Abstract
Background:
Developing a new reliable prognostic marker to predict the prognosis and supply better and more suitable therapy for patients with nasopharyngeal carcinoma (NPC) is urgent. Therefore, we performed this systematic review of the literature with meta-analysis to clarify and explore the associate expression of nm23-H1 with prognosis of NPC patients.
Methods:
Literature research in Cochrane Library, PubMed, and EMBASE was performed up to July 2016. Eligible case–control studies of associate expression of nm23-H1 with prognosis of NPC patients were included.
Results:
Nine studies met our inclusion criteria and were finally included for the analysis, involving 861 participants. Our meta-analysis revealed that the low expression of nm23-H1 in NPC was: RR = 2.13, 95% CI 1.15–3.95 and R = 2.56, 95% CI 2.03–3.22; and poorer overall survival (OS) rate was 3-year OS rate: RR: 0.55; 95% CI: 0.45–0.67 and 5-year OS rate: RR: 0.60; 95% CI: 0.52–0.69. Furthermore, the statistical significance was constant irrespective of different NPC subtypes.
Conclusion:
The low expression of nm23-H1 is associated with poorer prognosis in patients with NPC, suggesting that it is a prognostic factor and potential biomarker for survival in NPC.
Keywords: meta-analysis, nasopharyngeal carcinoma, nm23-H1
1. Introduction
Nasopharyngeal carcinoma (NPC) is an endemic malignancy in southern China, Hong Kong, Taiwan and Singapore where there is a strong genetic predisposition.[1,2] The mainstay of treatment for NPC is either radiotherapy or combined chemoradiotherapy, which demonstrates a cure rate of over 90% in early-stage patients.[3] However, the 5-year survival rate is only 40% to 50% even with the advanced radiotherapy technique and combined chemotherapy.[4,5] Besides, patients at the same status, for instance tumor differentiation, lymph node metastases, and TNM stage, may have diverse clinical outcomes. Therefore, we need to develop a new reliable prognostic marker to predict the prognosis and supply better and more suitable therapy for patients with NPC.
As the first metastasis suppressor protein of the 10 members of nm23 family, nm23-H1 has been found to be associated with the development and progression of various cancers.[6–8] Recently, a research by Wang et al[9] confirmed the low expression of nm23-H1 in head and neck squamous cell carcinoma (HNSCC) to be correlated with poor patients’ prognosis. In addition, nm23-H1 also plays a critical role in prognosis of patients with NPC, and the in vitro experiment found nm23-H1 expression correlated with the cellular and tumor radioresponse.[10] However, clinical studies on nm23-H1 expression in NPC did not draw a consistent conclusion. Most researches suggested that the low expression of nm23-H1 was associated with poorer survival in patients with NPC, while certain studies[11,12] showed no significant difference. Furthermore, none of these reports have been confirmed by systematic reviews with meta-analysis. Therefore, to clarify this question and explore its prognostic value, we performed this systematic review of the literature with meta-analysis.
2. Materials and methods
2.1. Publication search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The systematic literature search was performed through PubMed, Embase, and Cochran Library, covering all articles published up to July 2016. The following keywords were used to retrieve articles: nasopharyngeal carcinoma, nasopharyngeal neoplasms, and nm23. References of the retrieved publications were also screened. The language was English or Chinese. Only published studies with full-text articles were included. When overlapping articles were found, we only included the publications that reported the most extensive information.
2.2. Study selection and inclusion criteria
In our meta-analysis, the inclusion criteria for the studies were as follows: evaluating the association between nm23-H1expression and prognosis of patients with NPC; and reporting endpoints including tumor local recurrence, distant metastasis, or overall survival (OS) displaying outcomes in the form of relative risk (RR) with 95% confidence interval (CI).
2.3. Evaluation of study quality
The levels of evidence were estimated for all included studies with the Oxford Centre for Evidence-Based Medicine criteria, and the methodological quality evaluation of the studies was conducted using the Newcastle–Ottawa Scale for the cohort study. The specific quality assessment of prognosis studies was estimated according to the approach of Hayden et al.[13] The assessments were processed independently by 2 reviewers (CY and LX) and the final decision was achieved by consensus.
