Abstract
Rationale:
Endobronchial ultrasound as a powerful diagnostic technology can also be used to perform intratumoral chemotherapy for extraluminal tumor.
Patient concerns:
A 69-year-old man with chronic obstructive pulmonary disease (COPD) presented with worsening dyspnea. A pulmonary function test showed severe airway obstruction and that forced expiratory volume in 1 s was 43% of the predicted value after a bronchodilator.
Diagnoses:
A bronchoscopy and histopathological analyses revealed a squamous cell carcinoma mostly located outside of the lumen with central airway obstruction.
Interventions:
Due to the poor pulmonary function, he cannot tolerate conventional active treatments, such as surgery, full dose systemic chemotherapy, or radiotherapy. Local treatments including argon plasma coagulation, cryotherapy, and bronchoscopic endobronchial intratumoral chemotherapy with cisplatin were performed to debulk intraluminal component of the tumor and recanalize occlusive airways in the left upper lobe. Convex-probe endobronchial ultrasound-guided transbronchial needle injection (EBUS-TBNI) delivered cisplatin into the extraluminal component of the tumor to relieve the symptom of dyspnea and opened up the opportunity for systemic chemotherapy without severe systemic complications.
Outcomes:
The patient had a good response to the comprehensive therapy of 4 cycles of low-dose intravenous chemotherapy and bronchoscopic interventions.
Lessons:
EBUS-TBNI is proven an effective and safe method to treat inoperable extraluminal central pulmonary carcinoma complicated with severe COPD. In the future, EBUS-TBNI may offer more treatment indications outlined in the existing publications.
Keywords: chemotherapy, COPD, EBUS-TBNI, NSCLC
1. Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer are highly lethal disease and worldwide health problems, which were among the top 10 leading causes of death. Patients with COPD are at high risk of developing lung cancer. Approximately 30% of patients with lung cancer will develop malignant endobronchial obstruction, which often lead to postobstruction pneumonia and eventually to death.[1] Lung cancer developing in COPD patients has a lower survival rate.[2] The patients often cannot tolerate surgery due to poor lung function and symptoms of airway obstruction.[3] The management of patients with COPD and obstructive lung cancer remains a big challenge.
Tumor debulking and airway recanalization are the main strategies to prolong survival and improve the quality of life in patients with obstructive inoperable lung cancer. The commonly used bronchoscopic interventions include electrocautery, argon plasma coagulation (APC), cryotherapy, photodynamic therapy, laser, stents, and brachytherapy.[4] Each method has its value and special indications. A multimodality therapy is an optimal choice for long-term survival, including the use of different systemic or local treatments and the combination of systemic treatment and local interventions. The survivals of patients with malignant airway obstruction who received therapeutic bronchoscopy and systemic chemotherapy are similar to those without central airway obstruction treated with chemotherapy alone, regardless of the histologic subtype.[5]
During the past few years, an increasing number of clinical data have demonstrated that bronchoscopic endobronchial intratumoral chemotherapy (EITC) is a feasible and effective way to improve the potency of obstructive airway for intraluminal tumor.[6,7] For extraluminal tumor, it is difficult to precisely locate the extent of the tumor. The endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was first induced by Mark Krasnik in 2003.[8] The usefulness of EBUS-TBNA for diagnosis of solid lesions and lymph node staging and recurrence of lung cancer is well known during the past 12 years.[9,10] However, endobronchial ultrasound-guided transbronchial needle injection (EBUS-TBNI) is a brand new intervention to treat airway obstruction and debulk extraluminal tumor. Here, we report the novel use of EBUS-TBNI for the local treatment in a severe COPD patient with extraluminal central pulmonary squamous cell carcinoma.
2. Case description
A 69-year-old man diagnosed with COPD, presented to the hospital with symptoms of cough, sputum production, fever, and worsening dyspnea for 3 weeks. He is a nonsmoker and has no significant medical history and family history. He is not taking any medications and has no known allergies. Physical examination revealed a normal temperature of 36.6°C; respiration, 20 breaths/min; pulse, 82 beats/min; blood pressure 119/75 mm Hg; and SaO2 97%. An arterial blood gas analysis obtained with the patient receiving oxygen via nasal cannula at 2 L/min showed pH 7.378, pCO2 39.4 mm Hg, and pO2 98 mm Hg. On pulmonary auscultation, there were bilateral decreased breath sounds with rhonchi in the left upper lung field.
