Abstract
Background:
Robot-assisted minimally invasive surgery (RVATS) is a relatively new technique applied for thymectomies. Only few studies directly compare RVATS to the mainstay therapy, open surgery (sternotomy).
Methods:
A systematic search of the literature was performed in October 2016. The meta-analysis includes studies comparing robotassisted and open thymectomy regarding operation time, length of hospitalization, intraoperative blood loss, and chest-in-tube days, postoperative complications, reoperation, arrhythmic events, pleural effusion, and postoperative bleeding.
Results:
Of 626 studies preliminary screened, 7 articles were included. There were no significant differences in comparison of operation time (−3.19 minutes [95% confidence interval, 95% CI −112.43 to 106.05]; P = .94), but patients undergoing RVATS spent significantly less time in hospital (−4.06 days [95% CI −7.98 to −0.13], P = .046). There were fewer chests-in-tube days (−2.50 days [95% CI −15.01 to 10.01]; P = .24) and less intraoperative blood loss (−256.84 mL [95% CI −627.47 to 113.80]; P = .10) observed in the RVATS group; due to a small number of studies, these results were not statistically significant. There were also less post-operative complications in the RVATS group (12 complications in 209 patients vs 51 complications in 259 patients); however, this difference was not statistical significant (odds ratio 0.27, 95% CI 0.07–1.12; P = .06).
Conclusions:
Patients undergoing RVATS spent less time in hospital than patients treated by open surgery (sternotomy). These patients tended to have less postoperative complications, less intraoperative blood loss, and fewer chest-in-tube days. We found evidence for the safety and feasibility of RVATS compared with open surgery, which has to be further confirmed in randomised controlled trials.
Keywords: robot-assisted minimally invasive surgery, thoracic surgery, thymectomy
1. Introduction
Thymectomy is considered the standard of care in younger (<50 years) nonthymoma patients with myasthenia gravis [1] and cornerstone of treatment for thymoma patients.[2] In thymoma, the therapeutic approach depends mainly on the Masaoka classification,[3] which combines perioperative and histopathological findings. However, due to the rarity of thymoma, no prospective randomized trials on thymoma are available and the potential improvement of new treatment methods has mainly got to be evaluated in collaborative studies.[2] Surgical thymectomy is nowadays considered the mainstay of treatment in thymoma and thymic carcinoma.[4]
Robotic video assisted thoracic surgery (RVATS) was first established as a new operation technique in thoracic surgery 2003 for lobectomy [5] and other complex thoracoscopic procedures.[6] When operating with the da Vinci surgical system, the surgeon's movements are transferred to the tip of the instruments via a console, making use of highly sensitive motion sensors.[7] Advantages and disadvantages of the da Vinci Surgical System have been summed up by the Ontario Health Technology Advisory Committee [8] as follows: “The main advantages of use of the robotic device are: 1) the precision of the instrument and improved dexterity due to the use of “wristed” instruments; 2) three-dimensional imaging, with improved ability to locate blood vessels, nerves and tissues; 3) the surgeon's console, which reduces fatigue accompanied with conventional laparoscopy surgery and allows for tremor-free manipulation. The main disadvantages of use of the robotic device are the costs including instrument costs ($2.6 million in US dollars), cost per use ($200 per use), the costs associated with training surgeons and operating room personnel, and the lack of tactile feedback, with the trade-off being increased visual feedback.“
There have been a number of studies establishing a role of RVATS in surgery of the pelvis, for example, in benignant gynecology and rectal and colonic cancer.[9–11] Also, there has recently been evidence for a potential role of RVATS in the treatment of lung cancer; in lobectomy, RVATS was associated with shorter hospital stay, shorter chest tube duration, and less blood loss compared with open lobectomy.[12–14]
A first series of mediastinal resections including 9 thymectomies was published in 2004,[15] suggesting suitability of the procedure for complete thymectomy, and thereby also for the treatment of thymoma. In 2006, the authors of a case series of 22 thymectomies [16] concluded that the mediastinum should remain an area of special interest for robotic surgeons.
