Abstract
Rationale:
Pulmonary spindle cell carcinoma (PSCC) is a highly malignant carcinoma that often exhibits the histopathological characteristic of cell pleomorphism.
Patient concerns:
Here, we report a PSCC case in a 59-year-old woman with multiple metastases. The pathological manifestation of this case did not show high-grade pleomorphism or atypia, and was easily mimicked by other borderline or low-grade malignant tumors such as an inflammatory myofibroblastic tumor (IMT).
Diagnoses:
Based on the results of immunohistochemistry and fluorescence in situ hybridization after the operation, IMT was ruled out, and PSCC was confirmed.
Interventions:
Resection of the right middle lobe and dissection of the lymph nodes were performed. The patient was given 2 cycles of chemotherapy with cisplatin and 1 cycle of radiotherapy after the operation.
Outcomes:
Metastatic lesions in the left kidney, the right lung, the first thoracic vertebrae, the retroperitoneal lymph nodes, and the mediastinal lymph nodes were identified by positron emission tomography/computed tomography 4 months after the operation, and the patient died 2 months later.
Lessons:
PSCC is a highly malignant carcinoma, but it rarely shows a low-grade malignant histological morphology. So, efforts should be focused on differentiating it from other borderline or low malignant tumors (such as IMT) to avoid misdiagnosis.
Keywords: lung, sarcomatoid carcinoma, spindle cell carcinoma
1. Introduction
Pulmonary spindle cell carcinoma (PSCC), also known as sarcomatoid carcinoma (SC) or pseudosarcoma, is a high malignant variant of carcinoma, which is derived from epithelium, but exhibits characteristics of mesenchymal differentiation.[1] SCC occurs in the oral cavity, larynx, breast, kidney, uterus, conjunctiva, prostate, other organs, and occasionally in the lungs.[1–4] PSCC can be manifested by a variety of histological and morphological characteristics and is easily misdiagnosed. Currently, there are few reports of PSCC, and the clinical characteristics, diagnosis, and treatment strategy of PSCC are not well-known. Here, we report a rare case of PSCC that was initially misdiagnosed as an inflammatory myofibroblastic tumor (IMT), and combined with previously reported cases, we summarize the clinical manifestations, diagnosis, and treatment of PSCC.
2. Case report
The patient is a 59-year-old woman with a 20-year smoking history. She was presented with a cough without obvious cause for 8 days, and the cough was associated with the production of 20 mL of bloody sputum per day. Since its onset, she occasionally had a low fever with a body temperature of 37–38°C. Contrast-enhanced computed tomography (CT) showed a circular, low-density shadow of 4.8 × 4.0 cm without discernible borders in the right lung (Fig. 1A). Slightly enhanced borders were observed after enhancement (Fig. 1B). Head CT, bone scintigraphy, and ultrasound of the liver, gallbladder, spleen, pancreas, and kidneys showed no sign of tumors. Because of suspected lung tumor lesions, the patient underwent tumor resection under general anesthesia. The tumor was 3.5 × 3 cm with a gray resection surface, surrounded by a yellow-white fish-like mass, and pleural dissemination was not found. The result of intraoperative frozen section showed a diffuse distribution of a short spindle cell tumor without obvious atypism, which was accompanied by inflammatory cell infiltration. Therefore, it was initially diagnosed as an IMT. Resection of the right middle lobe and dissection of the lymph nodes were performed.
Figure 1.
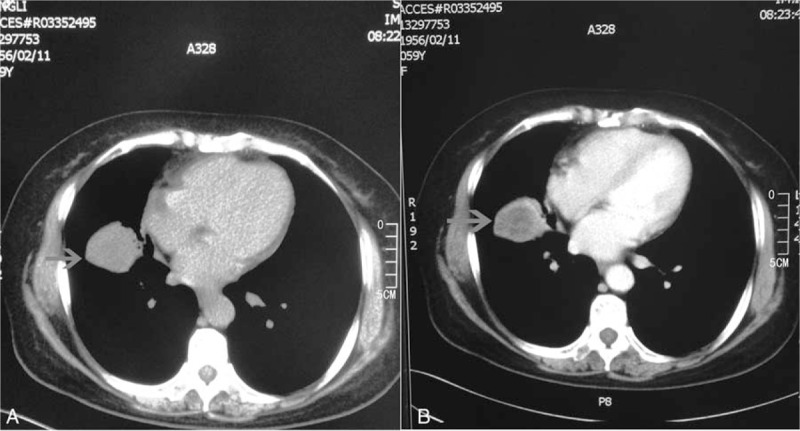
Computed tomography (CT) scan and enhanced CT findings. There is no shadow on the bronchus of the middle right lung. (A) A circular 4.8 × 4.0 cm low-density shadow in the lung field is visible (arrows), with a density similar to a liquid with indistinguishable borders. (B) Enhanced CT enhances the borders but not the center, thus forming a central low-density area.
