Abstract
This study was aimed to determine the risk factors of endoscopic variceal ligation-(EVL) induced ulcer bleeding.
The prevalence of EVL-induced ulcer bleeding is reported to be 3.6%. However, there are only limited reports of this serious complication, and the risk factors and the treatment methods are not well established.
A total of 430 patients who had undergone EVL in Chonnam National University Hospital from January 2014 to October 2016 were studied. EVL was performed for prophylaxis or acute hemorrhage. The patients were classified into 2 groups: a bleeding group (n = 33) and a non-bleeding group (n = 397). The patients who had endoscopically confirmed EVL-induced ulcer bleeding were included in the bleeding group.
EVL-induced ulcer bleeding occurred in 7.7% (n = 33) of the patients. In a multivariate analysis, model for end-stage liver disease (MELD) score >10 (odds ratio [OR]: 3.42, 95% confidence interval [CI]: 1.10–10.64), concomitant GV F3 (OR: 14.1, 95% CI: 2.84–71.43), and detachment of o-ring bands on follow-up endoscopy (OR: 8.06, 95% CI: 2.55–25.64) were independent predictive factors of EVL-induced ulcer bleeding. Various endoscopic modalities were attempted for hemostasis (EVL in 8 cases [24.2%], endoscopic variceal obturation [EVO] with cyanoacrylate in 6 cases [18.2%], argon plasma coagulation [APC] in 1 case (3%), Sengstaken–Blakemore (SB) tube in 3 cases [9.1%]), and proton pump inhibitor therapy only in 15 cases (45.5%).
MELD score >10, concomitant GV F3, and detachment of o-ring bands on follow-up endoscopy are risk factors for EVL-induced ulcer bleeding.
Keywords: endoscopic variceal ligation, esophageal varices, hemorrhage, therapeutics, ulcer
1. Introduction
Gastroesophageal varices are one of the most common complications of liver cirrhosis. Their prevalence is 40% of Child A patients and increases up to 85% of Child C patients.[1] Despite various efforts over the past decades, the mortality from esophageal variceal bleeding still remains 15% to 20%.[2] Currently, non-selective beta-blockers (BBs) or endoscopic variceal ligation (EVL) is recommended for primary prophylaxis of esophageal variceal bleeding. When active esophageal variceal bleeding occurs, the initial treatment of choice is EVL with pharmacologic treatment.[3]
The prevalence of EVL-induced ulcer bleeding is reported to be 3.6% to 15%,[4–7] and in some cases, this bleeding is fatal.[8,9] On the day after EVL, thrombi begin to develop in the strangulated vessels.[10] Approximately, 3 to 7 days after the banding, the rubber bands slip off and esophageal ulcerations develop, which heal within 2 to 3 weeks.[11] When early slippage of the rubber bands occurs, before the occlusion of the varix with a mature thrombus, rebleeding from the ulceration may occur. However, there are only limited reports of this serious complication and the risk factors and treatment methods are not well established. Vanbiervliet et al[12] have suggested that previous upper variceal digestive bleeding, peptic esophagitis, high AST to platelet ratio index (APRI) score, and low prothrombin time (PT index) are the risk factors for EVL-induced ulcer bleeding. Several traditional treatment methods, including cyanoacrylate injection, EVL, and transjugular intrahepatic portosystemic shunt,[13,14] and novel treatment methods, such as hemospray[15,16] and esophageal stent[17] have been suggested in past studies, but these reports included only a small number of patients. The aim of this study was to assess the risk factors of EVL-induced ulcer bleeding, and find appropriate treatment methods.
2. Material and methods
2.1. Patients
This study was a retrospective case-control study. Four hundred and thirty patients who had undergone EVL in Chonnam National University Hospital from January 2014 to October 2016 were studied. EVL was performed for prophylaxis or acute hemorrhage of the esophageal varices (EV). Following EVL, all patients received pantoprazole 40 mg intravenously for at least 3 days. Food intake was allowed 12 hours after prophylactic EVL and at the discretion of the physician after EVL of a bleeding EV. All patients received broad-spectrum antibiotics and vasoactive drugs according to the current guidelines.[3] Patients with a high risk of bleeding (EV form 3 [F3] or red-color signs) underwent follow-up endoscopy 1 to 2 weeks after EVL, in accordance with the policy in our institution (Fig. 1).
Figure 1.
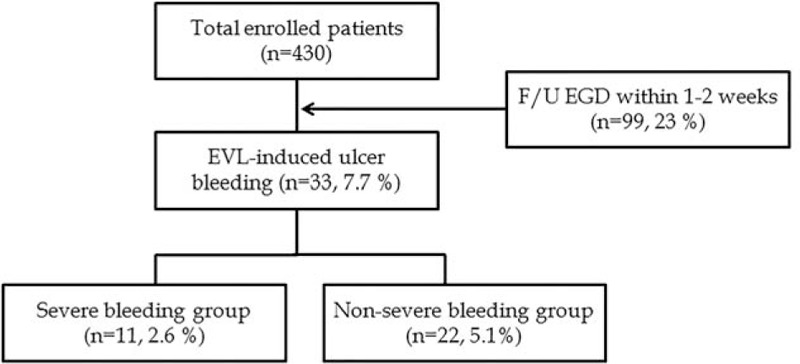
Flowchart of the enrolled patients.