In 2013, a case series of 100 patients undergoing robotic thymectomy was published and described the procedure as safe and effective; they also observed a neurological benefit for the majority of patients, especially in early stages of myasthenia gravis.[17] A multicenter observational study [18] described RVATS thymectomy in 79 patients with stage I and II thymoma as a safe procedure with a short hospital stay, low postoperative complication rate, and good oncologic outcomes at a median follow-up of 40 months.
According to our literature search, no randomized trials comparing RVATS to open surgery in thymectomy or treatment of thymoma have as yet been performed. Therefore, we hereby attempt to offer the best available evidence by reviewing data from all relevant comparative studies available. We will also perform meta-analyses wherein data quality is accordingly sensible.
2. Methods
2.1. Search strategies and data collection
To identify relevant studies, a systematic literature review was performed by searching PubMed on October 25, 2016, using the search terms ((“thymectomy” OR “thymoma” OR “thymus”) AND (“open” OR “open surgery” OR “sternotomy” OR “transsternal” OR “thoracotomy”) AND (“robotic” OR “robot” OR “robot assisted” OR “da Vinci” OR “daVinci”)). No language restriction and no filters were applied. In addition, the following literature databases were screened: The Cochrane Library, BioMed Central and Science Direct, and the “MEDLINE related articles” option was used to identify further relevant studies. All abstracts were screened for inclusion and exclusion criteria by 2 investigators (CS and AE). The basic inclusion criterion was the comparison of outcomes in robot-assisted versus open surgery thymectomy. Detailed inclusion criteria were suitable reporting of the surgical outcomes, operation time, length of hospitalization, intraoperative blood loss, and chest-in-tube days, postoperative complications, reoperation, arrhythmic events, pleural effusion, or postoperative bleeding. Studies were excluded if no data suitable for statistical analysis were available. Only data of already published studies found through online research were used for meta-analysis, and we did not require the approval of the local ethics committee.
After a preliminary screening of 626 potentially relevant articles by 2 researchers, we considered 10 studies for meta-analysis. Three studies were excluded for the following reasons: In the study of Orsini et al,[19] there was no separation between patients treated with RVATS and patients treated with VATS; in another study, only patients treated with RVATS were included without control group [20]; and the study of Cakar et al[21] was excluded as no data on patients characteristics were available. Details on study identification and selection are shown in Fig. 1.
Figure 1.
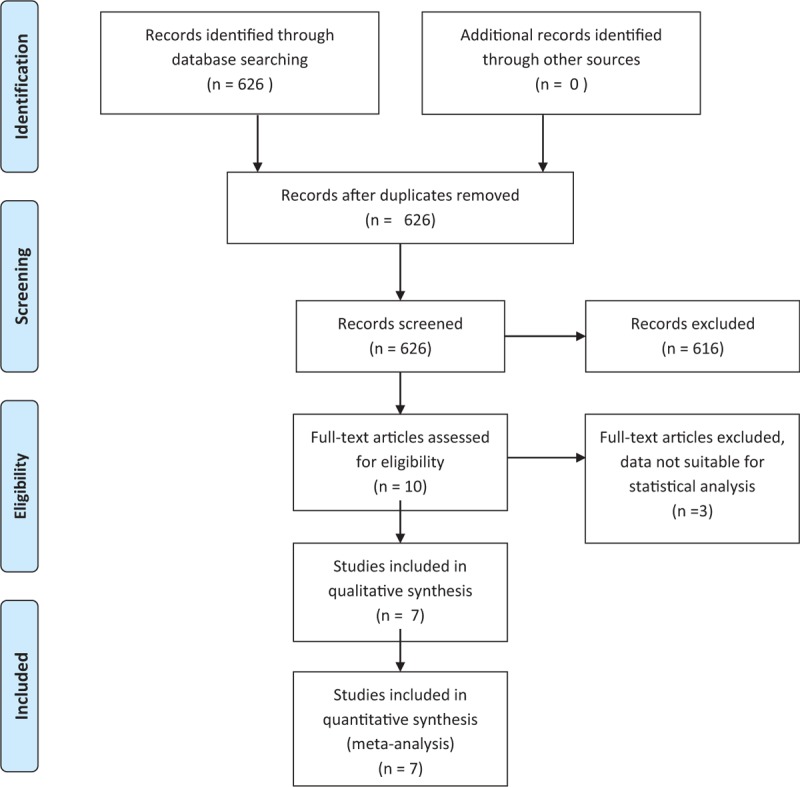
Flow diagram of studies identified, included, excluded.
