Abstract
Background:
This meta-analysis aimed to evaluate the efficiency and safety of dexamethasone administration in total knee and hip arthroplasties.
Methods:
Two researchers search the relevant studies independently including Embase (1980–017.04), PubMed (1966–017.04), ScienceDirect (1985–017.04), Web of Science (1950–2017.03), and Cochrane Library for potential relevant studies. After testing for heterogeneity between studies, data were aggregated for random-effects models when necessary. The results of dichotomous outcomes were expressed as risk difference (RD) with a 95% confidence intervals (CIs). For continuous various outcomes, mean difference (MD) or standard mean difference (SMD) with a 95% confidence intervals (CIs) was applied for assessment. Meta-analysis was performed using Stata 11.0 software.
Results:
Four randomized controlled trials (RCTs) including 361 patients met the inclusion criteria. The present meta-analysis indicated that there were significant differences between groups in terms of visual analogue scale (VAS) score at 12 hours (SMD = −0.579, 95% CI: −0.780 to −0.357, P = .000), 24 hours (SMD = −0.820, 95% CI: −1.036 to −0.604, P = .000), and 48 hours (SMD = −0.661, 95% CI: −1.149 to −0.172, P = .008). Dexamethasone was associated with a lower opioid consumption at 12 hours (SMD = −0.245, 95% CI: −0.465 to −0.025, P = .029), 24 hours (SMD = −0.285, 95% CI: −0.505 to −0.064, P = .011), and 48 hours (SMD = −0.989, 95% CI: −1.710 to −0.267, P = .007).
Conclusion:
Dexamethasone could significantly reduce postoperative pain scores and opioid consumption within the 1st 48 hours following total joint arthroplasty (TJA). The overall evidence quality was moderate to low, further high-quality RCTs are needed to identify the optimal dose of dexamethasone for reducing pain after TJA.
Keywords: dexamethasone, meta-analysis, pain control, total joint arthroplasty
1. Introduction
Total joint arthroplasty (TJA) is considered to be successful procedure for the treatment of degenerative arthritis. However, following the procedure, 30% to 77% of patients suffer moderate to severe postoperative pain.[1] Moreover, nausea and vomiting are the most frequent adverse effects of the anesthesia, with a reported incidence of 20% to 83%.[2,3] Appropriate postoperative pain control is essential for early recovery and better functional outcomes. Besides, optimal pain management may decrease length of stay (LOS) and the risk of thromboembolism, such as deep vein thrombosis and pulmonary embolism. Several strategies have been proposed to minimize postoperative pain, nausea, and vomiting following TJA including peripheral nerve block, local infiltration anesthesia, and intrathecal morphine.[4–6] However, additional opioid administration, which is frequently performed as a beneficial adjunct to multimodal regimes for pain relief, may result in adverse effects, for instance, nausea, vomiting, respiratory depression, and urinary retention. Postoperative pain management has been an interesting topic for a few decades, and optimal anesthesia remains controversial.
Dexamethasone is a high-potency, long-acting glucocorticoid that has been widely used in the field of orthopedics. Dexamethasone has been reported to inhibit peripheral phospholipase, which decreases the pain-aggravating agents from the cyclooxygenase and lipoxygenase pathways.[7] Previous studies have shown the analgesic effect of dexamethasone in general and orthopedic surgery. In addition, preoperative administration of dexamethasone has been reported to reduce postoperative nausea and vomiting.[8,9] However, some potential side effects associated with dexamethasone administration limit its regular use in surgical procedures, although Salerno and Hermann[1] demonstrated that it was effective and safe for pain management.
Currently, whether the administration of dexamethasone is effective and safe in reducing pain score and opioid consumption in TJAs remains, unclear due to a lack of reliable evidence from high-quality randomized controlled trials (RCTs). Therefore, we performed the present systemic review and meta-analysis to evaluate the efficiency and safety of dexamethasone administration in total knee and hip arthroplasties.
2. Methods
This systematic review was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. The study was approved by the ethics committee of the First People's Hospital of Jining.
2.1. Search strategy
Two researchers search the relevant studies independently including Embase (1980–017.04), PubMed (1966–017.04), ScienceDirect (1985–017.04), Web of Science (1950–2017.03), and Cochrane Library for potential relevant studies. Reference lists of all the potential included studies and relevant reviews were hand-searched for any additional trials. No restrictions were imposed on language. The Mesh terms and their combinations used in the search were as follows: “analgesia” OR “pain management” OR “pain control” OR “total knee arthroplasty or replacement” OR “total hip arthroplasty or replacement” AND “dexamethasone”. A 3rd reviewer acted as a judge if there was any disagreement. The retrieval process is presented in Fig. 1.
Figure 1.
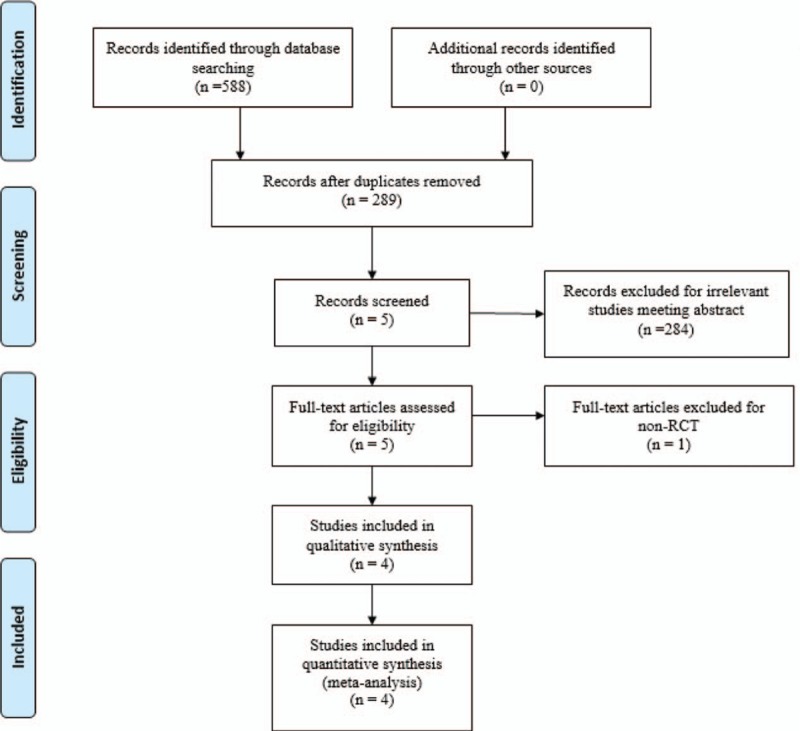
Search results and the selection procedure.