2.2. Endoscopic treatment
Esophagogastroduodenoscopy (EGD) was performed using a forward-viewing endoscope (GIF Q 260, Olympus, Tokyo, Japan). EVL (using a 6 shooter Saeed multiband ligator, Cook Medical Endoscopy, Limerick, Ireland) was performed by occluding the protruding variceal column with elastic rubber rings, using a transparent cap attached to the distal end of the endoscope. N-Butyl-2-cyanoacrylate (Histoacryl; B. Braun Dexon, Spangenberg, Germany) was mixed with ethiodized oil (Lipiodol; Guerbert, Roissy, France) and was injected as a bolus dose of 0.5 to 2 mL, depending on the amount of the bleeding from the EVL-induced ulcer. Argon plasma coagulation (APC) was performed through the working channel of the endoscope under direct visualization by using an electrosurgical generator (VIO 300D, Erbe Elektromedizin GmbH, Tuebingen, Germany) and a 2.3 mm probe.
2.3. Definitions
EVL-induced ulcer bleeding was defined as endoscopically confirmed active bleeding (spurting or oozing) from an ulcer that was formed due to the slippage of the rubber band (Fig. 2). We confirmed that there was no other upper gastrointestinal bleeding source. Severe bleeding was defined as bleeding which resulted in hypotension (blood pressure <90 mm Hg) or death after EVL. Bleeding-related death was defined as death within 6 weeks of the index bleeding.[18] The size of EV was classified as small, straight (F1); enlarged, tortuous (F2); or large, coil-shaped that occupy more than one-third of the lumen (F3).[19,20] The morphology of the gastric varices (GV) was classified according to the system proposed by Hashizume et al[21]: tortuous (F1), nodular (F2), or tumorous (F3).
Figure 2.
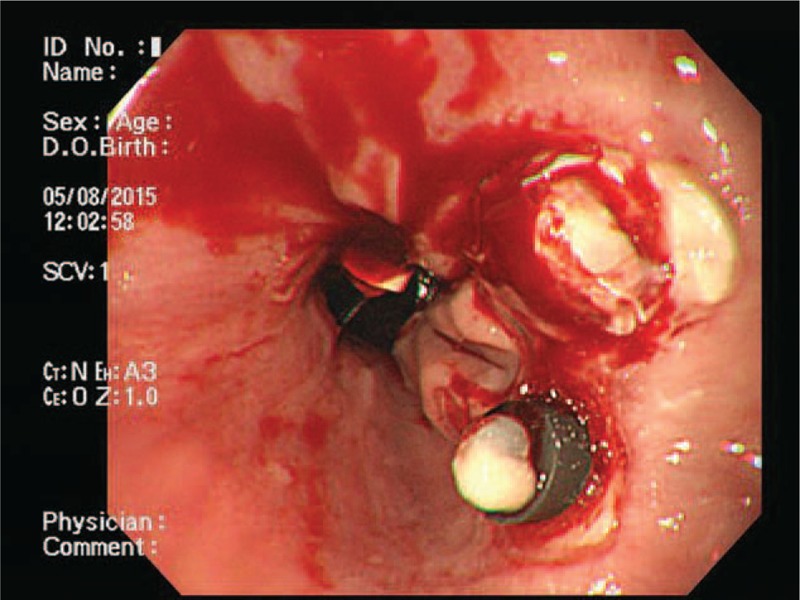
Esophagogastroduodenoscopy (EGD) showed active oozing from endoscopic variceal ligation (EVL)-induced ulcer site.
2.4. Ethical considerations
The present study was conducted in accordance to the ethical guidelines of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No.: CNUH-2016-208). All patients gave informed consents.
2.5. Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Inc., IBM, Chicago, IL). Continuous data are shown as mean ± SD, and categorical data as absolute and relative frequencies. Continuous variables between the bleeding and non-bleeding groups were analyzed using Student t test. Categorical data were examined using the Fisher exact test or χ2 test with Yates correction. In the multivariate analysis, binary logistic regression models were used to investigate the risk factors associated with EVL-induced ulcer bleeding. Variables with a P value ≤.05 at the univariate analysis were selected for possible inclusion in the multivariate analysis. Data for regression analysis are presented as odds ratio with 95% confidence intervals.
