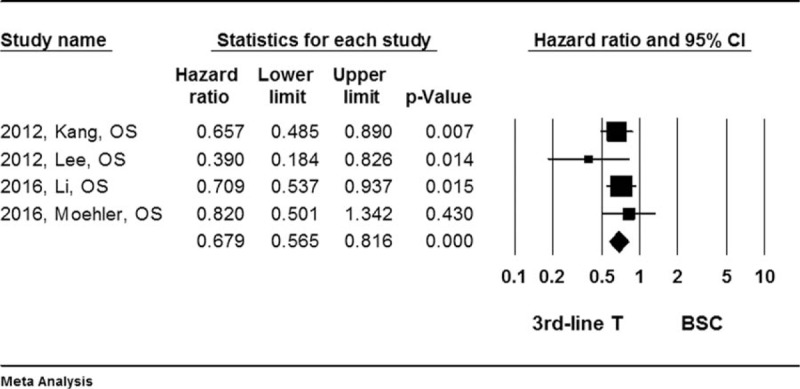
Forest plot for hazard ratio (HR) of overall survival (OS) rate. A fixed effect model was used due to non-significant heterogeneity of publications (I2 = 0.12, P = .424). The effect size was assessed by hazard ratio (HR) and 95% CI, and the OS rate was in favors of third-line chemotherapy (HR = 0.679, 95%CI: 0.565–0.816, P < .001). 3rd-line T = third-line chemotherapy, BSC = best supportive care, CI = confidence interval, HR = hazard ratio, OS = overall survival.
Figure 5.
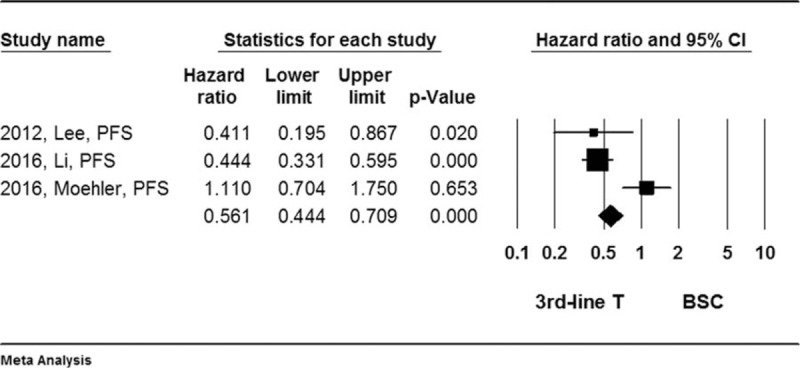
Forest plot for hazard ratio (HR) of progress free survival (PFS) rate. A random effect model was used due to non-significant heterogeneity of publications (I2 = 82.9%, P = .04). The effect size was assessed by hazard ratio (HR) and 95% CI, and the OS rate was in favor of third-line chemotherapy (HR = 0.561, 95%CI: 0.444–0.709, P < .001). 3rd-line T = third-line chemotherapy, BSC = best supportive care, CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progress free survival.
Of the 14 non-controlled clinical studies, while 1 study reported that third-line therapy did not have objective response,[19] the rest 12 studies indicated that the third-line chemotherapy seemed to be able extend patients’ life. For instance, Kang et al[14] treated 158 cases of advanced gastric cancer patients with the combination chemotherapy of 5-fluorouracil (5-FU), leucovorin, and irinotecan (FOLFIRI regimen) after failure of fluoropyrimidine, platinum, and taxane, and found that overall response rate was 9.6% in patients with measurable lesions, the median overall survival (OS) and progression free survival (PFS) were 5.6 months (95% CI, 4.7–6.5) and 2.1 months (95% CI, 1.7–2.5), respectively. Most recently, Fushida et al[28] examined paclitaxel plus valproic acid (an inhibitor of histone deacetylase) versus paclitaxel alone as second- or third-line therapy for advanced gastric cancer in a randomized phase II clinical trial and found that there was no statistically significant difference between paclitaxel alone and paclitaxel plus valproic acid. Similarly, Pasquini et al[31] reported 42% of disease control rate by third-line chemotherapy with irinotecan plus 5-Fluorouracil (FOLFIRI) in gastric cancer patients pretreated with platinum derivatives, fluoropyrimidine, and taxanes.