2.2. Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria: 1) Published clinical RCTs; 2) Patients undergoing TJAs, experiment group received intravenous or topical injection of dexamethasone for pain management and control group received placebo or nothing; 3) Reported surgical outcomes, including visual analogue scale (VAS) scores, opioid consumption, LOS and postoperative adverse effects including the risk of nausea, vomiting. Studies would be excluded from present meta-analysis for incomplete data, case reports, conference abstract, or review articles.
2.3. Selection criteria
Two authors independently reviewed all the abstracts of the potential studies identified by the above searches. After an initial decision, full text of the studies that potentially met the inclusion criteria were reviewed and final decision was made. A senior reviewer is consult in case of disagreement regarding which studies to include.
2.4. Date extraction
A standard form for date extraction is printed for date extraction. Two authors independently extracted the relevant data from the included articles. Details of incomplete data of included studies are obtained by consulting the corresponding author. Following data were extracted: First author names, published year, sample size, study design, comparable baseline, dosage of dexamethasone, and duration of follow-up. Other relevant data were also extracted from individual studies.
2.5. Quality assessment
Quality assessment of the included RCTs was assessed by 2 authors independently which used the Cochrane Collaboration tool. We conducted “risk of bias” table including the following key points: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting, and other bias, each item was recorded by “Yes,” “No,” or “Unclear.”
The qualities of evidence of main outcomes in present meta-analysis were evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system including the following items: risk of bias, inconsistency, indirectness, imprecision, and publication bias which was assessed according to guideline panels of GRADEpro software. The recommendation level of evidence is classified into the following categories: high, which means that further research is unlikely to change confidence in the effect estimate; moderate, which means that further research is likely to significantly change confidence in the effect estimate and may change the estimate; low, which means that further research is likely to significantly change confidence in the effect estimate and to change the estimate; and very low, which means that any effect estimate is uncertain.
2.6. Data analysis and statistical methods
All calculations were carried out with Stata 11.0 (The Cochrane Collaboration, Oxford, UK). Statistical heterogeneity was assessed based on the value of P and I2 using the standard chi-square test. When I2 > 50%, P < .1 was considered to be significant heterogeneous. The random-effect model was performed for meta-analysis; otherwise, the fixed-effect model was used. When possible, subgroup analyses were conducted to explore the origins of the heterogeneity. The results of dichotomous outcomes (postoperative adverse effects, including the risk of nausea and vomiting) were expressed as risk difference (RD) with 95% confidence intervals (CIs). For continuous various outcomes (VAS scores, opioid consumption, and LOS), mean difference (MD) or standard mean difference (SMD) with a 95% confidence intervals (CIs) was applied for assessment. Subgroup analysis was conducted according to the dosage of dexamethasone.
3. Results
3.1. Search result
A total of 588 studies were preliminarily reviewed. By screening the titles and reading the abstracts and entire contents, 584 reports were excluded from present meta-analysis following inclusion criteria. No gray reference was included. Finally, 4 RCTs[10–13] which had been published between 2008 and 2016 were enrolled in present meta-analysis and include 183 participates in the dexamethasone groups and 178 patients in the control groups.
3.2. Study characteristics
Demographic characteristics, the details about the included studies, are summarized in Table 1. The sample size of the included studies ranged from 40 to 193. All of them evaluated the efficiency and safety of dexamethasone for reducing postoperative pain in TJA. Experimental groups received intravenous or topical dexamethasone, while control groups received placebo or none. There is a variation in dosage of dexamethasone in experimental groups. Three studies[10,11,13] performed general anesthesia and one[12] performed spinal anesthesia. Patient control analgesia with opioid was applied in all participates for concomitant pain management. The follow-up period ranged from 1 to 12 months.
Table 1.
Trials characteristics.

3.3. Risk of bias assessment
Cochrane Collaboration tool was used to assess the RCTs (Table 2). All the RCTs[10–14] provided clear inclusion and exclusion criteria, and suggested a methodology of randomization, demonstrating that the randomization algorithm was generated by a computer program. Furthermore, all RCTs stated allocation concealment was achieved by the sealed envelope approach. Double blinding was provided in 3 RCTs.[11–13] Three studies[10–12] attempted to blind the assessors. All of them suggest the outcomes for at least 95% of the patients. Each risk of bias item was presented as a percentage across all included studies. The percentage indicated the proportion of different levels of risk of bias for each item (Table 3).
Table 2.
Methodological quality of the randomized controlled trials.