The histopathological features after operation were as follows: the tumor had clear boundaries (Fig. 2A); the tumor cells were entirely composed of spindle cells in diffuse or storiform patterns. Typical features of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma were not observed. Cell pleomorphism was not obvious (Fig. 2B), but mitosis was easily detected in some regions (about 8/10 high power fields [HPFs]; Fig. 2C). The tumor was associated with regions of coagulation necrosis (Fig. 2D) with nuclear hyperchromasia. The inflammatory cells in the tumor stroma could be seen, which was similar to IMT. Immunohistochemistry results were as follows: epithelial membrane antigen (EMA) (E29; Maixin Biology, Fuzhou, China), CK (pan) (MX005; Maixin Biology, Fuzhou, China) (Fig. 3A), and CK7 (OV-TL 12/30; Dako; Copenhagen, Denmark) (Fig. 3B) showed positive in tumor cells; thyroid transcription factor-1 (8G7G3/1; Dako; Copenhagen, Denmark) was expressed only in type II alveolar epithelial cells, but was negative in tumor cells (Fig. 3C). Vimentin (V9; Dako; Copenhagen, Denmark) in tumor cells were positive (Fig. 3D); Napsin A (MX015; Maixin Biology, Fuzhou, China), P63 (MX013; Maixin Biology, Fuzhou, China), P40 (ZR8, Maixin Biology, Fuzhou, China), actin (SMA) (1A4; Dako; Copenhagen, Denmark), CD68 (KP1; Maixin Biology, Fuzhou, China), CD34 (QBEnd 10; Dako; Copenhagen, Denmark) (Fig. 3E), signal transducer and activator of transcription 6 (STAT6) (YE361, Abcam, United States), CD99 (12E7; Dako; Copenhagen, Denmark), CK5/6 (D5/16B4; Maixin Biology, Fuzhou, China), Calretinin (DAK-Calret 1; Dako; Copenhagen, Denmark), D2–40 (D2–40; Maixin Biology, Fuzhou, China), WT1 (6F-H2; Dako; Copenhagen, Denmark), Desmin (D33; Maixin Biology, Fuzhou, China), MyoD1 (5.8A; Dako; Copenhagen, Denmark), S-100 (16/f5; Maixin Biology, Fuzhou, China), and transducer-like enhancer of split 1 (TLE1; 1F5, Maixin Biology, Fuzhou, China) were all negative in tumor cells. β-catenin (β-Catenin-1; Dako, Copenhagen, Denmark) was shown to be cytoplasm positive in tumor cells, but in tumor cells nuclei were negative (Fig. 3F). Anaplastic lymphoma kinase (ALK) (ALK-1; Dako, Copenhagen, Denmark) was also negative in tumor cells (Fig. 3G). Ki67 (MIB-1; Dako, Copenhagen, Denmark) indicated a high tumor proliferation index (about 70%+) (Fig. 3H). Fluorescence in situ hybridization (FISH) detection of ALK t(2P23) rearrangements indicated that <5% of tumor cells had separate red and green signals, and the synovial sarcoma-associated t(X;18)(p11;q11) translocation was also not observed. Based on the immunohistochemical and FISH findings, the patient was diagnosed with primary PSCC. There was no tumor involvement in the resected lymph nodes. The staging was pT2aN0M0 (Ib stage). The patient received 2 cycles of chemotherapy with cisplatin and docetaxel and 1 cycle of radiotherapy. Radiological examination after completion of treatment showed no signs of tumor recurrence and metastasis; however, the patient experienced weight loss and fatigue 4 months after the operation. Positron emission tomography (PET)/CT identified metastatic lesions in the kidney, lung, liver, thoracic vertebrae, retroperitoneal lymph nodes, and mediastinal lymph nodes (Fig. 4A–F). The patient refused to continue treatment and died 2 months later. This study was approved by the Institution Review Board of China Medical University.
Figure 2.