3.3. Side effects of the third-line chemotherapy
Although most of the 19 articles enrolled into the current systematic review and meta-analysis reported variety kinds of hematological and non-hematological toxicities, the most common adverse events associated with third-line chemotherapy was neutropenia, anemia, thrombocytopenia, and anorexia.[19,22,24–26,28]
4. Discussion
Survival outcomes of gastric cancer are poor. Although salvage chemotherapy after first-line chemotherapy is currently used in patients with advanced gastric cancer, it is lack of standard treatment regimens for these patients. This review systematically reviewed and analyzed outcomes of the third-line chemotherapy in the patients with progressive or recurrent gastric cancer. Meta-analysis on the results of the 5 randomized clinical trials revealed that average of the median overall survival (OS) length and progress free survival (PFS) length were significantly longer in the patients treated with third-line chemotherapy compared to the patients received best supportive care, suggesting salvage chemotherapy is superior to best supportive care in advanced gastric cancer patients.
Since early 1990s, it has been accepted that palliative chemotherapy can significantly prolong the survival of patients with advanced gastric cancer, compared to supportive care alone.[32,33] The second-line chemotherapy has been proved to be effective in the patients who were not responsive to first-line chemotherapy.[9,28,34] However, the third-line chemotherapy and its regimens for progressive gastric cancer after the second-line chemotherapy is largely not evidence based, and treatment varies considerably in terms reagent selection and treatment plan. The current systematic review found that the most commonly used single reagent for third-line chemotherapy is irinotecan or paclitaxel/docetaxel. Recently, Fushida et al[28] compared the therapeutic effect of paclitaxel (PTX) alone versus PT plus valproic acid (VPA), an inhibitor of histone deacetylase in 66 randomly assigned gastric cancer patients, and found that no statistically significant difference was observed between PTX alone and PTX plus VPA. These findings indicated that the third line chemotherapy reagent remains to be standardized in the near future.
In the treatment of advanced or recurrent gastric cancer, the prolongation of survival as well as quality of patient's life depends on not only the effectiveness of second-line or third-line chemotherapy, but also the side-effect of the cytotoxic drugs. Systematic review on the 20 studies of salvage chemotherapy enrolled into this review indicated that the most common hematological adverse events associated with third-line chemotherapy were neutropenia, anemia, and thrombocytopenia, and non-hematological toxicities were anorexia, nausea and vomiting, and fatigue.[19,22,24–26,28] Even if most of the patients experienced aforementioned adverse effect, they were still able to continue and complete the treatment with third-line chemotherapy. However, none of the studies, which were included in this review, evaluated life quality of the patient. Therefore, whether it is worthy to sacrifice patient's quality of life in exchange of prolonging several months of survival remains to be further evaluated in the future.
There are limitations in the current review and meta-analysis. First, the patients included may not be characteristic of the overall population of patients with gastric cancer because limited number of cases was enrolled into each study. Second, reagents and regimens used as the third-line chemotherapy in the studies enrolled into this review were not standardized. Third, limited number of randomized clinical trials as well as small number of cases in each trial was used to conduct meta-analysis. Fourth, there was publication bias in those publications enrolled into this systematic review and meta-analysis as evidenced by funnel plot (Supplement Figure 1). Finally, patient selection for third-line chemotherapy in each study was not standardized and the life quality of the patients was not evaluated.
Although the current study suggests that third-line chemotherapy is effective in advanced gastric cancer patient, side effects resulted from systemic chemotherapy may preclude large number of patients from the salvage chemotherapy. Thus, it is necessary to explore alternative reagents and drug delivery ways. In this regard, biological reagents such as tyrosine kinase inhibitors and immune regulators are potentially used as third-line chemotherapy in advanced gastric cancer. Furthermore, based on survival predictors including performance status, chemotherapy-free interval, and response duration, local drug delivery strategy such as hyperthermic intraperitoneal chemotherapy (HIPEC) for progressive or recurrent gastric cancer with malignant ascites can be considered as alternative and palliative therapy.
Taken together, irrespective of the positive impact of any presently available chemotherapy or immunotherapy, the prognosis of patients with advanced gastric cancer remains desperate, with less than a year of median survival rate. Although the benefit of salvage therapy with third-line chemotherapy may be greater or variable in individual patients, appropriate treatment measures including selection of patients and reagents should be standardized to become the principles of palliative care.
Supplementary Material
Footnotes
Abbreviations: BSC = best supportive care, HIPEC = hyperthermic intraperitoneal chemotherapy, OS = oval survival, PFS = progress free survival, VPA = valproic acid.