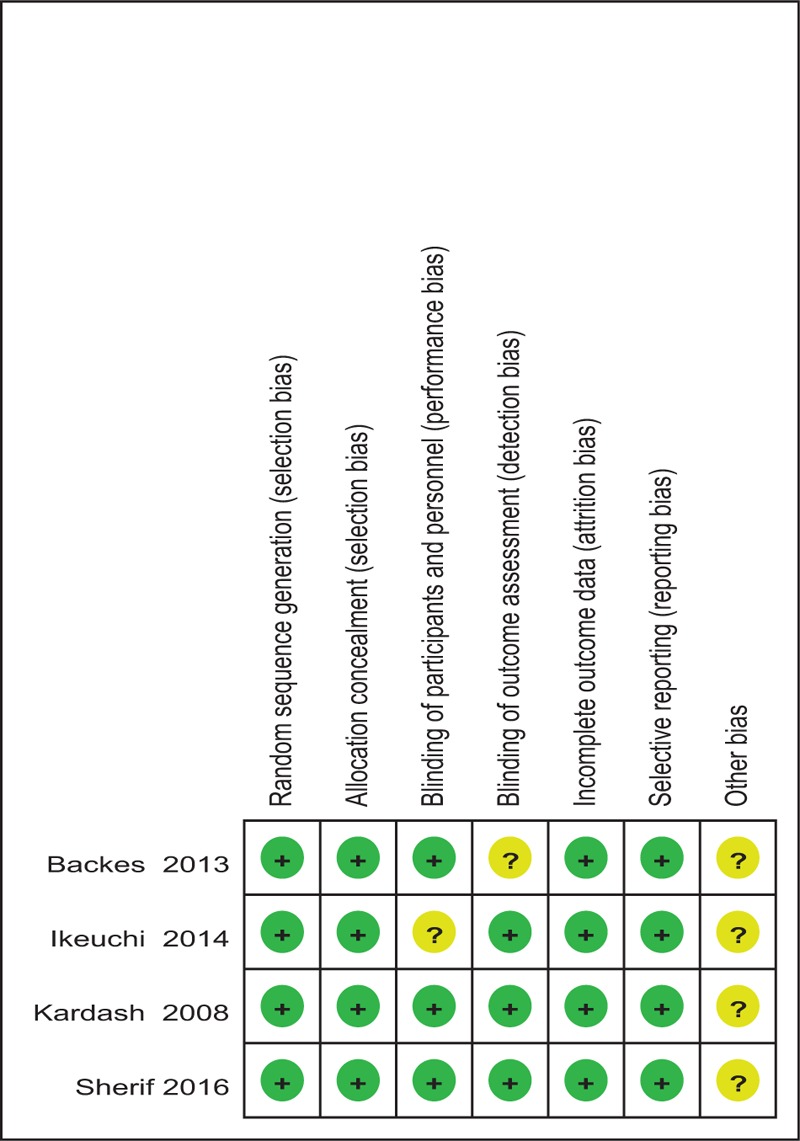
Table 3.
Risk of bias.
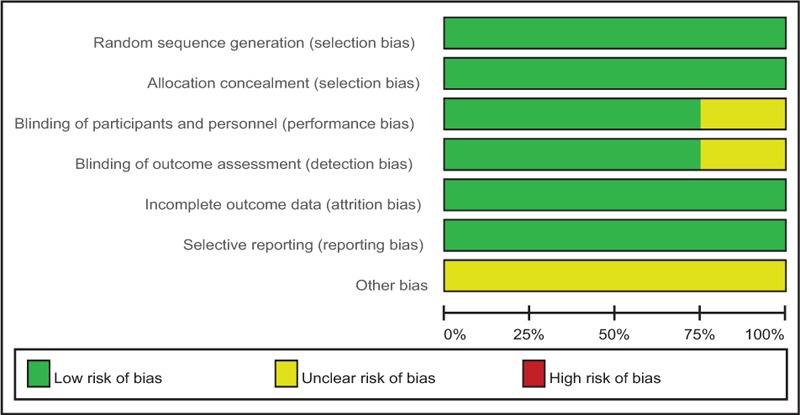
3.4. Outcomes for meta-analysis
3.4.1. VAS scores at 12 hours
Four articles[10–13] reported the outcomes of VAS scores at 12 hours following TJA. There was no significant heterogeneity among the studies (χ2 = 4.09, df = 3, I2 = 26.7%, P = .252); therefore, a fixed-effects model was used. Pooled results demonstrated that VAS scores at 12 hours in control groups was significant higher than in dexamethasone groups (SMD = −0.579, 95% CI: −0.780 to −0.357, P = .000; Fig. 2).
Figure 2.
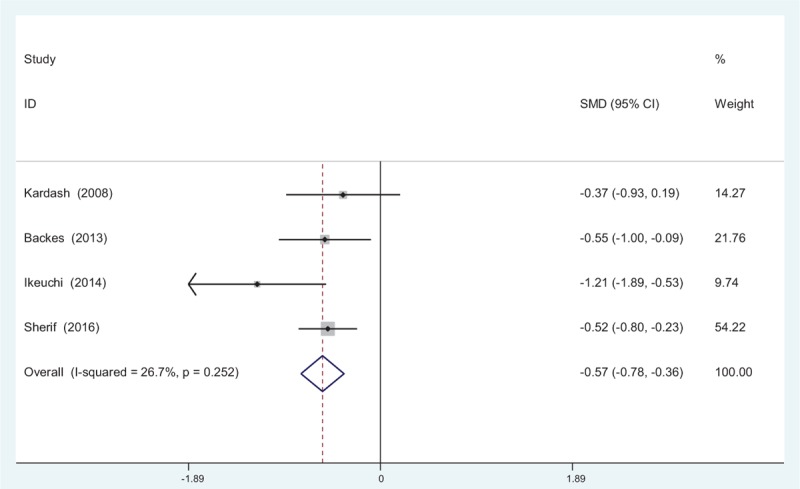
Forest plot diagram showing VAS scores at 12 hours following TJA. TJA = total joint arthroplasty, VAS = visual analogue scale.