2.4. Data extraction
Articles were independently reviewed by 2 investigators (CY and LX) for data extraction. Any discrepancy was discussed further to reach a consensus. The data were independently extracted from eligible studies by 2 investigators (CY and LX). The primary data were survival outcomes of OS. The additional data obtained from the studies included first author, publication year, number of patients, patients’ mean age, median follow-up time, TNM stage, histological classification, sampling time, sampling site, and test method.
2.5. Statistical analysis
In order to pool the various outcomes included studies, firstly, heterogeneity was assessed using the Q and I2 statistics. Significant heterogeneity was defined as Q statistic P value <.10 or I2 value >50%. A fixed-effect model was used if there was no evidence of heterogeneity, otherwise, a random-effect model was performed. Results were reported as pooled as RR and their 95% CI and presented by forest plots, and the P value less than .05 was considered significant. Publication bias was evaluated though funnel plots. All analyses were performed using STATA 13.0 (Stata Corporation, College Station, TX) and Review Manager (version 5.3, the Cochrane collaboration), using 2-sided P values.
3. Results
3.1. Study characteristics
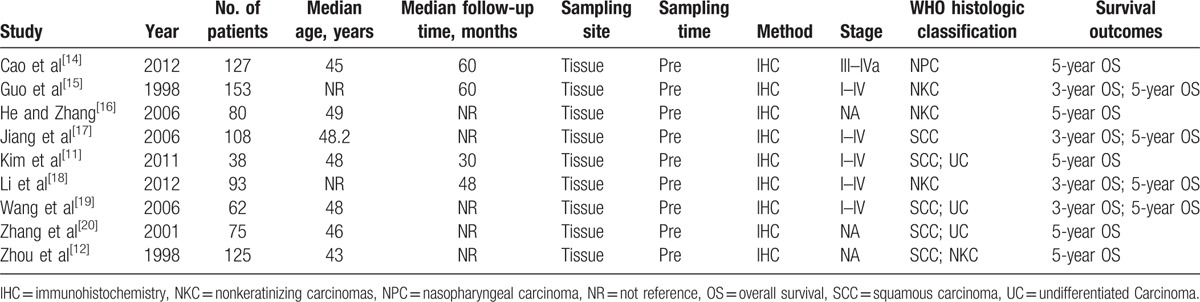
The detailed information of included studies is summarized in Table 1. Nine studies[11,12,14–20] met our inclusion criteria and were finally included for the analysis, involving 3 different subtypes (squamous carcinoma (SCC), nonkeratinizing carcinomas (NKC), and undifferentiated carcinoma). A total of 861 participants were analyzed for the association between nm23-H1 expression and disease prognosis, of which 416 (53.54%) and 861 (100.00%) ones were respectively included into 3-year OS and 5-year OS analyses.
Table 1.
Characteristics of published studies included in this meta-analysis.
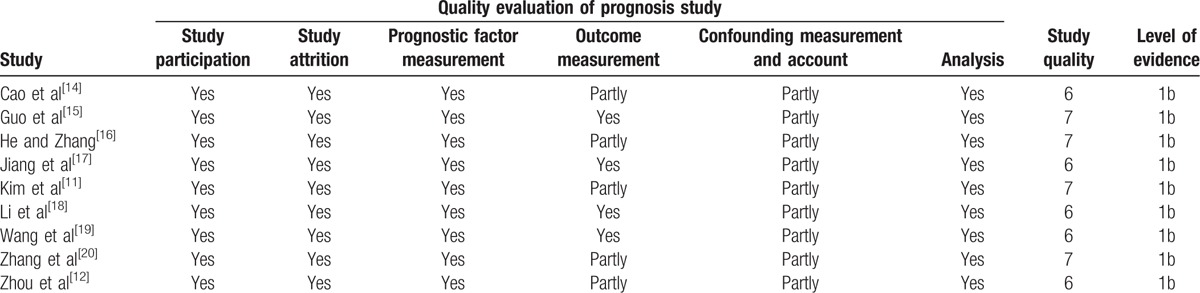
According to the guidelines for assessing quality in prognostic studies, the evaluation results of each item with potential bias are in Table 2. The key baseline characteristics of patients were adequately presented and the adopted statistical analyses were appropriate in all included studies.
Table 2.
Quality assessment of the studies included in the meta-analysis.