Funding: This study was supported by the Scientific Research Fund of Zhejiang Education Department (No. Y201224441).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. doi:10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- [2].Hess LM, Michael D, Mytelka DS, et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer 2016;19:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao G, Ford ES, Ahluwalia IB, et al. Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. J Gen Intern Med 2009;24:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu J, Sun L, Gong Y. Risk factors of precancerous gastric lesions in a population at high risk of gastric cancer. Chin J Cancer Res 2010;22:267–73. [Google Scholar]
- [5].Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol 2006;12:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bu Z, Ji J. A current view of gastric cancer in China. Transl Gastrointest Cancer 2013;2:1–4. [Google Scholar]
- [7].Howlader N, et al. National Cancer Institute, Available at: http://seer.cancer.gov/csr/1975_2013, Accessed 2016. [Google Scholar]
- [8].NCCN. in V.1.2014. Available at: http://www.nccn.org., Accessed 2014. [Google Scholar]
- [9].Park JS, Lim JY, Park SK, et al. Prognostic factors of second and third line chemotherapy using 5-fu with platinum, irinotecan, and taxane for advanced gastric cancer. Cancer Res Treat 2011;43:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–14. [DOI] [PubMed] [Google Scholar]
- [11].Koo DH, Ryu MH, Ryoo BY, et al. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer 2015;18:346–53. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [13].Barone C, Basso M, Schinzari G, et al. Docetaxel and oxaliplatin combination in second-line treatment of patients with advanced gastric cancer. Gastric Cancer 2007;10:104–11. [DOI] [PubMed] [Google Scholar]
- [14].Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–8. [DOI] [PubMed] [Google Scholar]
- [15].Lee MJ, Hwang IG, Jang JS, et al. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat 2012;44:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [17].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [18].Moehler M, Gepfner-Tuma I, Maderer A, et al. Sunitinib added to FOLFIRI versus FOLFIRI in patients with chemorefractory advanced adenocarcinoma of the stomach or lower esophagus: a randomized, placebo-controlled phase II AIO trial with serum biomarker program. BMC Cancer 2016;16:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shin SJ, Jeung HC, Ahn JB, et al. Capecitabine and doxorubicin combination chemotherapy as salvage therapy in pretreated advanced gastric cancer. Cancer Chemother Pharmacol 2008;61:157–65. [DOI] [PubMed] [Google Scholar]
- [20].Yi JH, Lee J, Lee J, et al. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer 2012;106:1469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kang EJ, Im SA, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013;16:581–9. [DOI] [PubMed] [Google Scholar]
- [22].Lee JH, Kim SH, Oh SY, et al. Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med 2013;28:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kang YK, Muro K, Ryu MH, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs 2014;32:355–61. [DOI] [PubMed] [Google Scholar]
- [24].Yamaguchi K, Nakagawa S, Yabusaki H, et al. Combination chemotherapy with 5-fluorouracil, cisplatin and paclitaxel for pretreated patients with advanced gastric cancer. Anticancer Res 2007;27:3535–9. [PubMed] [Google Scholar]
- [25].Shimoyama S, Kaminishi M. A three-year survivor case of gastric cancer with peritoneal dissemination--an outpatient with second-line weekly paclitaxel. Gan To Kagaku Ryoho 2008;35:1003–7. [PubMed] [Google Scholar]
- [26].Hironaka S, Ueda S, Yasul H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–44. [DOI] [PubMed] [Google Scholar]
- [27].Kimura M, Usami E, Kanematsu T, et al. Safety and continuity of second- and third-line therapy with paclitaxel or irinotecan for advanced and recurrent gastric cancer. Mol Clin Oncol 2014;2:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fushida S, Kinoshita J, Kaji M, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second- or third-line therapy for advanced gastric cancer: a randomized Phase II trial. Drug Des Devel Ther 2016;10:2353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawakami T, Machida N, Yasui H, et al. Efficacy and safety of irinotecan monotherapy as third-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol 2016;78:809–14. [DOI] [PubMed] [Google Scholar]
- [30].Liu L, Yu H, Huang L, et al. Progression-free survival as a surrogate endpoint for overall survival in patients with third-line or later-line chemotherapy for advanced gastric cancer. Onco Targets Ther 2015;8:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pasquini G, Vasile E, Caparello C, et al. Third-line chemotherapy with irinotecan plus 5-fluorouracil in caucasian metastatic gastric cancer patients. Oncology 2016;91:311–6. [DOI] [PubMed] [Google Scholar]
- [32].Pyrhonen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163–8. [DOI] [PubMed] [Google Scholar]
- [34].Teker F, Yilmaz B, Kemal Y, et al. Efficacy and safety of docetaxel or epirubicin, combined with cisplatin and fluorouracil (DCF and ECF), regimens as first line chemotherapy for advanced gastric cancer: a retrospective analysis from Turkey. Asian Pac J Cancer Prev 2014;15:6727–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