3.4.2. VAS scores at 24 hours
Four studies[10–15] reported VAS scores at 24 hours following TJA. There was no significant heterogeneity among the studies (χ2 = 5.72, df = 3, I2 = 47.6%, P = .126); therefore, a fixed-effects model was applied. Pooled results demonstrated that the VAS scores at 24 hours in control groups were significantly higher than that in experimental groups (SMD = −0.820, 95% CI: −1.036 to −0.604, P = .000; Fig. 3).
Figure 3.
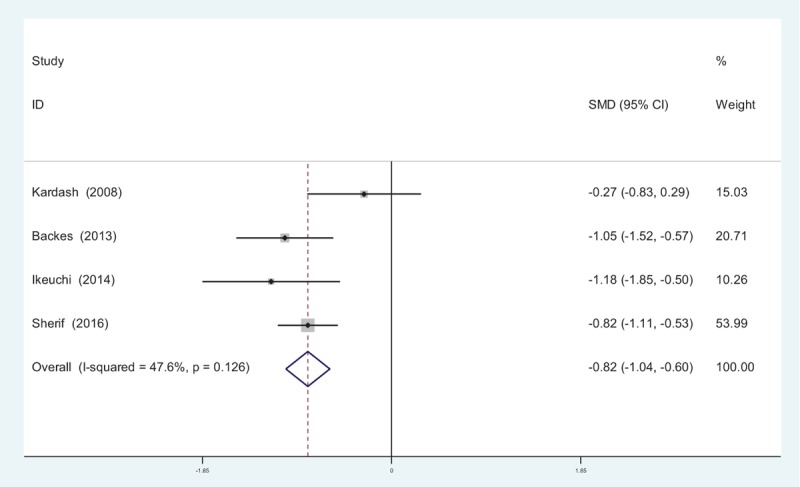
Forest plot diagram showing VAS scores at 24 hours following TJA. TJA = total joint arthroplasty, VAS = visual analogue scale.
3.4.3. VAS scores at 48 hours
Four reports[10–15] provided the outcomes of VAS scores at 48 hours following TJA. There was significant heterogeneity among these studies; therefore, a random-effects model was used (χ2 = 12.71, df = 3, I2 = 76.4%, P = .005). Pooled results demonstrated that VAS scores at 48 hours in control groups were significantly higher than in experimental groups (SMD = −0.661, 95% CI: −1.149 to −0.172, P = .008; Fig. 4).
Figure 4.
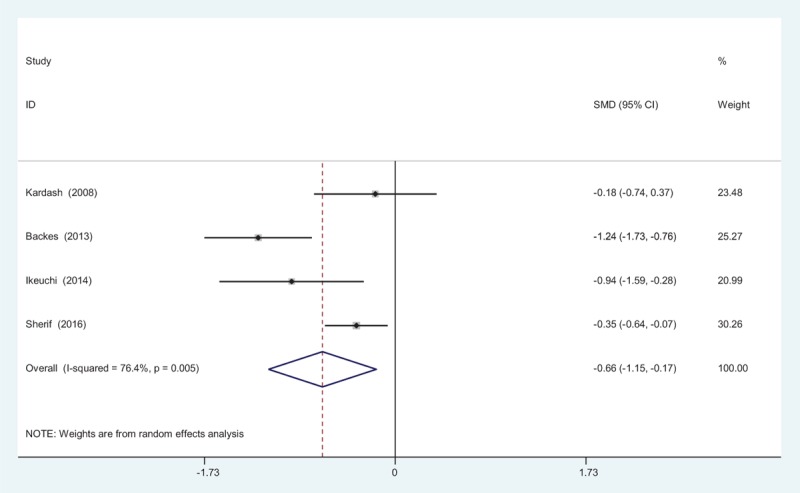
Forest plot diagram showing VAS scores at 48 hours following TJA. TJA = total joint arthroplasty, VAS = visual analogue scale.
3.4.4. Opioid consumption at 12 hours
Opioid consumption at 12 hours after TJA was provided in 3 articles.[11–13] No significant heterogeneity among these studies was found (χ2 = 1.53, df = 2, I2 = 0%, P = .465); therefore, a fixed-effects model was used. Opioid consumption at 12 hours in control groups was significantly higher than in experimental groups (SMD = −0.245, 95% CI: −0.465 to −0.025, P = .029; Fig. 5).
Figure 5.
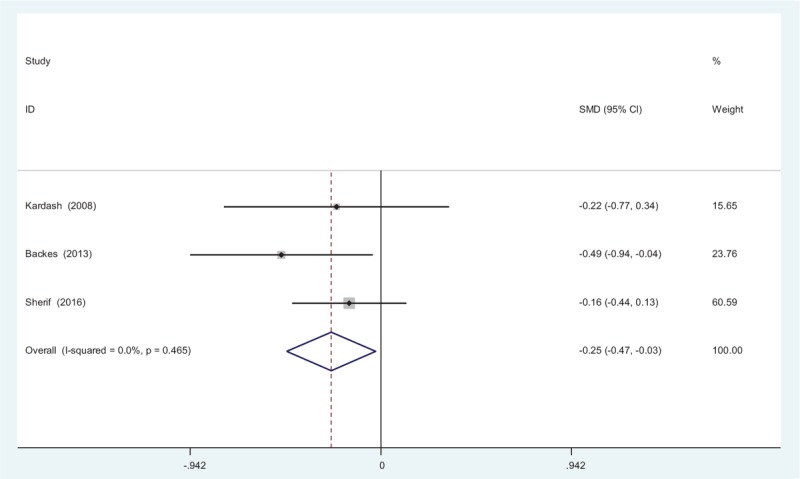
Forest plot diagram showing opioid consumption at 12 hours following total joint arthroplasty (TJA).