Data extraction was performed by 1 investigator (JH) and independently verified by another (CS); disagreements were resolved by consensus between these 2 researchers.
Data were extracted on the surgical outcomes as well as study characteristics, including the number of patients, age, proportion of females and males, and types of surgery method.
2.2. Statistical analysis
Meta-analyses were conducted separately for each surgical outcome. If the outcome was a continuous measure (e.g., operation time, length of hospitalization, intraoperative blood loss, and chest-in-tube days), the number of patients in each group, the mean value and standard error, and the mean differences were used (inverse variance method).
For dichotomous variables (e.g., postoperative complications, reoperation, arrhythmic events, pleural effusion, and postoperative bleeding), the number of events and the number of patients in the groups were used. The groups were compared by considering logarithmic odds ratios.
Due to the clinical and methodological heterogeneity between studies, random-effects models were used to allow the combination of data from different studies.[22] In the case of zero counts (zero count per cell) in a contingency table, a continuity correction of 0.5 was added to all cells.[23] In 1 study, only the P value relating to the mean differences for continuous outcomes was given but not the standard deviation.[24] In this case, standard deviations were obtained from the P value and t value according to the description in the Cochrane Handbook. Between-study variance and its uncertainty was estimated by the method proposed by Paule and Mandel [25,26] and the Cochran Q test to assess heterogeneity was used. In order to account for the small number of studies available for the meta-analyses and varying studies’ precisions, the modified Knapp–Hartung approach was used to combine effect estimates and to derive associated confidence intervals.[27] Computations were performed using R [28] and the metafor package.[29] Forest plots were generated with forestplot package [30] in R.
3. Results
3.1. Study characteristics
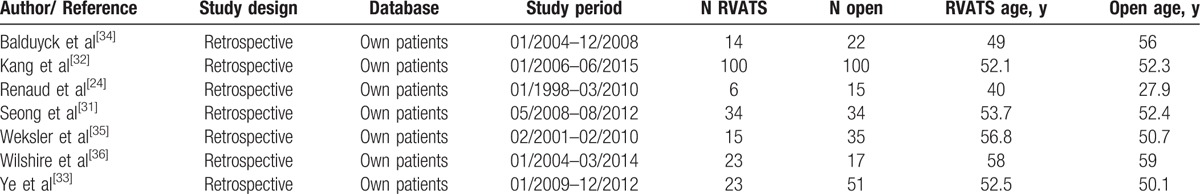
Seven retrospective studies published between 2010 and 2016 were analyzed for this study. Characteristics are listed in Table 1.[31–36] Of a total of 489 patients, 215 (44%) were undergoing RVATS, while 274 (56%) experienced open surgery. The number of patients included into these studies varied between 6 and 100 per study for RVATS and between 15 and 100 for open sternotomy. Mean (or in some cases median) age of patients included ranged from 40 to 58 years for RVATS and 27.9 to 59 years per study for open surgery. Two out of seven studies used propensity score matching to reduce bias.[31,32] Indication for open surgery or RVATS were anterior mediastinal masses,[31,32] thymoma [33–36] (classified using the Masaoka [33] or the WHO classification),[34] thymic cysts, thymic hyperplasia, [34,35] or myasthenia gravis.[24] Out of 215 interventions performed by RVATS (7 studies included), 4 were converted to open sternotomy. The following parameters were included into meta-analyses: operation time, length of hospitalization, intraoperative blood loss, chest-in-tube days, postoperative complications, reoperation, arrhythmic events, pleural effusion, and postoperative bleeding.