A computed tomography (CT) scan of the chest was performed in a local hospital a week ago revealed a large left hilar mass. Pulmonary function test identified a severe mixed obstructive and restrictive abnormality. Spirometry values were forced vital capacity (FVC) 3.01 L (71% predicated); forced expiratory volume in 1 s (FEV1) 1.24 L (43% predicated); FEV1/FVC 41%; FEV1. The patient is unable to complete the 12-s maximal voluntary ventilation (MVV) test. Several tumor markers for lung cancer are elevated. The serum values of carcinoembryonic antigen, neuron-specific enolase, and squamous cell carcinoma antigen are 7.18 ng/mL (reference range: 0.5–5.0 ng/mL), 25.24 ng/mL (reference range: 0.05–16.3 ng/mL), and 1.9 ng/mL (reference range: 0.1–1.5 ng/mL), respectively. A bronchoscopy was carried out on the day after admission and showed that the origin of the left lingular bronchus was completely occluded (Fig. 1A), with stenosis of the left upper lobe bronchus and the presence of a mucosal hypertrophy and unobstructed left lower lobe bronchi. A diagnostic biopsy confirmed the presence of a squamous cell carcinoma. A following positron emission tomography-CT scan showed a high metabolic activity (standardized uptake value max. = 9.3) of a 4.1 cm × 3.1 cm tumor mass (Fig. 2A), no fluorodeoxyglucose accumulation in the mediastinal lymph nodes and no distant metastases.
Figure 1.
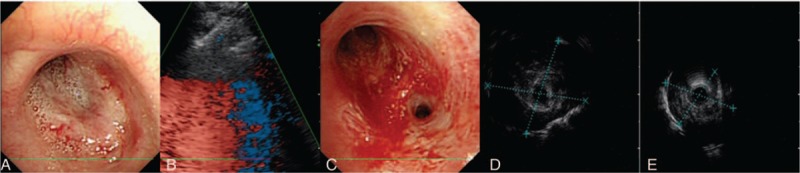
Bronchoscopy and endobronchial ultrasound images during the local intratumoral treatments. (A) A bronchoscopy image in the left upper lobe before local treatment with lingular segment totally collapsed. (B) Endobronchial ultrasound image during the injection of cisplatin through endobronchial ultrasound-guided transbronchial needle injection (EBUS-TBNI). (C) A bronchoscopy image shows a patent left upper lobe bronchus with lingular segment opened after 4 weeks of initial EBUS-TBNI treatment. A radial probe EBUS in lingular segment shows an abnormal region measuring 1.8 cm × 1.9 cm (D) and 0.8 cm × 1.3 cm (E) in the third and fourth bronchoscopy, respectively.
Figure 2.
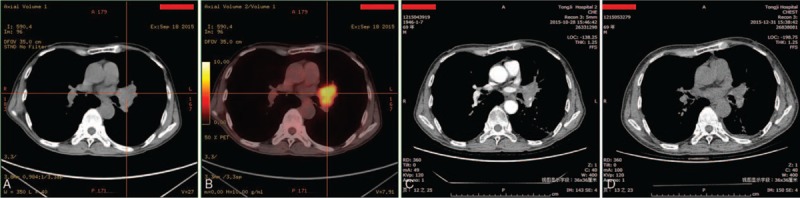
Positron emission tomography-computed tomography (PET-CT) and CT images of the region of lung cancer. (A) PET-CT scan with evidence of lung cancer in the left upper lobe upon diagnosis. (B) A CT scan was performed 1 week after the second endobronchial ultrasound-guided transbronchial needle injection (EBUS-TBNI) intratumoral treatment. (C) A CT scan at 1 month after the fourth EBUS-TBNI intratumoral treatment.
Because of the respiratory symptoms and the risks of postoperative pulmonary complication, the patient was reluctant to surgery treatment. The KPS and Zubrod scores of the patient were 50 and 3, respectively. Thus, the patient cannot tolerate other conventional active treatments including chemotherapy and radiotherapy.[11]
Local treatment was determined to relieve dyspnea and increase survival for this patient. Since the tumor mostly grows outside the bronchial lumen, conventional endobronchial therapy such as APC or cryotherapy is not able to eliminate the tumor. EBUS-TBNI delivery of cisplatin at the University of Vermont College of Medicine was clinically proven to be an safe and effective treatment. After reviewing cases of local control of lung cancer, a proposal was brought to the table that EBUS-TBNI to deliver cisplatin into the tumor located outside of the left lobe bronchi to debulk tumor.
3. Procedure methods
Bronchoscopic evaluation revealed complete lingular obstruction with partial left upper bronchus obstruction. Bronchoscopy with APC and intratumoral injection cisplatin (2.5 mL and 4 mg/mL) using an NM-200L needle (Olympus America, Corporate Center Drive, Melville, NY) were performed to ablate the intraluminal component of the tumor.[6,12]
For eliminating the component outside the lumen, EBUS-guided extraluminal intratumoral chemotherapy was performed 4 times within 6 weeks. Under convex-probe EBUS (Olympus America, NA-201SX-4021), a well-defined abnormal echoic mass was identified outside of the junction of the upper and lower left lobe bronchi. A 21-gauge EBUS needle was placed into the mass outside the bronchus (Fig. 1B). Doppler ultrasound verified that there was no vascular signal within the target area. About 1.5 mL cisplatin (4 mg/mL) was then injected at 4 sites within the mass. No leakage of the cisplatin into the airway was noted.