3.4.5. Opioid consumption at 24 hours
Three studies[11–13] provided data regarding opioid consumption at 24 hours after TJA. There was no significant heterogeneity among the pooled data (χ2 = 1.42, df = 2, I2 = 0%, P = .491), therefore a fixed-effects model was used. There was significance between the 2 groups in opioid consumption at 24 hours after TJA (SMD = −0.285, 95% CI: −0.505 to −0.064, P = .011; Fig. 6).
Figure 6.
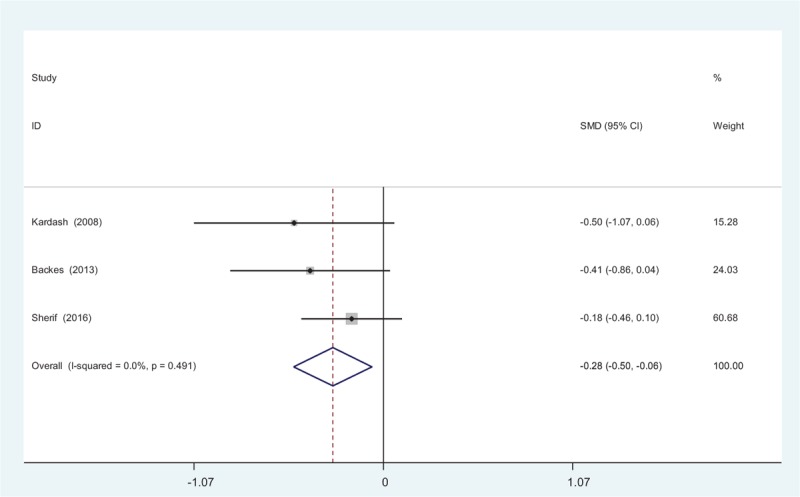
Forest plot diagram showing opioid consumption at 24 hours following total joint arthroplasty (TJA).
3.4.6. Opioid consumption at 48 hours
Four studies[11–13] reported the outcomes of opioid consumption at 48 hours following TJA. There was significant heterogeneity among these studies; therefore, a random-effects model was used (χ2 = 15.14, df = 2, I2 = 86.8%, P = .001). Pooled results demonstrated that opioid consumption at 48 hours in control groups was significantly higher than in experimental groups (SMD = −0.989, 95% CI: −1.710 to −0.267, P = .007; Fig. 7).
Figure 7.
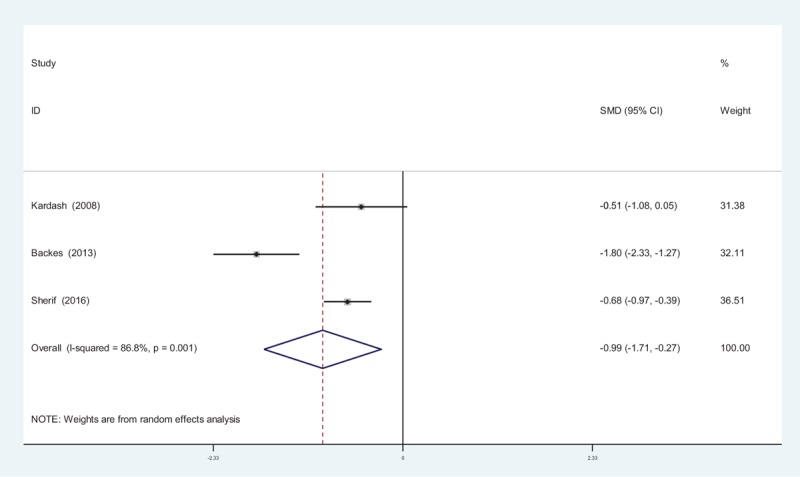
Forest plot diagram showing opioid consumption at 48 hours following total joint arthroplasty (TJA).
3.4.7. Length of hospital stay (LOS)
Four studies[10–13] reported the length of hospital stay between groups. Significant heterogeneity was identified in the pooled results; therefore, a random-effects model was used (χ2 = 22.08, df = 3, I2 = 86.4%, P = .000). There was no significant difference between the 2 groups (SMD = −0.373, 95% CI: −1.002 to 0.256, P = .245; Fig. 8).
Figure 8.
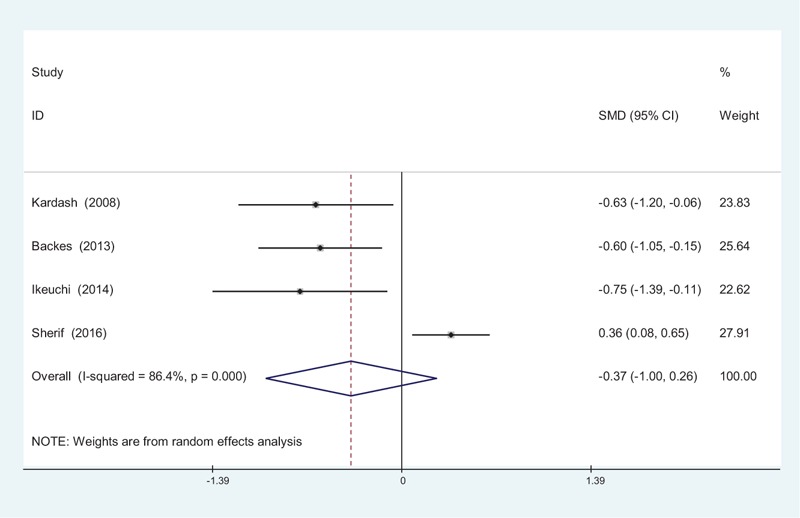
Forest plot diagram showing length of stay following total joint arthroplasty (TJA).