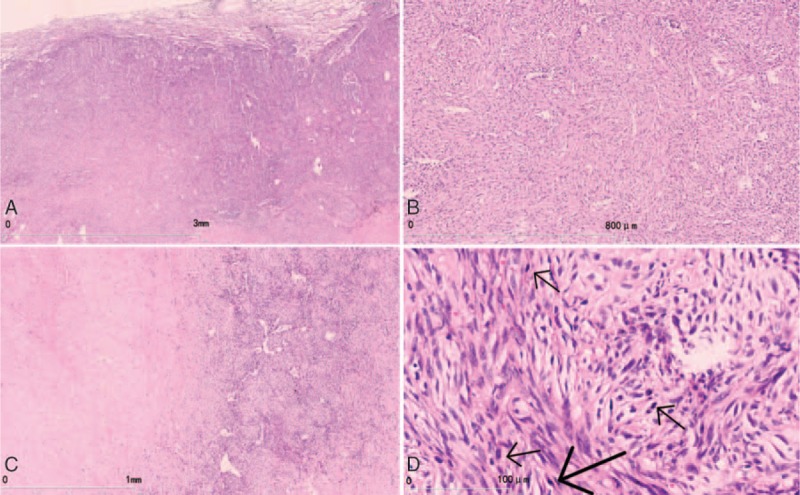
Histopathological findings. (A) The tumor tissue borders were clear. (B) The tumor cells are completely composed of spindle cells arranged in bundles and swirls. No obvious cell pleomorphism is observed. (C) Mitosis is obvious in some regions. (D) Coagulation necrosis is observed in some regions.
Figure 3.
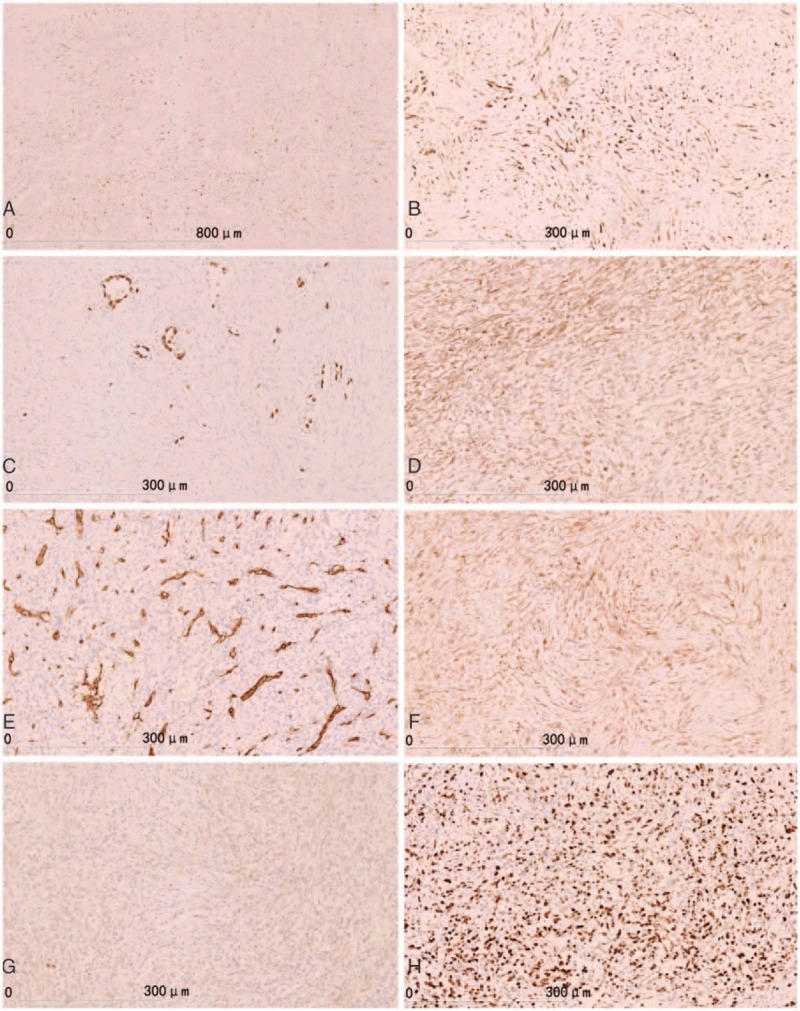
Immunohistochemical staining. (A) CK (pan) showed tumor cells were positive. (B) CK7 showed positive in tumor cells. (C) TTF-1 was expressed in only type II alveolar epithelial cells, but tumors cells were negative. (D) Vimentin showed positive tumor cells. (E) CD34 showing vascular positive and tumor cells (−). (F) β-Catenin showed as cytoplasm positive in tumor cells, but were negative in tumor cells nuclei. (G) ALK showed tumor cells were negative. (H) Ki-67 showed a high tumor proliferative index (about 70%+). ALK = anaplastic lymphoma kinase, TTF-1 = thyroid transcription factor-1.