3. Results
3.1. Baseline characteristics of the enrolled patients
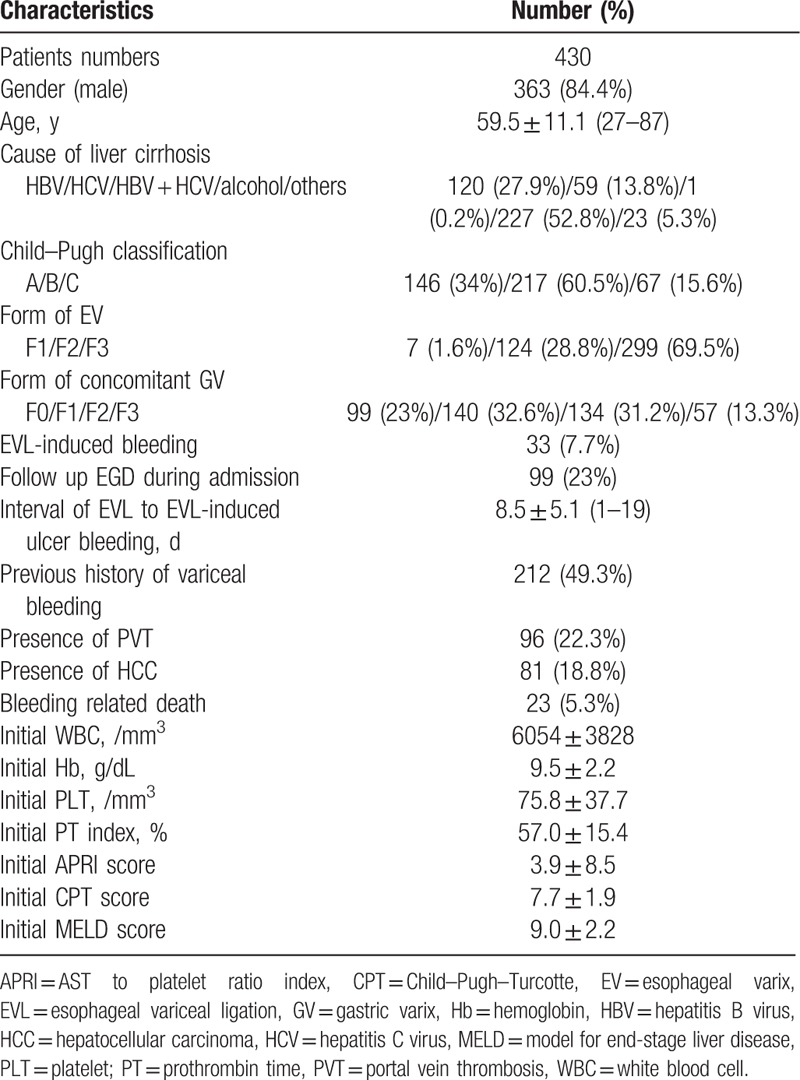
The patients included 363 (84.4%) men and 67 (15.6%) women. The mean age of the enrolled patients was 59.5 ± 11.1 years (range, 27–87 years). The Child–Pugh classification of enrolled patients was A in 146 (34%) patients, B in 217 (60.5%) patients, and C in 67 (15.6%) patients. The form of EV was classified as F1 in 7 (1.6%) patients, F2 in 124 (28.8%) patients, and F3 in 299 (69.5%) patients. The form of concomitant GV was classified as F0 in 99 (23%) patients, F1 in 140 (32.6%) patients, F2 in 134 (31.2%) patients, and F3 in 57 (13.3%) patients. EVL-induced ulcer bleeding was observed in 33 (7.7%) patients. The mean interval of EVL to EVL-induced ulcer bleeding was 8.5 ± 5.1 days (range, 1–19 days). Two hundred and twelve (49.3%) patients had a previous history of variceal hemorrhage, 96 (22.3%) patients had portal vein thrombosis, and 81 (18.8%) patients had hepatocellular carcinoma (HCC). Bleeding-related death rate was 5.3% (23/430). Initial PT index of the enrolled patients was 57.0 ± 15.4%, initial APRI score was 3.9 ± 8.5, initial Child–Pugh–Turcotte score was 7.7 ± 1.9, and initial model for end-stage liver disease (MELD) score was 9.0 ± 2.2. The baseline clinical characteristics of the enrolled patients are shown in Table 1.
Table 1.
Baseline clinical characteristics of the enrolled patients.