3.4.8. The occurrence of nausea
The occurrence of nausea was provided in 4 studies.[10–13] No significant heterogeneity among these studies was found; therefore, a fixed-effects model was used (χ2 = 1.10, df = 3, I2 = 0%, P = .778). There was a significant difference between the 2 groups (RD = −0.148, 95% CI: −0.218 to −0.078, P = .000; Fig. 9).
Figure 9.
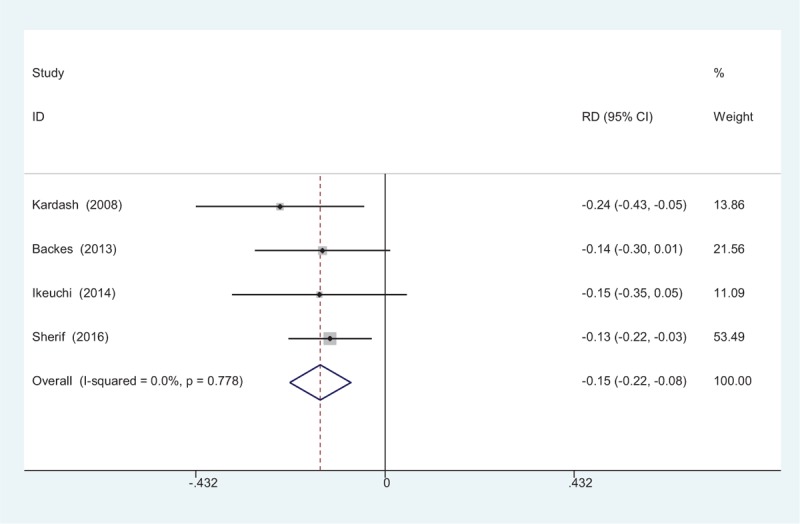
Forest plot diagram showing incidence of nausea following total joint arthroplasty (TJA).
3.4.9. The occurrence of vomiting
Four studies reported the incidence of vomiting.[10–13] We found no statistical heterogeneity, and a fixed-effects model was applied (χ2 = 1.25, df = 3, I2 = 0%, P = .742). The meta-analysis showed significant difference between groups. (RD = −0.166, 95% CI: −0.237 to −0.095, P = .000; Fig. 10).
Figure 10.
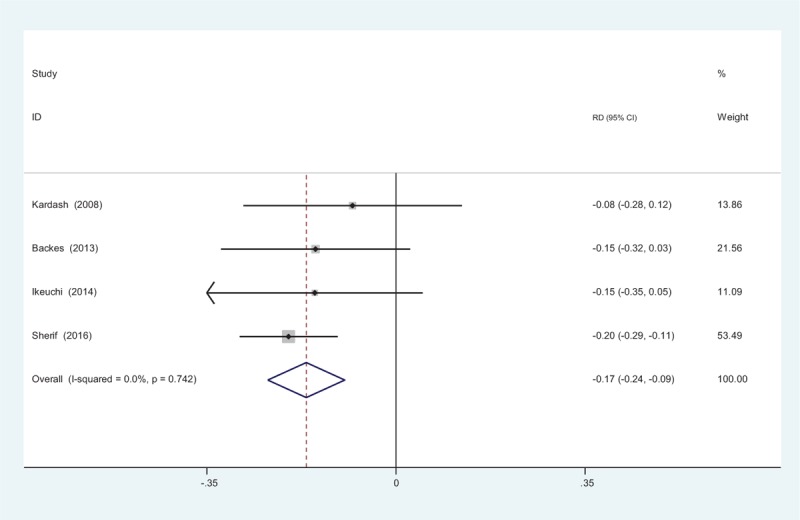
Forest plot diagram showing incidence of vomiting following total joint arthroplasty (TJA).
3.4.10. Subgroup analysis and evidence level
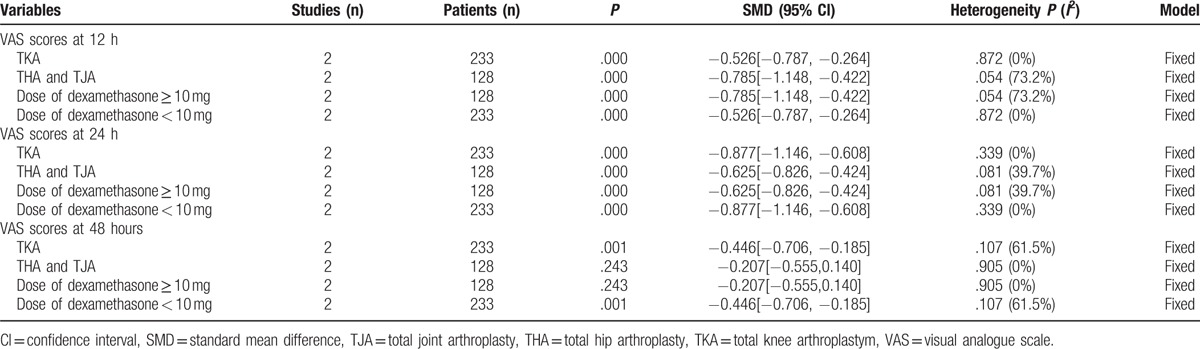
The subgroup analysis was performed about the dose and for 2 types of arthroplasty in order to determine the outcome of VAS scores (Table 4). All subgroups were analyzed using the fixed model, which indicated that the 2 factors were the potential sources of heterogeneity. Only 3 studies reported opioid consumption, thus it was not suitable to conduct subgroup analysis. More RCTs were required for further analyses.
Table 4.
The outcomes of subgroup analysis for VAS scores.
All main outcomes in this meta-analysis were evaluated using the GRADE system (Table 5).