Table 1.
Study characteristics.
A high risk of bias of the studies is most likely, as the included studies are retrospective studies with small sample sizes. A selection bias due to missing randomization and biased allocation to the treatment groups, a performance bias due to knowledge of the allocated interventions by participants and personnel during the study, and a reporting bias due to possible selective outcome reporting cannot be excluded. Two studies reported data on propensity score matching analyses.[31,32] In this case, the matched data were used for meta-analysis.
3.2. Surgery outcomes
Mean operation time was reported in 5 studies. In the RVATS groups, the operation time ranged between 97 and 224.2 minutes [N (RVATS) = 177], and in open surgery groups, between 55 and 243.8 minutes [N (open) = 222]. The estimated mean differences are shown in Fig. 2. Comparing operation time between both approaches resulted in a mean difference of -3.19 minutes [95% confidence interval, 95% CI -112.43 to 106.05]; P = .94, indicating no substantial difference between the RVATS and open surgery group. We observed a substantial between-study heterogeneity (tau = 85.8; I2 = 98.9%; P < .0001).
Figure 2.
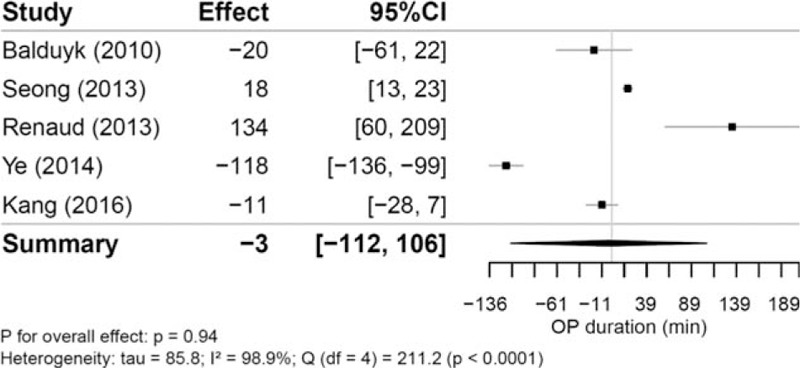
Mean differences of operation time (in min) between robotic and open thymectomy and combined estimate of the operation time.
Four studies reported data on hospitalization [N (RVATS) = 77, N (open) = 122; Fig. 3]. Mean number of days in hospital were reported between 3.7 and 9.6 days in the RVATS group and 5.5 and 11.8 days in the open surgery group. Patients undergoing RVATS spent significantly less time hospitalized than patients undergoing sternotomy [-4.06 days (95% CI −7.98 to −0.13); P = .046]. Between the studies, we also found a significant heterogeneity (tau = 2.1; I2 = 79.9%; P = .007).
Figure 3.
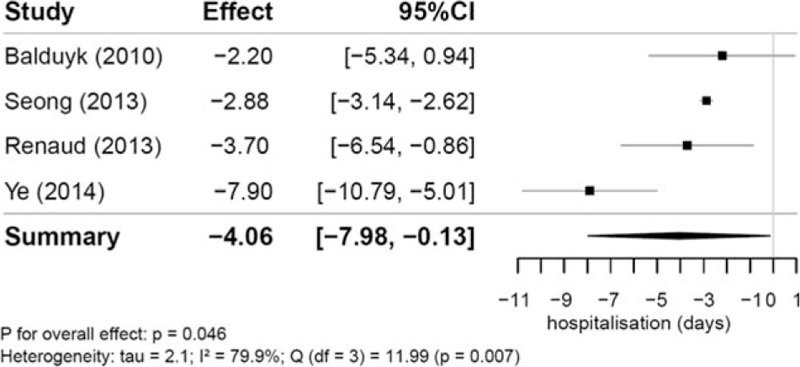
Mean differences in length of hospitalization (in d) between robotic and open thymectomy and combined estimate of the length of hospitalization.