3.2. Comparison of baseline characteristics between the patient groups
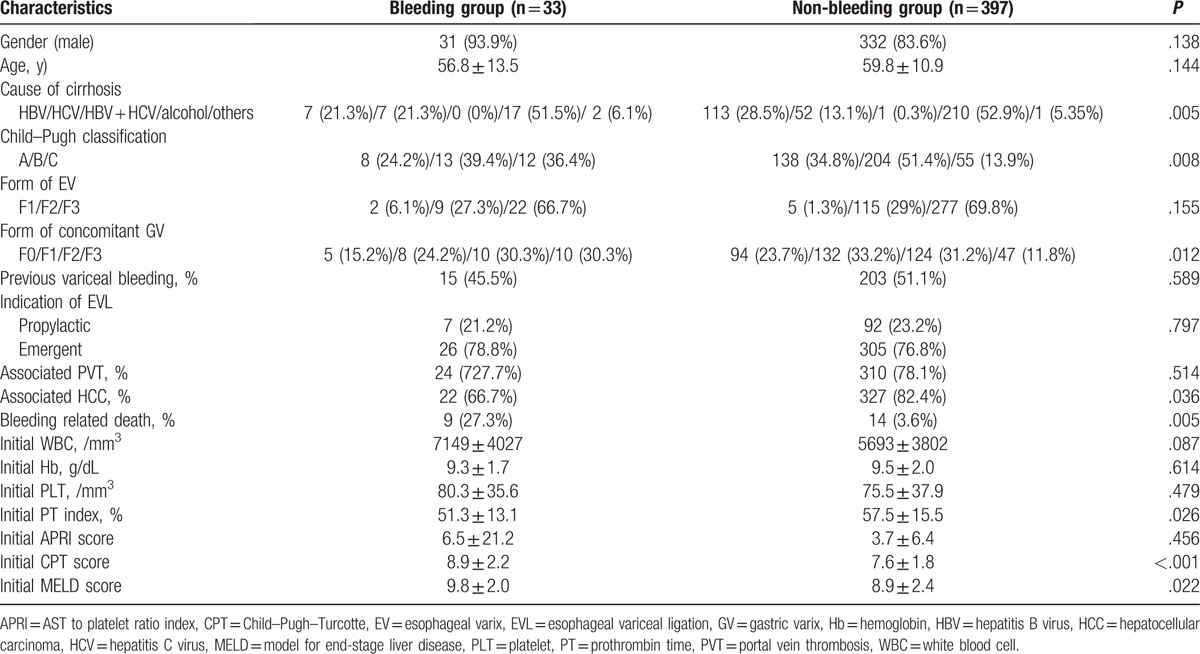
Of the 430 enrolled patients, 33 patients who experienced EVL-induced ulcer bleeding were classified as the “bleeding group,” and the other 397 patients were classified as the “non-bleeding group” (Fig. 1). The analysis of both groups is shown in Table 2. There were more patients with hepatitis C virus (HCV) infection (21.3% vs. 13.1%, P = .005), Child–Pugh classification C (36.4% vs. 13.9%, P = .008), and concomitant GV F3 (30.3% vs. 11.8%, P = .012) in the bleeding group, than the non-bleeding group. HCC was more frequently present in the non-bleeding group (66.7% vs. 87.4%, P = .036). Initial PT index, Child–Pugh score, and MELD score were associated with EVL-induced ulcer bleeding (P = .026, P < .05, and P = .022, respectively). More bleeding-related death was observed in the bleeding group than the non-bleeding group (27.3% vs. 3.6%, P = .005). Other baseline clinical characteristics were not significantly different between the 2 groups. A total of 99 patients (33 patients in the bleeding group and 66 patients in the non-bleeding group) received follow-up endoscopy within 1 to 2 weeks after EVL. On follow-up endoscopy, 28 patients (84.8%) had detachment of the rubber bands in the bleeding group, but this was also observed in 26 patients (39.4%) in the non-bleeding group (P < .001). (These data are not shown in the table).
Table 2.
Comparison of baseline characteristics between the patient groups.
3.3. Analysis of potential risk factors for EVL-induced ulcer bleeding
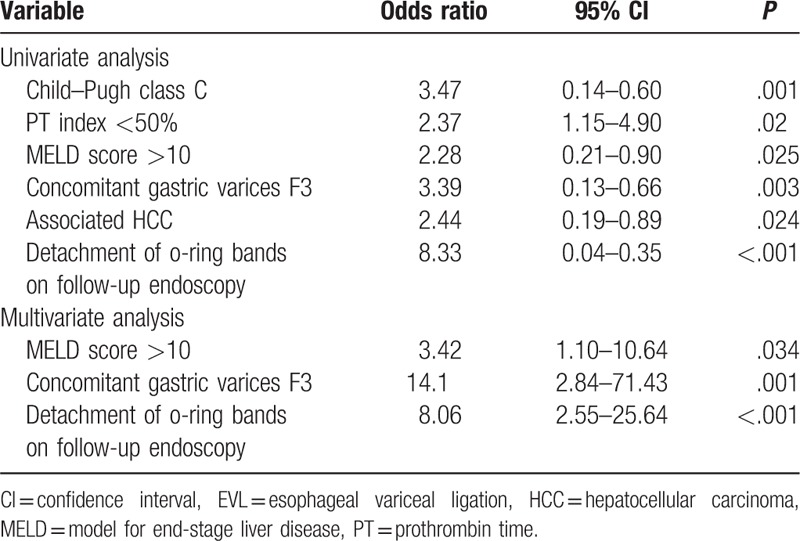
We evaluated potential risk factors for EVL-induced ulcer bleeding. In a univariate analysis, Child–Pugh class C (P = .001), PT index <50% (P = .02), MELD score >10 (P = .025), concomitant GV F3 (P = .003), presence of HCC (P = .024), and detachment of o-ring bands on follow-up EGD (P < .001) were associated with EVL-induced ulcer bleeding (Table 3). In multivariate analysis, MELD score >10 (P = .034), concomitant GV F3 (P = .001), and detachment of o-ring bands on follow-up EGD (P < .001) were independent predictive factors of EVL-induced ulcer bleeding (Table 3).