Figure 4.
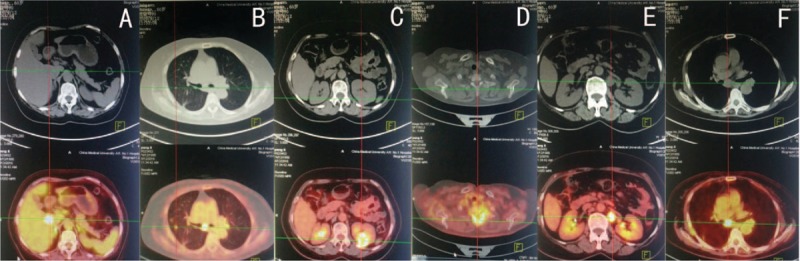
Positron emission tomography (PET)/computed tomography (CT) findings. (A) PET/CT (bottom) showing an area of abnormally increased radiopharmaceutical uptake in the liver (indicated by the coordinate line). The maximum SUV was 17.0. No obvious abnormality is visible in the corresponding CT (top). (B) CT showing right pulmonary nodules. FDG uptake is increased and the maximum SUV was 3.6. (C) Increased circular FDG uptake in the left kidney. The maximum SUV was 11.4. (D) Increased uptake in the first thoracic vertebrae. The maximum SUV was 7.8. (E) PET/CT showing increased nodular FDG uptake in the left renal anterior region. The maximum SUV was 13.5. (F) PET/CT showing increased FDG uptake in the mediastinum. The maximum SUV was 9.2. FDG = fluorodeoxyglucose, SUV = standardized uptake value.
3. Discussion
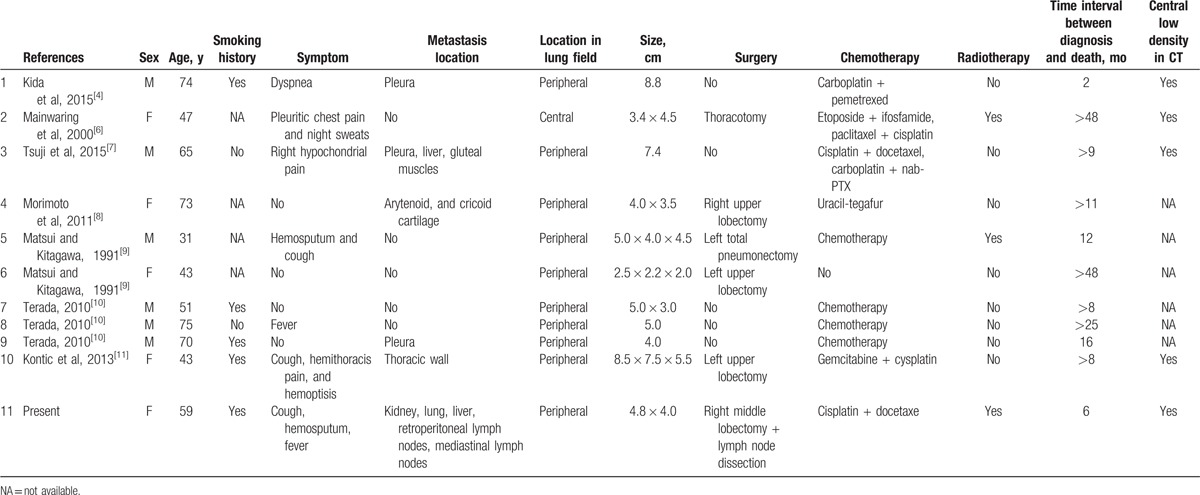
According to the 2015 WHO histological classification of lung tumors,[5] pulmonary sarcomatoid carcinoma (PSC) includes PSCC, pleomorphic carcinoma (PC), giant cell carcinoma (GCC), carcinosarcoma, and pulmonary blastoma. All forms of SC have a low incidence, and PSCC is very rare, accounting for only 0.2% to 0.3% of all lung cancers.[6] We reviewed the PSCC case reports published in English,[4,6–11] and our present case, and summarized the clinical features of PSCC (Table 1). A total of 11 cases were analyzed, including 6 men and 5 women. Their ages ranged from 31 to 75 years with an average of 57.4 years. In the 7 cases with information on smoking history, 6 patients smoked, suggesting that smoking may be related to PSCC. It has to be noticed that there were 4 cases without any obvious clinical symptoms,[8–10] and their lesions were detected by routine chest radiography. These patients were treated promptly and the outcomes were relatively good. Ten of the 11 cases were peripheral and only 1 was central. In all 5 PSCC cases with CT scan images, central, low-density regions were found in the lesions, which might be the most specific imaging feature of PSCC. Kida et al[4] suggested that this feature was related to hemorrhage at the center of the lesion. Five patients had involvement in the pleura or other distal organs. PET/CT had a significant advantage in detecting metastatic lesions.[7,12] Six patients received surgical treatments, and the rest did not, mainly due to their poor conditions that rendered them unable to tolerate the operations. One case was treated with only surgery and got a satisfactory result (disease-free observation >48 months).[9] So, we recommend that patients with no distal metastases and in good physical condition choose surgery. Ten patients received chemotherapy, and 3 of them also received radiotherapy. Because of the differences of the tumor staging and physical conditions of these patients, the chemotherapy programs were also not the same, so their outcomes differed greatly (the survival periods were from 2 months to more than 48 months). Most of the time, radiotherapy and chemotherapy could alleviate the diseases, but these treatments may not be particularly suitable for patients in poor physical conditions.