Intraoperative blood loss was reported in 3 studies ranging from 41.7 to 100.9 mL and from 151.4 to 466.1 mL for RVATS versus sternotomy [N (RVATS) = 138, N (open) = 186]. The analysis of these studies resulted in a pooled mean difference of -256.84 mL (95% CI −627.47 to 113.80; P = .10) and a between-study heterogeneity of tau = 146.6 (I2 = 97.9%; P < .0001) (Fig. 4).
Figure 4.
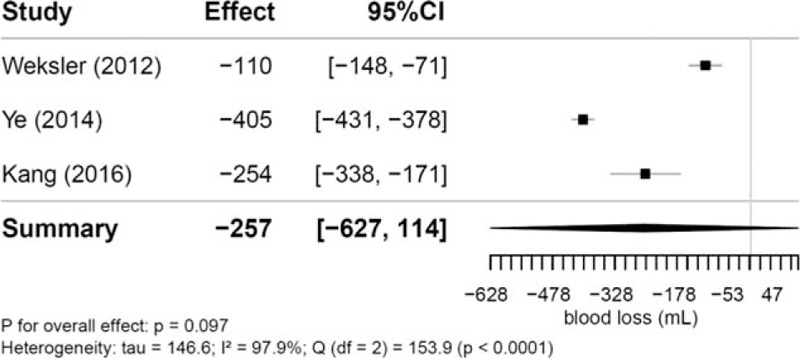
Mean differences in intraoperative blood loss (in mL) between robotic and open thymectomy and combined estimate of the intraoperative blood loss.
Two studies reported chest-in-tube days [N (RVATS) = 57, N (open) = 85], which was shorter for patients undergoing RVATS [−2.50 days (95% CI -15.01 to 10.01); P = .24], although this difference is statistically not significant. A significant between-study heterogeneity (tau = 1.4; I2 = 98.3%; P < .0001) was present.
Six studies reported data on postoperative complications [N (RVATS) = 209, N (open) = 259]. Overall, there were fewer complications observed in the RVATS group, which is also apparent in the pooled odds ratio (OR) [0.27 (95% CI 0.07–1.12)]; however, this is not significant (P = .06). It has to be noted that in the study of Weksler et al,[35] a high proportion of complications in the open surgery group was observed compared with the other studies (Fig. 5). The recorded complications in the study of Weksler et al[35] were supraventricular arrhythmia, atelectasis, respiratory failure, renal failure, sternal dehiscence, change in mental status, severe subcutaneous emphysema, and chyle leak. In a sensitivity analysis, this study was excluded of the meta-analysis; however, the results did not change substantially (OR 0.37, 95% CI 0.09–1.68; P = .15).
Figure 5.
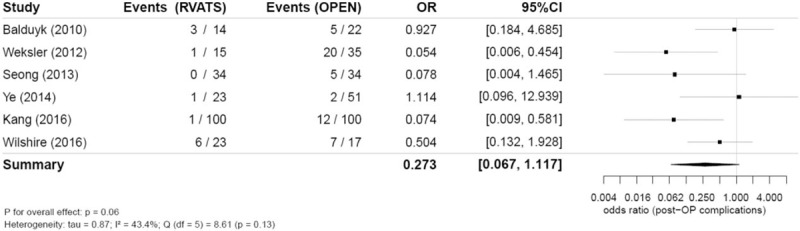
Combined postoperative complications (odds ratio) in robotic and open thymectomy and combined estimate of the complications.
Out of all observed postoperative complications, arrhythmic events, pleural effusion, and postoperative bleeding were reported separately as follows.
Atrial fibrillation and supraventricular arrhythmia were classified as arrhythmic events. In total, 4 studies reported arrhythmic events and 3 events were recorded for RVATS [N (RVTAS) = 166] and 7 for open surgery [N (open) = 192]. We did not detect significant difference between both approaches (OR 0.76, 95% CI 0.07–7.69; P = .72).