Table 5.
The GRADE evidence quality for main outcome.
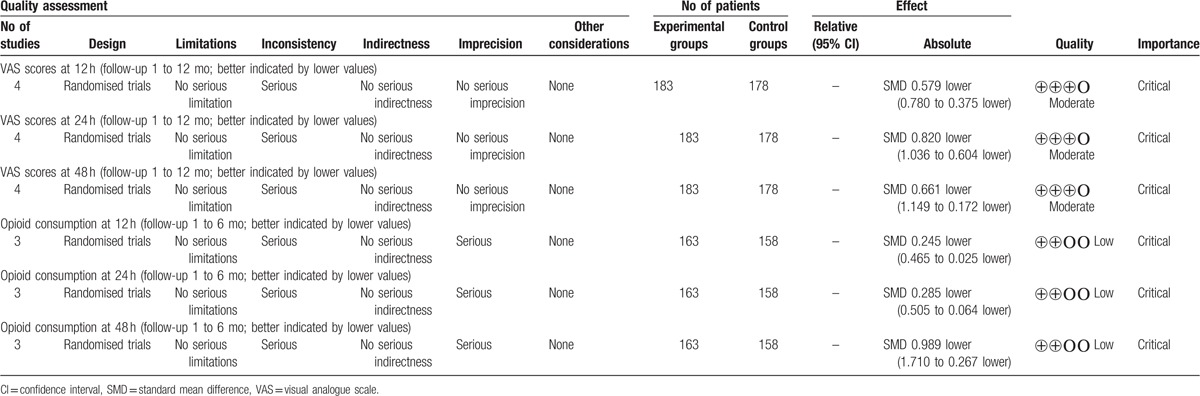
Inconsistency: when heterogeneity exists, but investigators fail to identify a plausible explanation, the quality of evidence should be downgraded by 1 or 2 levels. Limitations: low risk of bias would indicate “no serious limitations;” unclear risk of bias would indicate either “no serious limitations” or “serious limitations.” Indirectness: indirect population, intervention, comparator, or outcome may downgrade the quality rating by 1 or even 2 levels. Imprecise: results are imprecise when studies include relatively few patients and few events and thus have wide confidence intervals around the estimate of the effect. The quality of evidence should be downgraded by 1 or 2 levels. The overall evidence quality for each outcome was moderate to low which means that further research is likely to significantly change confidence in the effect estimate and to change the estimate. This finding may lower the confidence in any recommendations.
4. Discussion
The occurrence of joint osteoarthritis has increased with an increase in the ageing population, and TJA is a successful treatment that can be widely performed to address joint osteoarthritis. However, pain control following TJA can be very challenging, yet, optimal analgesia may shorten hospital stays and result in decreased medical costs. Furthermore, early rehabilitation exercise contributes to an early recovery and satisfactory functional outcome. Multiple perioperative pain management strategies have been implemented following TJA, including femoral nerve block (FNB), spinal analgesia, and periarticular or intraarticular injection of anesthetics.[14–16]
Dexamethasone is a long-acting glucocorticoid that has been widely used as an analgesic, as well as an effective preventive treatment of postoperative nausea and vomiting. Henzi et al[17] reported that a dose of 8 to 10 mg intravenous dexamethasone could further decrease the risk of postoperative nausea. Recently, studies have demonstrated that glucocorticoids show similar analgesic effects with supraclavicular blocks and interscalene blocks, an observation that has been attributed to their antiinflammatory effect. Sean et al[18] demonstrated that triamcinolone acetonide combined with bupivacaine improved the analgesic effect and early recovery. For patients undergoing TJA, dexamethasone is injected as an adjunct to FNB and local infiltration analgesia for pain control. Kim et al[19] demonstrated that dexamethasone injected in the interscalene brachial plexus could significantly reduce early postoperative pain for shoulder surgery. However, Christensen et al[20] showed that dexamethasone had no beneficial effect on pain relief for patients undergoing total knee arthroplasty (TKA). It is plausible that different types and doses of glucocorticoid may alter the clinical efficiency of a specific glucocorticoid. The present meta-analysis indicated that the administration of dexamethasone could significantly reduce postoperative pain scores within the 1st 48 hours following TJA.
TJA is usually associated with severe pain in 60% and moderate pain in 30% of patients, especially in the 1st 48 hours.[21] Although various analgesic methods have been implemented, they are insufficient in most cases and additional opioid, including oral or patient-controlled analgesia administration, have been applied as concomitant pain management. Opioid consumption is identified as an objective method to measure pain. Opioid-related adverse effects, such as dizziness, nausea, vomiting, orthostatic hypotension, and respiratory depression have been frequently reported in the literature.[22,23] Besides the side effects described above, drug dependence is also an important issue that should be considered. Reducing opioid consumption could improve patients’ satisfaction and expedite mobilization and rehabilitation. The present meta-analysis indicated that there was a significant reduction in opioid consumption in dexamethasone groups compared with controls. Further investigations are required due to the small sample size of the included studies.
Nausea and vomiting are common side effects that are frequently associated with oral or intravenous opioid. The overall incidence of nausea in the dexamethasone group (12/183) was low compared with that in the controls (38/178). We also identified a decreased risk of postoperative complications, which was partly attributable to less opioid consumption and the use of dexamethasone. Despite the benefits mentioned, dexamethasone's routine use in surgical procedures is still limited because of concerns over the numerous side effects associated with glucocorticoids. After reviewing the previous studies, we found no reliable evidence regarding the side effects of glucocorticoids after single-dose administration of dexamethasone[17,24]; however, we would like to emphasize that only 4 studies were included in our meta-analysis. Furthermore, due to the small sample size, we did not perform investigations on dose-dependence.