[4] Only 3 of the 11 patients had a survival of more than 24 months, which indicates that PSCC is a highly malignant tumor, and the prognosis of PSCC is poor, even in early stage. Recent comprehensive genomic profiling studies showed that genomic alterations of tumor protein p53 (74%), V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (34%), hepatocyte growth factor receptor gene (MET) (13.6%), epidermal growth factor receptor (8.8%), V-raf murine sarcoma viral oncogene homolog B (7.2%), human epidermal growth factor receptor 2 (1.6%), or ret proto-oncogene (0.8%) were identified in PSCs, and tyrosine-protein kinase Met (C-MET) exon 14 alterations were more frequently in PSC (12%) than in non-PSC non-small cell lung cancers (3%).[13] Some of the PSC patients were successfully treated with a C-MET inhibitor were also reported.[14] In addition, Kim et al[15] reported that programmed death-1 (PD-1)/PD ligand 1 (PD-L1) were highly expressed in PSCs. Immunotherapy targeting of the immune checkpoint inhibitors, such as PD-1/PD-L1 antibodies, may represent a potential therapeutic candidate for PSCs.[15]
Table 1.
Data summary of case series.
Pulmonary spindle cell carcinoma should be distinguished from other spindle cell tumors as follows:
-
1.
Inflammatory myofibroblastic tumor: IMT is generally more common in children and young people. The basic structures of IMT are composed of inflammatory cells and myofibroblasts (spindle-like or epithelioid). The interstitial space may exhibit edematous, mucous, fibrous, or hyaline changes. Mitoses are usually rare (<2/10 HPFs). Immunohistochemical features include different degrees of actin (SMA), ALK, and desmin positivity. ALK gene rearrangement is a feature of IMT.[16] In this case, PSCC was misdiagnosed as IMT based on the result of the intraoperative frozen section, but the further immunohistochemistry and FISH findings confirmed it as a PSCC.
-
2.
Malignant solitary fibrous tumor: The diameter of a malignant solitary fibrous tumor is often >10 cm, and it often contains dense areas of tumor cells with obvious sarcomatoid and atypical features. The nuclei are pleomorphic with mitosis >4/10 HPF. These tumors are STAT6-positive and EMA-negative. In our case, STAT6 and CD99 were negative and EMA was positive, and thus did not meet the criteria for a solitary fibrous tumor.
-
3.
Synovial sarcoma: In this kind of tumor, the spindle cells are homogenous and relatively small with an oval-shaped and lightly stained nucleus, and the nucleolus is not obvious. The cytoplasm is sparse and the cell boundaries are unclear. Most cells are arranged in short bundles. It has a characteristic t (X;18)(p11;q11) translocation that produces an SYT-SSX fusion gene. Immunohistochemical features include expression of EMA and broad-spectrum CK, CD99, and TLE1.
-
4.
Sarcomatoid mesothelioma: Clinical manifestations of this tumor often include pleural effusions, pleural nodules, and pleural thickening. Microscopic features include a bundle or cluster arrangement of the spindle cells, similar to fibrosarcoma tumor cells. D2–40 and WT1 are positive. In our case, D2–40 and WT1 were negative. That did not support the diagnosis of sarcomatoid mesothelioma.
-
5.