Table 3.
Univariate and multivariate analysis of potential risk factors for EVL-induced ulcer bleeding.
3.4. Treatment methods and clinical outcomes of EVL-induced ulcer bleeding
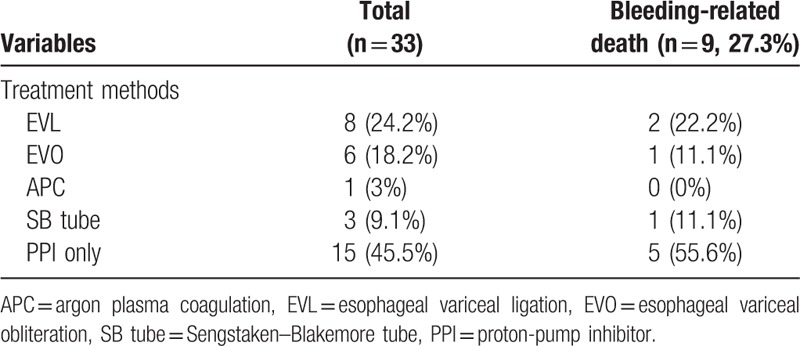
Of the 33 patients who were confirmed to have EVL-induced ulcer bleeding, 24.2% of patients underwent EVL and 18.2%, 3%, 9.1%, and 45.5% of patients received endoscopic variceal obturation (EVO), APC, Sengstaken–Blakemore (SB) tube, and proton pump inhibitor (PPI) only as the rescue therapy for EVL-induced ulcer bleeding, respectively (Table 4). EVL was the most commonly performed rescue therapy, performed in 24.2% of the patients. Bleeding-related death was observed in 27.3% of the bleeding group. Mortality was highest in the PPI only treated group (55.6%).
Table 4.
Treatment methods and clinical outcomes of EVL-induced ulcer bleeding.
3.5. Subgroup analysis of EVL-induced ulcer bleeding
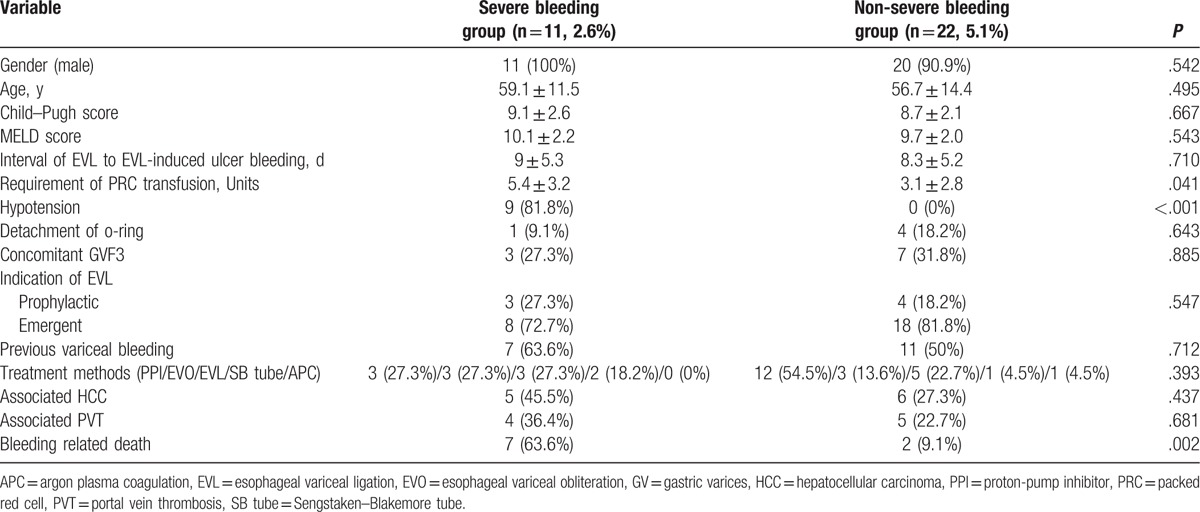
The definition of severe bleeding was bleeding that resulted in hypotension or death after EVL. The subgroup analysis showed that 11 patients (2.6%) were in the severe bleeding group and 22 patients (5.1%) were in the non-severe bleeding group. Baseline clinical characteristics were not significantly different between the 2 groups (Table 5). Requirement of packed red blood cell (PRC) transfusion, hypotension, and death were more frequently observed in the severe bleeding group. Sex, age, Child–Pugh score, early detachment of o-ring, presence of HCC, portal vein thrombosis, and emergent EVL were not associated with severe bleeding.