3.2. Tumor local recurrence and distant metastasis
A total of 4 studies reported the relationship between nm23-H1 expression and tumor recurrence, and 6 reflected distant metastasis of NPC. Notably, our meta-analysis revealed that the low expression of nm23-H1 in NPC was significantly associated with a high local recurrence and distant metastasis (RR = 2.13, 95% CI 1.15–3.95, Fig. 1; RR = 2.56, 95% CI 2.03–3.22, Fig. 2).
Figure 1.
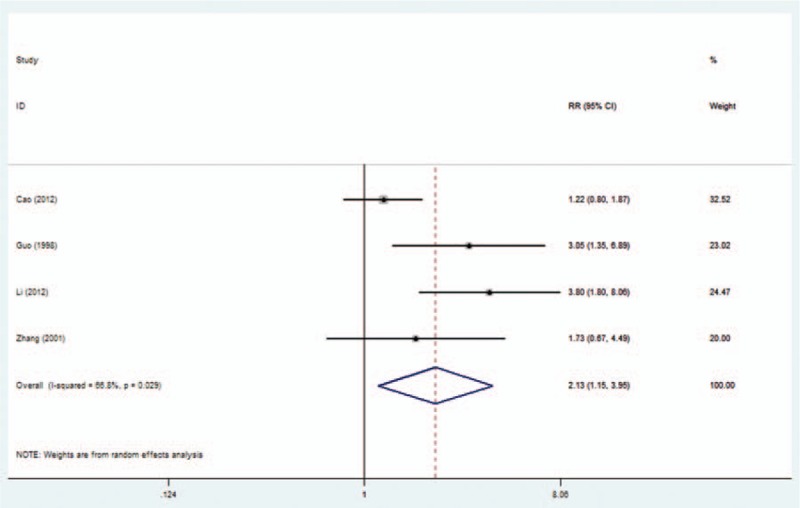
Forest plot evaluating the association of nm23-H1 expression and local recurrence in NPC patients. NPC = nasopharyngeal carcinoma.
Figure 2.
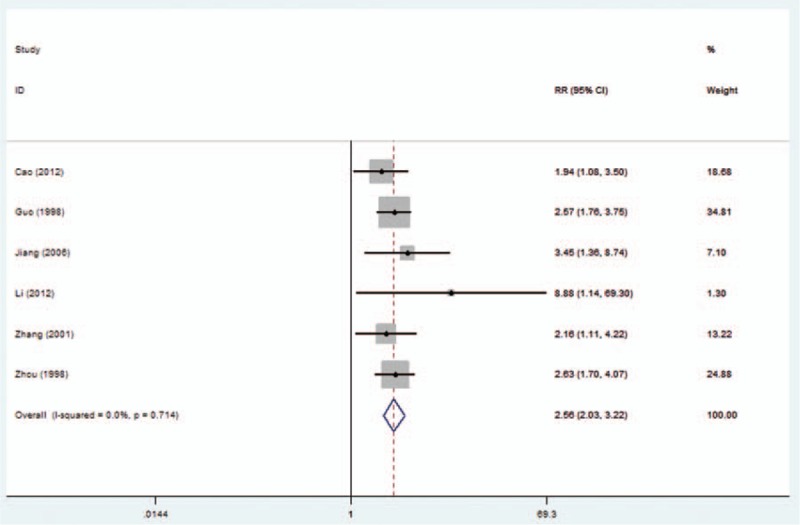
Forest plot evaluating the association of nm23-H1 expression and distant metastasis in NPC patients. NPC = nasopharyngeal carcinoma.
3.3. Survival outcome
In all, 9 studies included data on OS in 3 types of cancer. Homogeneity tests showed evidence of nonsignificant heterogeneity among studies in all the OS analyses. The low expression of nm23-H1 was significantly associated with a poorer OS in patients with NPC (3-year OS rate: RR: 0.55; 95% CI: 0.45–0.67, Fig. 3 and 5-year OS rate: RR: 0.60; 95% CI: 0.52–0.69, Fig. 4). Furthermore, the statistical significance was constant irrespective of different NPC subtypes containing NKC (3-year OS rate: RR: 0.25, 95% CI: 0.14–0.44 and 5-year OS rate: RR: 0.23, 95% CI, 0.14–0.38) and SCC (3-year OS rate: RR: 0.26, 95% CI: 0.11–0.63 and 5-year OS rate: RR: 0.26, 95% CI: 0.11–0.63).
Figure 3.