Pleural effusion and postoperative bleeding [3 studies, N (RVATS) = 157 and N (open) = 151] were observed solely in the open surgery group, although this did still not result in statistically significant differences between the 2 treatment groups [postoperative bleeding (OR 0.21, 95% CI 0.004–11.46; P = .24) and pleural effusion (OR 0.29, 95% CI 0.005–17.71; P = .33)].
4. Discussion
Decisions about the operative strategies for a thymectomy should depend on the size, location, local invasion of the lesion, experience of the surgeons, and condition of the patient. Until recently, sternotomy was the only approach to guarantee complete removal of the thymus. But in the last years, robotic-assisted thoracoscopy has become more commonplace.[2,37]
Robotic assistance using the da Vinci system allows a more delicate approach to the mediastinum. The excellent 3D vision with magnification is utilized, and the endoscopic “arms” with their 7 degrees of freedom are more maneuverable than typical thoracoscopic instruments.[38] This seems to suggest the possibility of less potential trauma to mediastinal structures and less postoperative pain while still allowing complete resection of the thymus. RVATS is described as a safe and feasible technique for thymectomy. Overall, 15 to 20 thymectomies may be required for a surgeon to learn and adequately perform this technique.[39]
Several systematic reviews and meta-analysis have evaluated the efficacy and safety of robotic-assisted surgery versus open surgery in lung cancer, rectal cancer, and kidney surgery.[40–42] During the past 5 years, many authors from established general thoracic centers reported studies that supported the feasibility, reproducibility, effectiveness, and safety of the robotic-assisted procedures compared with the open thymectomy.[24,31–36] To the best of our knowledge, this is the first meta-analysis comparing these 2 surgical techniques of thymectomy.
In the present study, we analyzed data of 215 and 274 patients undergoing either RVATS or open surgery. Seven observational studies were included into this meta-analysis. No difference in operation time was found indicating that not the approach used for thymectomy but other factors, for example, the experience of the surgeon, the equipment of the operation theater, or patients chosen for surgery influence operation time. It should be considered that the definition of “operation time” differed between studies, for example, Balduyck et al described room occupation time.
Patients undergoing RVATS spent significantly less time in hospital than patients undergoing sternotomy. Although a lower hospitalization rate might be used as an argument for RVATS, one should consider that hospitalization rates are not generalizable. Length of stay standards may vary between countries, as does medical training insurances coverage, cultural and societal values as well as hospital-specific protocols all influencing discharge timing.[43]
While the difference of intraoperative blood loss between those 2 approaches was not significant, less intraoperative blood loss was observed for RVATS (mean range 41.7–100.9 mL for RVATS, 151.4–466.1 mL for open surgery). We also observed a shorter chest-in-tube time for patient treated with RVATS (mean range RVATS 1.3–1.53 days, mean range open surgery 3–4.8 days). The studies reporting intraoperative blood loss and chest-in tube days indicated that RVATS is favorable to open surgery. Due to the small number of studies included and the appropriate use of the modified Knapp–Hartung approach, a conservative approach yielding a large confidence interval, the advantageous effects of RVTAS were statistically not significant. A larger number of studies are required to determine if this observation might be significant.
A further advantage of RVATS is the smaller complication rate than open surgery thymectomy (12 complications in 209 patients vs 51 complications in 259 patients), albeit this difference was not significant.