Table 5.
Subgroup analysis of EVL-induced ulcer bleeding group.
4. Discussion
Significant EVL-induced ulcer bleeding occurs in 3.6% to 15% of cases,[4–7] and the mortality is reported to be as high as 52%.[12] However, the risk factors of EVL-induced ulcer bleeding have not been clearly identified, and there are currently no guidelines for the treatment of this potentially lethal complication.
This study is unique in that the incidence rate, risk factors, and treatment methods of the EVL-induced ulcer bleeding were comprehensively evaluated. In addition, this is a large number cohort study including 430 patients who received prophylactic or emergent EVL, with follow-up endoscopies in 99 patients (23%) within 1 to 2 weeks following EVL. The detachment of rubber bands was also assessed for the first time.
The incidence of EVL-induced ulcer bleeding in our study was 7.7% of all EVL episodes. The reason for EVL did not affect the incidence of EVL-induced ulcer bleeding. Among the 99 patients who received prophylactic EVL, 7 patients (7.1%) had EVL-induced ulcer bleeding, and among the 331 patients who received emergent EVL, 26 (7.9%) experienced EVL-induced ulcer bleeding. The incidence of EVL-induced ulcer bleeding in our study is higher than recently published rates of about 2.8%.[12,22] However, the rate of severe bleeding, which was defined as bleeding associated with hypotension or death, was 2.6%, similar to the previous reports. The high proportion (23%) of follow-up endoscopies within 1 to 2 weeks after EVL made it possible to detect minor EVL-induced ulcer bleeding and may explain the high prevalence in our study.
The mortality rate of the EVL-induced ulcer bleeding was 27.3% in our study, which is significantly higher than the mortality rate (3.6%) observed in the patients without EVL-induced ulcer bleeding. This mortality rate was similar to a previous report of 28% by Sinclair et al,[22] but lower than another report of 52% by Vanbiervliet et al.[12] In the study by Sinclair et al, the use of prophylactic antibiotics was not recorded. However, in the study by Vanbiervliet et al, the cause of death in the majority of the patients was sepsis, and this was explained by the low rate of prophylactic antibiotic usage (62%). The patients in our study received prophylactic antibiotics according to the guidelines,[3] and this may explain the lower mortality rate and emphasizes the importance of prophylactic antibiotics when performing emergent EVL.
In our study, MELD score >10, concomitant GV F3, and detachment of o-ring bands were independent risk factors for the EVL-induced ulcer bleeding. Previously reported independent predictive factors such as emergent EVL, previous history and treatment of upper variceal bleeding, high APRI score, and low PT index,[12,22] were not risk factors in our study. Poor liver function has been well known as a predictive factor for bleeding in patients with liver cirrhosis,[6,7] and a previous study reported MELD score as a risk factor for EVL-induced bleeding as well.[22] Our study confirmed that a higher MELD score is a risk factor for EVL-induced ulcer bleeding.
Interestingly, concomitant GV F3 was a predictive factor for EVL-induced ulcer bleeding in our study. This is a new risk factor, which has not been evaluated in previous studies. When the liver becomes cirrhotic and portal hypertension develops, portosystemic collaterals develop in an attempt to decompress the pressure. Increased blood flow and increased blood volume worsen the formation of collaterals.[23] The blood reflux in the left gastric vein that originally drained into the portal vein, results in esophageal variceal formation. The retrograde blood flow in the short gastric veins that drain into the splenic vein makes GVs. EVL is performed for the reduction of the blood vessel size to prevent bleeding, not for the reduction in the blood volume or pressure. Thus, the still increased blood volume or pressure should be compensated by other parts of the cephalad collaterals (e.g., splenic vein) until the EVL-induced ulceration heals to prevent rebleeding from the ulcer sites that are more fragile than the original mucosa. However, when there is a large GV F3, with retrograde blood flow and high pressure in the splenic vein, compensation cannot occur properly and remaining blood volume and pressure overload in the EV may result in rebleeding at the more vulnerable ulcer sites (EVL-induced ulcer bleeding). This hypothesis corresponds well with our previous findings, in which higher rebleeding rates were observed after sclerotherapy of GV in cases with large EV.[24] Thus, the potential to compensate for the increased blood volume and pressure in the whole cephalad collateral system may be important in the acute phase after EVL or sclerotherapy of gastroesophageal varices.