The present meta-analysis exists some limitations that should be noted.
-
(1)
Only 4 RCTs were included in present meta-analysis, the sample size is relatively small.
-
(2)
Functional outcome is an important parameter, due to the insufficiency of relevant data, we fail to perform a meta-analysis.
-
(3)
Dose of dexamethasone is varied from each other, which may influence the results of the meta-analysis.
-
(4)
The duration of follow-up is relatively short which leads to underestimating complications.
-
(5)
Publication bias in present meta-analysis may influence the results.
Despite the limitations above, this is the 1st meta-analysis of recent RCTs to evaluate the efficiency and safety of dexamethasone administration in total knee and hip arthroplasties. Further large well-designed RCTs are still needed to validate this research.
5. Conclusion
Dexamethasone could significantly reduce postoperative pain scores and opioid consumption within the 1st 48 hours following TJA. The overall evidence quality was moderate to low, further high-quality RCTs are needed to identify the optimal dose of dexamethasone for reducing pain after TJA.
Footnotes
Abbreviations: LOS = length of stay, RCT = randomized controlled trial, TJA = total joint arthroplasty, VAS = visual analogue scale.
Authorship: JM and LL conceived the design of the study. JM and LL performed and collected the data and contributed to the design of the study. JM finished the manuscript. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am 2006;88:1361–72. [DOI] [PubMed] [Google Scholar]
- [2].Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003;97:62–71. table of contents. [DOI] [PubMed] [Google Scholar]
- [3].DiIorio TM, Sharkey PF, Hewitt AM, et al. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res 2010;468:2405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thorsell M, Holst P, Hyldahl HC, et al. Pain control after total knee arthroplasty: a prospective study comparing local infiltration anesthesia and epidural anesthesia. Orthopedics 2010;33:75–80. [DOI] [PubMed] [Google Scholar]
- [5].Kuchalik J, Granath B, Ljunggren A, et al. Postoperative pain relief after total hip arthroplasty: a randomized, double-blind comparison between intrathecal morphine and local infiltration analgesia. Br J Anaesth 2013;111:793–9. [DOI] [PubMed] [Google Scholar]
- [6].Chan MH, Chen WH, Tung YW, et al. Single-injection femoral nerve block lacks preemptive effect on postoperative pain and morphine consumption in total knee arthroplasty. Acta Anaesthesiol Taiwan 2012;50:54–8. [DOI] [PubMed] [Google Scholar]
- [7].Bahammam MA, Kayal RA, Alasmari DS, et al. Comparison between dexamethasone and ibuprofen for postoperative pain prevention and control after surgical implant placement: a double-masked, parallel-group, placebo-controlled randomized clinical trial. J Periodontol 2017;88:69–77. [DOI] [PubMed] [Google Scholar]
- [8].Naja Z, Kanawati S, Al Khatib R, et al. The effect of IV dexamethasone versus local anesthetic infiltration technique in postoperative nausea and vomiting after tonsillectomy in children: a randomized double-blind clinical trial. Int J Pediatr Otorhinolaryngol 2017;92:21–6. [DOI] [PubMed] [Google Scholar]
- [9].Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 2016;24:675–82. [DOI] [PubMed] [Google Scholar]
- [10].Ikeuchi M, Kamimoto Y, Izumi M, et al. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2014;22:1638–43. [DOI] [PubMed] [Google Scholar]
- [11].Sherif AA, Elsersy HE. Dexamethasone as adjuvant for femoral nerve block following knee arthroplasty: a randomized, controlled study. Acta Anaesthesiol Scand 2016;60:977–87. [DOI] [PubMed] [Google Scholar]
- [12].Kardash KJ, Sarrazin F, Tessler MJ, et al. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 2008;106:1253–7. [DOI] [PubMed] [Google Scholar]
- [13].Backes JR, Bentley JC, Politi JR, et al. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty 2013;28(8 Suppl):11–7. [DOI] [PubMed] [Google Scholar]
- [14].Kurosaka K, Tsukada S, Seino D, et al. Local infiltration analgesia versus continuous femoral nerve block in pain relief after total knee arthroplasty: a randomized controlled trial. J Arthroplasty 2016;31:913–7. [DOI] [PubMed] [Google Scholar]
- [15].Ishida K, Shibanuma N, Matsumoto T, et al. Periarticular multimodal drug injection improves post-operative pain and functional recovery after total knee arthroplasty. J Orthop Sci 2016;21:178–83. [DOI] [PubMed] [Google Scholar]
- [16].D’Ambrosio A, Spadaro S, Natale C, et al. Continuous spinal analgesia with levobupivacaine for postoperative pain management: Comparison of 0.125% versus 0.0625% in elective total knee and hip replacement: a double-blind randomized study. J Anaesthesiol Clin Pharmacol 2015;31:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000;90:186–94. [DOI] [PubMed] [Google Scholar]
- [18].Sean VW, Chin PL, Chia SL, et al. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J 2011;52:19–23. [PubMed] [Google Scholar]
- [19].Kim YJ, Lee GY, Kim DY, et al. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol 2012;62:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty. A double-blind randomized trial. J Bone Joint Surg Am 2009;91:2550–5. [DOI] [PubMed] [Google Scholar]
- [21].Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg 2016;36(Pt A):44–55. [DOI] [PubMed] [Google Scholar]
- [22].Tammachote N, Kanitnate S, Manuwong S, et al. Is pain after TKA better with periarticular injection or intrathecal morphine? Clin Orthop Relat Res 2013;471:1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain 2002;3:159–80. [DOI] [PubMed] [Google Scholar]
- [24].Sauerland S, Nagelschmidt M, Mallmann P, et al. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf 2000;23:449–61. [DOI] [PubMed] [Google Scholar]