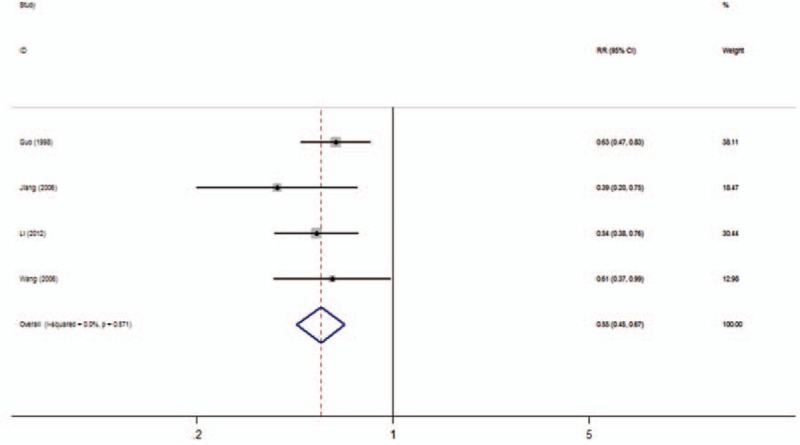
Forest plot evaluating the association of nm23-H1 expression and 3-year OS rate in NPC patients. NPC = nasopharyngeal carcinoma, OS = overall survival.
Figure 4.
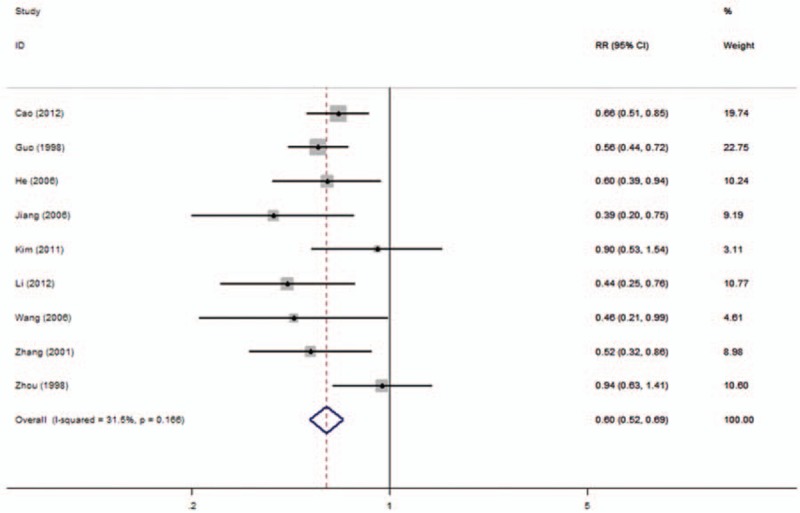
Forest plot evaluating the association of nm23-H1 expression and 5-year OS rate in NPC patients. NPC = nasopharyngeal carcinoma, OS = overall survival.
3.4. Sensitivity analysis
Sensitivity analysis was performed to estimate individual study's influence on the pooled RR, and the result of sensitivity analysis showed no other single study influenced the summary RR qualitatively, suggesting stability of the meta-analyses.
3.5. Heterogeneity and publication bias
There was no evidence of heterogeneity in all survival outcomes in the χ2 and I2 tests, and a fixed effect model was carried out. The funnel plot was performed to assess the publication bias in all the included studies for evaluation of the OS. As shown in Fig. 5, the funnel plot did not reveal any evidence of significant asymmetry.
Figure 5.

The funnel plot of publication bias.
4. Discussion
There has been a growing interest in evaluating prognostic markers for patients diagnosed with NPC, which might help conduct clinical therapies with regard to outcomes. In the present meta-analysis, 9 eligible studies including 861cases were analyzed. The results indicated that the low expression of nm23-H1 was significantly associated with a poorer 3-year and 5-year OS rate and higher local recurrence and distant metastasis in patients with NPC. Furthermore, the statistical significance was constant irrespective of different NPC subtypes containing NKC and SCC.