Detachment of the o-ring bands on follow-up endoscopy was another risk factor for EVL-induced ulcer bleeding in our study. In the bleeding group, 84.8% of patients had detachment of the rubber bands, but this was observed in 39.4% of patients in the non-bleeding group (P < .001). Most of the EVL-induced ulcer bleeding occurred within 2 weeks of the procedure (29/33, 87.9%) and most of the severe bleeding was observed in this period (10/11, 90.9%). These findings are comparable to previous reports suggesting that massive bleeding from EVL-induced ulcers usually occurs between 5 and 10 days when the o-ring bands were detached.[9,25,26] However, 4 patients (12.1%) had bleeding in 15 to 19 days after EVL and 1 of these patients died in our study. In a previous report of autopsy specimens from 6 patients, non-healed ulcers were observed 22 days after ligation.[10] Our finding is similar to this report, suggesting that signs for rebleeding should be observed for 3 weeks after EVL. Several studies have been conducted to evaluate the efficacy of PPIs or sucralfate in promoting ulcer healing and reducing bleeding after EVL, but the results were inconclusive.[12,22,27] Further studies to look for methods to enhance ulcer healing are needed.
It is not always possible to avoid EVL in patients with high MELD score and concomitant large GV. It is especially impossible in cases of emergent EVL. However, meta-analysis of EVL versus BB in primary prophylaxis shows that both treatments reduce bleeding and mortality significantly.[28] Thus, when primary prophylaxis of EV is needed in patients with these risk factors, BB might be considered rather than EVL. But this treatment strategy needs a well-designed randomized controlled trial.
In subgroup analysis of the EVL-induced ulcer bleeding group, the mortality was significantly higher in the severe bleeding group (63.6% vs. 9.1%). Associated factors for severe bleeding were presence of hypotension and requirement of PRC transfusion. Higher MELD or Child–Pugh score, concomitant GV F3, detachment of o-ring bands, previous history of variceal bleeding, and prophylactic or emergent EVL were not associated with severe bleeding. Therefore, there is no way to predict the severity of EVL-induced ulcer bleeding before EVL. When patients with EVL-induced bleeding experience hypotension and require more than 6 units of PRC transfusion, a high mortality rate can be predicted and other rescue therapies such as early transjugular intrahepatic portosystemic shunt or liver transplantation may be needed.
In our study, various treatment modalities including EVL, EVO with N-butyl-2-cyanoacrylate, APC, SB tube, or intravenous PPIs only were used to control EVL-induced ulcer bleeding. We noticed a trend towards increased mortality in patients who received PPIs only as a rescue therapy, but this was not statistically significant. Further large number, prospective randomized studies are warranted to find best treatment methods for EVL-induced ulcer bleeding.
There are several limitations of our study. First, this was a retrospective study; therefore, the findings in our study may not apply to the general population. Secondly, the treatment modalities applied to EVL-induced ulcer bleedings were dependent on the physician's preference and decision. Thirdly, novel treatment methods, such as hemospray or esophageal stents, were not used. In addition, the number of patients who received different rescue therapies was too small to find statistical significance. Lastly, follow-up EGD after EVL was not done at the same time, thus the status of detachment of o-ring bands was inconsistently observed. Further prospective studies, including strict follow-up EGD schedules, are needed to confirm this risk factor for EVL-induced bleeding.
In conclusion, EVL-induced ulcer bleeding is not a rare complication of EVL and has a relatively high mortality rate. MELD score >10, concomitant GV F3, and detachment of o-ring bands in follow-up endoscopy are the predictive factors for EVL-induced bleeding. This complication was most commonly observed within 2 weeks after EVL, but could also occur as late as 19 days. Therefore, pharmacologic treatment with BBs may be better as primary prophylaxis than EVL in patients with these risk factors, and when EVL is unavoidable, patients should be observed for signs of rebleeding for 3 weeks.
Footnotes
Abbreviations: APC = argon plasma coagulation, APRI = AST to platelet ratio index, BB = beta-blocker, EGD = esophagogastroduodenoscopy, EV = esophageal varices, EVL = endoscopic variceal ligation, EVO = endoscopic variceal obturation, GV = gastric varices, HCC = hepatocellular carcinoma, MELD = model for end-stage liver disease, PPI = proton pump inhibitor, PT = prothrombin time, SB tube = Sengstaken–Blakemore tube.
EC, CHJ, SBC, CHP, HSK, SKC, and JSR have no financial ties to disclose.
The authors report no conflicts of interest.