According to the proposal, intratumoral injections were commonly performed weekly for 1 to 2 months.[13] The patient was too debilitated and reluctant to be treated once a week. Therefore, this procedure was repeated 3 additional times within 6 weeks. At the third EBUS-TBNI therapy about 4 weeks later, the reopen of the lingular bronchus was noted even though the lumen was narrowed as aperture (Fig. 1C). Radial EBUS was able to detect an abnormal region measuring 1.8 cm × 1.9 cm around the proximal lingular bronchus. So, the additional cryotherapy was performed into the lingular bronchus. The fourth therapy after 6 weeks were EBUS-guided chemotherapy described above in conjunction with cryotherapy. All of the local treatments were well tolerated with minimal nonsystemic complications. Concurrent 4 cycles of low-dose intravenous chemotherapy (gemcitabine and cisplatin) every 4 weeks were pursed while the patient status got obvious improvement.
4. Outcome
On admission, the patient was unable to complete the 6-min walk test. After all of the procedures, the symptom of dyspnea was obviously alleviated. The mean distance walked was 309 m. The post-treatment KPS and Zubrod scores of the patient were re-evaluated and remarkably improved, which were 70 and 2, respectively. Pulmonary function test revealed an increase in the FEV1/FVC ratio (51.89%) and FEV1 (1.56 L), as well as an MVV of 64.01 L/min (55.9%). The serum values of carcinoembryonic antigen, neuron-specific enolase and squamous cell carcinoma antigen were decreased to the normal range. Surveillance CT at 3 weeks confirmed partial debulking of the tumor and detected no intrapulmonary metastasis (Fig. 2B). A chest CT scan obtained 16 weeks after the first local treatment revealed >50% tumor shrinkage (Fig. 2C). Bronchoscopy showed that airway patency in the left upper lobe bronchus was re-established with, cryotherapy and EITC (Fig. 1C). Radial probe EBUS were performed during the therapeutic bronchoscopic modalities to determine the volume of the tumor around lingular segment of upper lobe of left lung. The size of abnormal regions under ultrasound was 1.8 cm × 1.9 cm and 0.8 cm × 1.3 cm in the third and fourth bronchoscopy, respectively (Fig. 1D and E).
Two months after completion of intratumoral chemotherapy via EBUS-TBNI, a CT scan revealed no increase in the size of the mass in the left upper lobe. Bronchoscopy performed at the same time revealed a patent left upper lobe bronchus.
5. Discussion
Central airway obstruction is a severe and potentially life-threatening complication of lung cancer. Surgery is the gold standard treatment of lung cancer with malignant airway obstruction. Patients with technically inoperable (tracheal or proximal main-stem malignant neoplasm) or late-stage cancer or severe underlying obstructive pulmonary disease are not surgical candidates. Compared with conventional systemic chemotherapy or radiotherapy, local bronchoscopic treatments have a better performance in rapid restoration of airway patency in patients with malignant airway obstruction.[14]
Intratumoral chemotherapy is considered as a neoadjuvant chemotherapy of nonsmall lung cancer and plays an considerable role in increasing survival in patients. Endobronchial intratumoral chemotherapy is available for patients with all stages of nonsmall cell lung cancer (NSCLC) (stages I–IV). The procedure is regarded as safe and well-tolerated without serious systemic complications. The mode of injection varies depend on the location of the tumor. The needles were inserted directly into the bronchoscopically visible mass and inserted into the submucosal mass at an oblique angle. In case of extraluminal diseases (a mass or lymph nodes) that cause a compressive airway obstruction, the needle should be inserted to the wall at an angle of about 60° to 90°.[7] When the bronchial mucosa and lumen appears normal at endoscopy, it is difficult to identify the location of injection with the aid of CT scan. Furthermore, for injecting the drugs into extraluminal tumor, 21 gauge and 15 mm or longer needles are required. EBUS-TBNI is a more safe and effective method for delivering drugs with a longer needle length. For example, the maximum extruding stroke of NA-201SX-4021 used in this case is 40 mm. Real-time ultrasound guidance provided a visualization of parabronchial anatomy and a guarantee for optimal site selection to deliver the drug, reducing the risk of potential side effects by avoidance of regional vasculature.