The reason that the low expression of nm23-H1 is potent to be the prognostic marker might be due to its implication in NPC treatment failure by disturbing the normal patterns of the nasopharyngeal cells. The overexpression of nm23-H1 correlated with the cellular and tumor radioresponse, and the mechanisms is the nm23-H1 protein involved in the regulation of intracellular functions, including stress responses, cell proliferation, and DNA repair. The overexpression of nm23-H1 also correlated with favorable response to chemotherapy in various cancers,[21–23] and the low expression of nm23-H1 attenuated the chemosensitivity of cancer cells to cisplatin, which was associated with reduced cisplatin-induced S-phase accumulation.[9]
Furthermore, downregulated nm23-H1 plays a role in tumor metastasis. Hartsough's study has revealed that the expression level of nm23-H1 is negatively correlated with the metastatic potential of certain human tumors.[24] Nm23-H1 strongly inhibited the metastasis in nude mice and inhibited the epidermal growth factor-induced cell migration in vitro, which may relate to the regulation of the myosin light-chain phosphorylation.[25] Decreased nm23-H1 expression by small interfering RNA significantly increased in vitro invasive ability of NPC cell line. The results further supported that nm23-H1 behaves as a metastasis suppressor in human NPC. It is worth noting that the above conclusions are controversial for certain tumors. The overexpression of nm23-H1 might even facilitate disease progression and poor prognosis in colonic neoplasms, gastric carcinoma, T-cell lymphoma, etc.[26–29]
However, the limitations of this study cannot be ignored. Firstly, variation in definitions of clinical outcomes, measurements, and experimental procedures might contribute to between-study heterogeneity. Secondly, the 9 trials included in the meta-analysis were mainly concentrated in the Asian population, and this may be related to the high incidence of NPC in southern China, Hong Kong, Taiwan, and Singapore. Thirdly, there was limited information regarding some patients, and the total sample sizes were small.
In summary, the low expression of nm23-H1 is associated with poorer prognosis patients with various subtypes of NPC, suggesting that it is a prognostic factor and a potential biomarker for survival in NPC.
4.1. Ethical review
Ethical approval was not necessary, because this article is a meta-analysis and it does not involve the participation of ethics committee.
Footnotes
Abbreviations: CI = confidence interval, HNSCC = head and neck squamous cell carcinoma, NKC = nonkeratinizing carcinomas, NOS = Newcastle–Ottawa Scale, NPC = nasopharyngeal carcinoma, OS = overall survival, RR = relative risk, SCC = squamous carcinoma, UC = undifferentiated carcinoma.
CY and LX contributed equally to this work.
Funding: This study was funded by Natural Science Foundation of Hubei Province, China (grant number 2014CFB312).
The authors have no conflicts of interest to disclose.
References
- [1].Jia WH, Huang QH, Liao J, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20-25 year period (1978/1983–2002) in Sihui and Cangwu counties in southern China. BMC Cancer 2006;6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [3].Chan AT. Current treatment of nasopharyngeal carcinoma. Eur J Cancer (Oxford, England: 1990) 2011;47Suppl 3:S302–3. [DOI] [PubMed] [Google Scholar]
- [4].Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011;80:661–8. [DOI] [PubMed] [Google Scholar]
- [5].Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188–98. [DOI] [PubMed] [Google Scholar]
- [6].Steeg PS, Bevilacqua G, Kopper L, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988;80:200–4. [DOI] [PubMed] [Google Scholar]
- [7].Jensen SL, Wood DP, Jr, Banks ER, et al. Increased levels of nm23 H1/nucleoside diphosphate kinase A mRNA associated with adenocarcinoma of the prostate. World J Urol 1996;14Suppl 1:S21–5. [DOI] [PubMed] [Google Scholar]