References
- [1].Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007;102:2086–102. [DOI] [PubMed] [Google Scholar]
- [2].D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–31. [DOI] [PubMed] [Google Scholar]
- [3].de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- [4].Schmitz RJ, Sharma P, Badr AS, et al. Incidence and management of esophageal stricture formation, ulcer bleeding, perforation, and massive hematoma formation from sclerotherapy versus band ligation. Am J Gastroenterol 2001;96:437–41. [DOI] [PubMed] [Google Scholar]
- [5].Petrasch F, Grothaus J, Mossner J, et al. Differences in bleeding behavior after endoscopic band ligation: a retrospective analysis. BMC Gastroenterol 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bambha K, Kim WR, Pedersen R, et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008;57:814–20. [DOI] [PubMed] [Google Scholar]
- [7].D’Amico G, De Franchis R, Cooperative Study G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38:599–612. [DOI] [PubMed] [Google Scholar]
- [8].Mishin I, Dolghii A. Early spontaneous slippage of rubber bands with fatal bleeding: a rare complication of endoscopic variceal ligation. Endoscopy 2005;37:275–6. [DOI] [PubMed] [Google Scholar]
- [9].Toyoda H, Fukuda Y, Katano Y, et al. Fatal bleeding from a residual vein at the esophageal ulcer base after successful endoscopic variceal ligation. J Clin Gastroenterol 2001;32:158–60. [DOI] [PubMed] [Google Scholar]
- [10].Polski JM, Brunt EM, Saeed ZA. Chronology of histological changes after band ligation of esophageal varices in humans. Endoscopy 2001;33:443–7. [DOI] [PubMed] [Google Scholar]
- [11].Nijhawan S, Rai RR, Nepalia S, et al. Natural history of postligation ulcers. Am J Gastroenterol 1994;89:2281–2. [PubMed] [Google Scholar]
- [12].Vanbiervliet G, Giudicelli-Bornard S, Piche T, et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Aliment Pharmacol Ther 2010;32:225–32. [DOI] [PubMed] [Google Scholar]
- [13].Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Practice Guidelines Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922–38. [DOI] [PubMed] [Google Scholar]
- [14].Tierney A, Toriz BE, Mian S, et al. Interventions and outcomes of treatment of postbanding ulcer hemorrhage after endoscopic band ligation: a single-center case series. Gastrointest Endosc 2013;77:136 e131–40 e131. [DOI] [PubMed] [Google Scholar]
- [15].Ozaslan E, Purnak T, Yildiz A, et al. Bleeding due to slippage of elastic band during variceal ligation: successful use of Ankaferd blood stopper. Indian J Gastroenterol 2010;29:166–8. [DOI] [PubMed] [Google Scholar]
- [16].Ibrahim M, Lemmers A, Deviere J. Novel application of hemospray to achieve hemostasis in post-variceal banding esophageal ulcers that are actively bleeding. Endoscopy 2014;46suppl: UCTN:E263. [DOI] [PubMed] [Google Scholar]
- [17].Choudhary NS, Puri R, Saigal S, et al. Innovative approach of using esophageal stent for refractory post-band ligation esophageal ulcer bleed following living donor liver transplantation. J Clin Exp Hepatol 2016;6:149–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis 2001;5:645–63. [DOI] [PubMed] [Google Scholar]
- [19].Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med 1988;319:983–9. [DOI] [PubMed] [Google Scholar]
- [20].Beppu K, Inokuchi K, Koyanagi N, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 1981;27:213–8. [DOI] [PubMed] [Google Scholar]
- [21].Hashizume M, Kitano S, Yamaga H, et al. Endoscopic classification of gastric varices. Gastrointest Endosc 1990;36:276–80. [DOI] [PubMed] [Google Scholar]
- [22].Sinclair M, Vaughan R, Angus PW, et al. Risk factors for band-induced ulcer bleeding after prophylactic and therapeutic endoscopic variceal band ligation. Eur J Gastroenterol Hepatol 2015;27:928–32. [DOI] [PubMed] [Google Scholar]
- [23].Polio J, Groszmann RJ. Hemodynamic factors involved in the development and rupture of esophageal varices: a pathophysiologic approach to treatment. Semin Liver Dis 1986;6:318–31. [DOI] [PubMed] [Google Scholar]
- [24].Jun CH, Kim KR, Yoon JH, et al. Clinical outcomes of gastric variceal obliteration using N-butyl-2-cyanoacrylate in patients with acute gastric variceal hemorrhage. Korean J Intern Med 2014;29:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Van Vlierberghe H, De Vos M, Hautekeete M, et al. Severe bleeding following endoscopic variceal ligation: should EVL be avoided in Child C patients? Acta Gastroenterol Belg 1999;62:175–7. [PubMed] [Google Scholar]
- [26].Sakai P, Maluf Filho F, Melo JM, et al. Is endoscopic band ligation of esophageal varices contraindicated in Child-Pugh C patients? Endoscopy 1994;26:511–2. [DOI] [PubMed] [Google Scholar]
- [27].Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005;41:588–94. [DOI] [PubMed] [Google Scholar]
- [28].Khuroo MS, Khuroo NS, Farahat KL, et al. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005;21:347–61. [DOI] [PubMed] [Google Scholar]