There are only few studies comparing RVATS to open surgery. Mostly data on early follow-up were reported. For example, Seong et al[31] observed no recurrence in either group, albeit their follow-up period ranged between 1.11 ± 0.21 years for RVATS and 1.85 ± 0.19 years for open surgery. Kang et al[32] described a 3-year follow-up with 3 recurrences in the RVATS group and none in the open group. These results were not significant. Weksler et al[35] described 1 postoperative death in the open group. The median follow-up of Balduyck et al[34] was 44 months; 1 recurrence in the sternotomy group after 54 months was described. More long-term follow-up studies are required to address the question whether the long-term out-come of RVATS is different from the outcome of patients undergoing open surgery. Furthermore, there are no sufficient data available comparing incomplete to complete resection of the thymus. Overall complete resection of the thymus is the gold standard to achieve cure. Complete resection is a prognostic factor for recurrence helping to improve survival.[44]
As described above, small number of studies is a limitation of this meta-analysis. All studies included in this analysis were retrospective, and only 2 were propensity matched. Indications of patients included and patient characteristics were rather variable. As, therefore, performance bias and reporting bias cannot be excluded, our findings point out the necessity and usefulness of randomized studies comparing RVATS with open surgery to better define the potential advantages of the minimally invasive approaches for thymectomy.
The results of our meta-analyses show that patients undergoing RVATS spent less time in hospital than patients treated by open surgery. Although the results for chest-in-tube days, intraoperative blood loss, and postoperative complications were not significant, RVATS may potentially favorable compared with sternotomy. However, further randomized and controlled studies are necessary to support this hypothesis. We found evidence for the safety and feasibility of RVATS compared with open surgery. Thus, we suggest that RVATS is an appropriate alternative to open surgery for thymectomy. In the absence of randomized controlled trials comparing RVATS with open surgery, our findings represent the highest level of clinical evidence in the current literature on this issue.
Acknowledgment
We acknowledge support by the German Research Foundation and the Open Access Publication Funds of Göttingen University.
Footnotes
Abbreviation: RVATS = Robot-assisted minimally invasive surgery.
JB and CS contributed equally to this work.
Funding/support: University of Goettingen supports the Open Access activities of their scholars and has established an Open Access Publication Funding. The administrator of this fund is the University Library of Göttingen.
The authors have no conflict of interest.
References
- [1].Gold R, Schneider-Gold C. Current and future standards in treatment of myasthenia gravis. Neurother J Am Soc Exp Neurother 2008;5:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Girard N, Mornex F, Houtte PV, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119–26. [DOI] [PubMed] [Google Scholar]
- [3].Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485–92. [DOI] [PubMed] [Google Scholar]
- [4].Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332–50. [DOI] [PubMed] [Google Scholar]
- [5].Ashton RC, Connery CP, Swistel DG, et al. Robot-assisted lobectomy. J Thorac Cardiovasc Surg 2003;126:292–3. [DOI] [PubMed] [Google Scholar]
- [6].Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883–7. discussion 887. [DOI] [PubMed] [Google Scholar]
- [7].Weissenbacher A, Bodner J. Robotic surgery of the mediastinum. Thorac Surg Clin 2010;20:331–9. [DOI] [PubMed] [Google Scholar]
- [8].Health Quality Ontario. Robotic-assisted minimally invasive surgery for gynecologic and urologic oncology: an evidence-based analysis. Ont Health Technol Assess Ser 2010;10:1–18. [PMC free article] [PubMed] [Google Scholar]
- [9].Lee SH, Lim S, Kim JH, et al. Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res 2015;89:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim S, Kim JH, Baek S-J, et al. Comparison of perioperative and short-term outcomes between robotic and conventional laparoscopic surgery for colonic cancer: a systematic review and meta-analysis. Ann Surg Treat Res 2016;90:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sinha R, Sanjay M, Rupa B, et al. Robotic surgery in gynecology. J Minimal Access Surg 2015;11:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innov Phila Pa 2014;9:10–5. [DOI] [PubMed] [Google Scholar]