This patient was stage III COPD with severe bilateral emphysema, at risk of poor maintenance of endurance activity and quality of life. Performance status score, including KPS and Zubrod/Eastern Co-operative Oncology Group/WHO score, is shown to be correlated with overall survival of patients with NSCLC.[11] A patient with a performance status of 3 is advised to take palliative care rather than active treatment. The initial KPS and Zubrod scores of the patient on admission were 50 and 3, respectively, implying that he was not suitable for surgery, chemotherapy, or radiation. The interventional bronchoscopy significantly improved performance status of the patient. The goal of EBUS-TBNI for intratumoral therapy in the prior report is to achieve local control of recurrent lung cancer.[15] In this case, the use of EBUS-TBNI in this patient is committed to relieving the symptom of dyspnea and opening up the opportunity for systemic treatments. The tumor treated with combination of local interventions and systemic chemotherapy had a good response, 50% reduction of tumor. The completely occluded left upper lobe bronchus was fully patent.
There are some limitations in the application of EBUS-TBNI. Certain anatomical area is invisible and undetectable due to a 30° to 35° forward oblique view and 120° scope head flexion of EBUS bronchoscope, such as the upper lobe.[16] The effect of EBUS-TBNI for intratumoral treatment varies on the technical skills of the operators. However, most of the chest physicians are lack of training and practice due to the high cost of the equipment, which slows down its development.[17,18]
EBUS has great potential for development and became more promising in the expanding application filed. EBUS-TBNI is a new effective method for tumor debulking and airway recanalization in inoperable COPD patients with lung cancer. Further trials are warranted to evaluate the effectiveness and safety of EBUS-TBNI and determine the optimal individualized treatment.
6. Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
Footnotes
Abbreviations: APC = argon plasma coagulation, COPD = chronic obstructive pulmonary disease, CT = computed tomography, EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration, EBUS-TBNI = endobronchial ultrasound-guided transbronchial needle injection, EITC = bronchoscopic endobronchial intratumoral chemotherapy, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, MVV = maximal voluntary ventilation, NSCLC = nonsmall cell lung cancer.
This project was supported by grants from the Natural Science Foundation of China (81470002 and 81270106) and the Ministry of Science and Technology of China (2012BAI05B00).
The authors have no conflicts of interest to disclose.
References
- [1].Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg 2010;11:425–8. [DOI] [PubMed] [Google Scholar]
- [2].Sekine Y, Katsura H, Koh E, et al. Early detection of COPD is important for lung cancer surveillance. Eur Respir J 2012;39:1230–40. [DOI] [PubMed] [Google Scholar]
- [3].Raviv S, Hawkins KA, DeCamp MM, Jr, et al. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med 2011;183:1138–46. [DOI] [PubMed] [Google Scholar]
- [4].Daniels JM, Sutedja TG. Detection and minimally invasive treatment of early squamous lung cancer. Ther Adv Med Oncol 2013;5:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chhajed PN, Baty F, Pless M, et al. Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest 2006;130:1803–7. [DOI] [PubMed] [Google Scholar]
- [6].Mehta HJ, Begnaud A, Penley AM, et al. Restoration of patency to central airways occluded by malignant endobronchial tumors using intratumoral injection of cisplatin. Ann Am Thorac Soc 2015;12:1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Celikoglu F, Celikoglu SI, York AM, et al. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer 2006;51:225–36. [DOI] [PubMed] [Google Scholar]
- [8].Krasnik M, Vilmann P, Larsen SS, et al. Preliminary experience with a new method of endoscopic transbronchial real time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. Thorax 2003;58:1083–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakairi Y, Nakajima T, Iizasa T, et al. Diagnosis of lymph node metastasis by endobronchial ultrasound-guided transbronchial needle aspiration more than 1 year after lung cancer resection: report of a case. Surg Today 2011;41:983–5. [DOI] [PubMed] [Google Scholar]
- [10].Mehta HJ, Begnaud A, Penley AM, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer 2015;90:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blagden SP, Charman SC, Sharples LD, et al. Performance status score: do patients and their oncologists agree? Br J Cancer 2003;89:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Dev Ther 2013;7:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer 2008;61:1–2. [DOI] [PubMed] [Google Scholar]
- [14].Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278–97. [DOI] [PubMed] [Google Scholar]
- [15].Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101–4. [DOI] [PubMed] [Google Scholar]
- [16].Yarmus L, Akulian J, Ortiz R, et al. A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. J Thorac Dis 2015;7:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jalil BA, Yasufuku K, Khan AM. Uses, limitations, and complications of endobronchial ultrasound. Proc (Bayl Univ Med Cent) 2015;28:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li P, Zheng W, Zhao L. Convex probe endobronchial ultrasound: applications beyond conventional indications. J Thorac Dis 2015;7:E289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