Metastatic sarcoma: Metastatic sarcoma occurs after the primary tumor. Our patient had no history of cancer, and thus this possibility can be excluded.
4. Conclusions
Pulmonary spindle cell carcinoma is a rare disease, with clinical symptoms and imaging findings similar to other lung cancers with a lack of specificity. PSCC tends to involve distal metastasis, and therefore, relevant imaging examinations need to be performed. If the patient is in a suitable physical condition, comprehensive treatment including surgery, chemotherapy, and radiotherapy should be provided to improve the prognosis. As a highly malignant carcinoma, PSCC rarely shows a low-grade malignant tumor performance in histological morphology. Therefore, efforts should be focused on differentiating it from other tumors (such as IMT) during pathological examination to avoid misdiagnosis. Immunohistochemistry and genetic analysis are important for a definitive diagnosis of PSCC.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, CT = computed tomography, FISH = fluorescence in situ hybridization, GCC = giant cell carcinoma, HPFs = high power fields, IMT = inflammatory myofibroblastic tumor, PC = pleomorphic carcinoma, PET = positron emission tomography, PSCC = pulmonary spindle cell carcinoma, SC = sarcomatoid carcinoma, TLE 1 = transducer-like enhancer of split 1.
Funding: This study was supported by Program of Science and Technology Department of Liaoning Province (No. 201602877) and Natural Science Foundation of Liaoning Province of China (No: L2015598).
The authors declare that they have no competing interests.
References
- [1].Yun YL, Lee YC, Shih JY, et al. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001;34:91–7. [DOI] [PubMed] [Google Scholar]
- [2].Öztürk H. Primary spindle cell sarcoma of the prostate and (18)F-fluorodeoxyglucose-positron-emission tomography/computed tomography findings. Urol Ann 2015;7:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shigeta T, Minamikawa T, Matsui T, et al. Spindle cell carcinoma of the oral cavity: the impact of chemotherapy on pulmonary metastatic tumor doubling time. Kobe J Med Sci 2015;61:E64–70. [PubMed] [Google Scholar]
- [4].Kida J, Kanaji N, Kishi S, et al. An autopsy case of rapidly progressing spindle cell carcinoma of the lung accompanied with intratumor hemorrhage. Am J Case Rep 2015;16:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed2015;Lyon: International Agency for Research on Cancer, 9-96. [DOI] [PubMed] [Google Scholar]
- [6].Mainwaring MG, Poor C, Zander DS, et al. Complete remission of pulmonary spindle cell carcinoma after treatment with oral germanium sesquioxide. Chest 2000;117:591–3. [DOI] [PubMed] [Google Scholar]
- [7].Tsuji T, Kim YH, Ozasa H, et al. Successful treatment with carboplatin and nanoparticle albumin-bound paclitaxel in a patient with pulmonary spindle cell carcinoma. Respir Med Case Rep 2015;15:48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morimoto M, Osaki T, Kodate M, et al. Spindle cell carcinoma of the lung. Gen Thorac Cardiovasc Surg 2011;59:129–32. [DOI] [PubMed] [Google Scholar]
- [9].Matsui K, Kitagawa M. Spindle cell carcinoma of the lung. A clinicopathologic study of three cases. Cancer 1991;67:2361–7. [DOI] [PubMed] [Google Scholar]
- [10].Terada T. Spindle cell carcinoma of the lung: frequency, clinical features, and immunohistochemical studies of three cases. Respir Med CME 2010;3:241–5. [Google Scholar]
- [11].Kontic M, Stojsic J, Stevic R, et al. Could spindle cell lung carcinoma be considered and treated as sarcoma, according to its clinical course, morphology, immunophenotype and genetic finding? Pathol Oncol Res 2013;19:129–33. [DOI] [PubMed] [Google Scholar]
- [12].National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): non-small cell lung cancer. V5.2017 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; c2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed May 10, 2017.
- [13].Schrock AB, Li SD, Frampton GM, et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol 2017;12:932–42. [DOI] [PubMed] [Google Scholar]
- [14].Lee C, Usenko D, Frampton GM, et al. MET 14 deletion in sarcomatoid non-small-cell lung cancer detected by next-generation sequencing and successfully treated with a MET inhibitor. J Thorac Oncol 2015;10:e113–4. [DOI] [PubMed] [Google Scholar]
- [15].Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698–707. [DOI] [PubMed] [Google Scholar]
- [16].Alassiri AH, Ali RH, Shen Y, et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am J Surg Pathol 2016;40:1051–61. [DOI] [PubMed] [Google Scholar]