- [13].Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893–8. [DOI] [PubMed] [Google Scholar]
- [14].Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740–6. [DOI] [PubMed] [Google Scholar]
- [15].Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-265–66. [DOI] [PubMed] [Google Scholar]
- [16].Augustin F, Schmid T, Bodner J. The robotic approach for mediastinal lesions. Int J Med Robot Comput Assist Surg MRCAS 2006;2:262–70. [DOI] [PubMed] [Google Scholar]
- [17].Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730–6. [DOI] [PubMed] [Google Scholar]
- [18].Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125–32. [DOI] [PubMed] [Google Scholar]
- [19].Orsini B, Santelmo N, Pages PB, et al. Comparative study for surgical management of thymectomy for non-thymomatous myasthenia gravis from the French national database EPITHOR. Eur J Cardiothorac Surg 2016;50:418–22. [DOI] [PubMed] [Google Scholar]
- [20].Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501–4. [DOI] [PubMed] [Google Scholar]
- [22].Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- [23].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. Accessed March 28, 2017. [Google Scholar]
- [24].Renaud S, Santelmo N, Renaud M, et al. Robotic-assisted thymectomy with Da Vinci II versus sternotomy in the surgical treatment of non-thymomatous myasthenia gravis: early results. Rev Neurol (Paris) 2013;169:30–6. [DOI] [PubMed] [Google Scholar]
- [25].Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Paule R, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand 1982. 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol 2015;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2014. http://www.R-project.org/. [Google Scholar]
- [29].Conducting Meta-Analyses in R with the metafor Package | Viechtbauer | Journal of Statistical Software. Available at: https://www.jstatsoft.org/article/view/v036i03. Accessed January 31, 2017. [Google Scholar]
- [30].Gordon M, Lumley T. Advanced Forest Plot Using “grid” Graphics. Available at: https://rdrr.io/cran/forestplot/. Accessed March 28, 2017. [Google Scholar]
- [31].Seong YW, Kang CH, Choi J-W, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68–73. discussion e73. [DOI] [PubMed] [Google Scholar]
- [32].Kang CH, Hwang Y, Lee HJ, et al. Robotic thymectomy in anterior mediastinal mass: propensity score matching study with transsternal thymectomy. Ann Thorac Surg 2016;102:895–901. [DOI] [PubMed] [Google Scholar]
- [33].Ye B, Li W, Ge X-X, et al. Surgical treatment of early-stage thymomas: robot-assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc 2014;28:122–6. [DOI] [PubMed] [Google Scholar]
- [34].Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543–8. [DOI] [PubMed] [Google Scholar]
- [35].Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261–6. [DOI] [PubMed] [Google Scholar]
- [36].Wilshire CL, Vallières E, Shultz D, et al. Robotic resection of 3 cm and larger thymomas is associated with low perioperative morbidity and mortality. Innov Phila Pa 2016;11:321–6. [DOI] [PubMed] [Google Scholar]
- [37].Martinelli SM, Lateef BD, Long JM, et al. Challenges in the anesthetic management for a robotic thymectomy in a patient with myasthenia gravis: a case report. A A Case Rep 2017;8:222–5. [DOI] [PubMed] [Google Scholar]
- [38].Rea F, Schiavon M, Marulli G. Robotic thymectomy for myasthenia gravis. Ann Cardiothorac Surg 2015;4:558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kamel MK, Rahouma M, Stiles BM, et al. Robotic thymectomy: learning curve and associated perioperative outcomes. J Laparoendosc Adv Surg Tech A 2017;Jan 25. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [40].Zhang L, Gao S. Robot-assisted thoracic surgery versus open thoracic surgery for lung cancer: a system review and meta-analysis. Int J Clin Exp Med 2015;8:17804–10. [PMC free article] [PubMed] [Google Scholar]
- [41].Liao G, Li Y-B, Zhao Z, et al. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci Rep 2016;6:26981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chang S-J, Hsu C-K, Hsieh C-H, et al. Comparing the efficacy and safety between robotic-assisted versus open pyeloplasty in children: a systemic review and meta-analysis. World J Urol 2015;33:1855–65. [DOI] [PubMed] [Google Scholar]
- [43].Anderson GF, Reinhardt UE, Hussey PS, et al. It's the prices, stupid: why the United States is so different from other countries. Health Aff Proj Hope 2003;22:89–105. [DOI] [PubMed] [Google Scholar]
- [44].Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2010;37:13–25. [DOI] [PubMed] [Google Scholar]