- [8].Khan MH, Yasuda M, Higashino F, et al. nm23-H1 suppresses invasion of oral squamous cell carcinoma-derived cell lines without modifying matrix metalloproteinase-2 and matrix metalloproteinase-9 expression. Am J Pathol 2001;158:1785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang YF, Chang CJ, Chiu JH, et al. NM23-H1 expression of head and neck squamous cell carcinoma in association with the response to cisplatin treatment. Oncotarget 2014;5:7392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qu S, Li XY, Liang ZG, et al. Protein expression of nucleophosmin, annexin A3 and nm23-H1 correlates with human nasopharyngeal carcinoma radioresistance in vivo. Oncol Lett 2016;12:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim YJ, Go H, Wu HG, et al. Immunohistochemical study identifying prognostic biomolecular markers in nasopharyngeal carcinoma treated by radiotherapy. Head Neck 2011;33:1458–66. [DOI] [PubMed] [Google Scholar]
- [12].Zhou Y, Xu A, Ling X. The expression of nm23-H1 and p53 protein in nasopharyngeal carcinoma. J Clin Otorhinolaryngol 1998;12:243–6. [PubMed] [Google Scholar]
- [13].Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Int Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- [14].Cao XJ, Hao JF, Yang XH, et al. Prognostic value of expression of EGFR and nm23 for locoregionally advanced nasopharyngeal carcinoma. Med Oncol (Northwood, London, England) 2012;29:263–71. [DOI] [PubMed] [Google Scholar]
- [15].Guo X, Min HQ, Zeng MS, et al. nm23-H1 expression in nasopharyngeal carcinoma: correlation with clinical outcome. Int J Cancer 1998;79:596–600. [DOI] [PubMed] [Google Scholar]
- [16].He SQ, Zhang WY. Relationship between the expressions of KaI1, nm23, ETS-1, VEGF and microvascular density and clinical significance in nasopharyngeal carcinoma. Chin J Otorhinolaryngol Head Neck Surg 2006;41:813–7. [PubMed] [Google Scholar]
- [17].Jiang WZ, Liao YP, Zhao SP, et al. Expression clinical significance of nm23-H1 and vessel endothelium growth factor protein in nasopharyngeal carcinoma. Chin J Otorhinolaryngol Head Neck Surg 2006;41:200–4. [PubMed] [Google Scholar]
- [18].Li X, Hu R, Qu J, et al. Identification of nm23-H1 as a metastatic suppressor and prognostic factor in nasopharyngeal carcinoma by proteomic analysis. J Central South Univ Med Sci 2012;37:17–26. [DOI] [PubMed] [Google Scholar]
- [19].Wang SL, Huang HC, Jiang YS, et al. Expressions and correlation with radiotherapy's effect of five kinds of genes in nasopharyngeal carcinoma. Chin J Otorhinolaryngol Head Neck Surg 2006;41:542–5. [PubMed] [Google Scholar]
- [20].Zhang G, Zeng J, Gong L, et al. Expression of vascular endothelial growth factor and nm23 as prognostic factors in nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Ke Za Zhi 2001;36:372–5. [PubMed] [Google Scholar]
- [21].Ferguson AW, Flatow U, MacDonald NJ, et al. Increased sensitivity to cisplatin by nm23-transfected tumor cell lines. Cancer Res 1996;56:2931–5. [PubMed] [Google Scholar]
- [22].lizuka N, Miyamoto K, Tangoku A, et al. Downregulation of intracellular nm23-H1 prevents cisplatin-induced DNA damage in oesophageal cancer cells: possible association with Na(+), K(+)-ATPase. Brit J Cancer 2000;83:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang LS, Chow KC, Lien YC, et al. Prognostic significance of nm23-H1 expression in esophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2004;26:419–24. [DOI] [PubMed] [Google Scholar]
- [24].Hartsough MT, Steeg PS. Nm23/nucleoside diphosphate kinase in human cancers. J Bioenerg Biomembr 2000;32:301–8. [DOI] [PubMed] [Google Scholar]
- [25].Suzuki E, Ota T, Tsukuda K, et al. nm23-H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulating myosin light chain phosphorylation. Int J Cancer 2004;108:207–11. [DOI] [PubMed] [Google Scholar]
- [26].Haut M, Steeg PS, Willson JK, et al. Induction of nm23 gene expression in human colonic neoplasms and equal expression in colon tumors of high and low metastatic potential. J Natl Cancer Inst 1991;83:712–6. [DOI] [PubMed] [Google Scholar]
- [27].Muller W, Schneiders A, Hommel G, et al. Expression of nm23 in gastric carcinoma: association with tumor progression and poor prognosis. Cancer 1998;83:2481–7. [DOI] [PubMed] [Google Scholar]
- [28].Pavelic K, Kapitanovic S, Radosevic S, et al. Increased activity of nm23-H1 gene in squamous cell carcinoma of the head and neck is associated with advanced disease and poor prognosis. J Mol Med (Berlin, Germany) 2000;78:111–8. [DOI] [PubMed] [Google Scholar]
- [29].Niitsu N, Nakamine H, Okamoto M, et al. Expression of nm23-H1 is associated with poor prognosis in peripheral T-cell lymphoma. Brit J Haematol 2003;123:621–30. [DOI] [PubMed] [Google Scholar]


