Abstract
Most sensory systems are innervated by efferent neurons as well as by afferent neurons. The efferent innervation modulates the sensitivity of the receptor cells or of the sensory terminals. In the posterior lateral line system of the zebrafish, two efferent nuclei have been described in the hindbrain. Here we examine the development of the efferent neurons. We show that their axons are guided toward the target organ along the lateral line nerve while their cell bodies migrate posteriorward across rhombomeres to achieve their final position in rhombomeres 6/7. This migration depends on the SDF1 chemokine. We show that the migration of motor neurons of the facial nucleus from rhombomere 4 to 6 is also affected in sdf1a morphants (embryos injected with morpholine-conjugated antisense oligonucleotides). We propose that SDF1/CXCR4-mediated cell migration is preferentially associated with movement along the anteroposterior axis of the animal.
Keywords: cell migration, hindbrain, octavolateral efferents, axon guidance
A marked difference between vertebrate and invertebrate sensory systems is the presence of an efferent innervation in the former but not in the latter. Efferent neurons have their cell bodies in the central nervous system and extend their axons toward their target organs in the periphery. They may serve a variety of purposes. Some are “feedback” inhibitory devices that reduce the response to constant excitation and may in some cases protect the system from the cytotoxic effects of overstimulation. Others are “feedforward” systems that anticipate sensory stimulation due to voluntary movements and inhibit the response to such stimulation. Excitatory efferent systems may act to lower the threshold of the response to stimulation, thereby “sensitizing” the system.
The relationship between the development of sensory systems and the development of the corresponding efferent systems has not been much studied so far. Here we examine this question in the posterior lateral line system (PLL) of the zebrafish. The lateral line is a water-displacement-detecting system present in fish and amphibians and mediates a sense of “distant touch” whereby the animal is able to perceive movements in its vicinity (1). The lateral line system is closely related to the ear sensory epithelia as judged by the adjacent positions of the ear and lateral line placodes, by the presence of hair cells, and by the central projection of the neurons (2–4).
The sensory organs of the lateral line, the neuromasts, comprise mechanosensory hair cells surrounded by support cells. They can be enclosed in pouches, canals, or vesicles, but their simplest form is superficial. They are arranged on the body surface in reproducible, species-specific patterns. The neuromasts on the head form the anterior lateral line system, which originates from a preotic placode, whereas the neuromasts on the body and tail form the posterior lateral line originating from a postotic placode. In many teleost species, the embryonic PLL consists of a single line of 5–10 neuromasts extending along the horizontal myoseptum (5). This line is laid down by a primordium that originates from the head epithelium, just posterior to the otic placode, and migrates all the way to the tip of the tail (6). The migrating cells express the chemokine receptor CXCR4 and are guided by a stripe of cells that express the ligand SDF1 (7). During this migration afferent axons are towed by the primordium, thus establishing the PLL nerve (6–8), as originally observed in the amphibian PLL by Harrison (9).
In the zebrafish, two efferent nuclei have been described in the hindbrain (10), corresponding to the rostral and the caudal efferent nuclei described in the goldfish (11). Most motor neurons of the hindbrain are known to undergo migration events that are usually directed along the mediolateral or the anteroposterior axis. The molecular mechanism of anteroposterior neuronal migration is not known. Here we show that the PLL efferents migrate posteriorward from their rhombomere of origin, and that this migration requires the presence of the SDF1 chemokine. The same is true for the neurons of the facial nucleus, a group of hindbrain motor neurons from which the inner ear and lateral line efferents (octavolateral efferents) probably evolved (12). We conclude that SDF1 is independently required for the caudal migration of the PLL primordium along the fish body and of the PLL efferent and facial neurons in the hindbrain.
We further show that the efferent axons are guided toward the target organs along the PLL nerve, the formation of which also depends on SDF1.
Materials and Methods
Fish Maintenance. Zebrafish (Danio rerio) were maintained as described by Westerfield (13). Embryos were obtained from natural spawning and incubated at 28.5°C in tank water.
Gene Inactivation. The genes sdf1a and Zath1 were inactivated by injection of morpholino-antisense RNA at the one- or two-cell stage. The sequence of the anti-sdf1a morpholino oligonucleotide has been described in ref. 7; the sequence of the anti-Zath1 morpholino oligonucleotide will be described elsewhere (A. Sarrazin, E. Villablanca, A.G., and M. Allende, unpublished work).
Labeling of Hair Cells. Hair cells were labeled by incubating live, unanesthetized embryos in 5 μM 4-(4-diethylaminostyryl)-1-methylpyridinium iodide (4-Di-2-ASP; Sigma D 3418) in tank water for 10 min.
Labeling of Lateral Line Efferents. Efferent neurons were labeled by iontophoretic application of DiI (1,1′-dioctadecyl-3,3–3′,3′tetramethylindocarbocyanine perchlorate; Molecular Probes) by using an electrometer from WPI Instruments (Waltham, MA) and a fixed-stage Axioskop microscope equipped with a long-distance water-immersion ×40 objective. In most cases the injection was done at the level of the ganglion and aimed at the efferent axons as visualized by GFP fluorescence in the islet-GFP line (14). In 3–day-old and older larvae iontophoretic applications were also done in the anteriormost neuromast of the PLL. Fixed embryos were embedded in methylcellulose and examined under Nomarski optics to visualize the neuromasts of the PLL.
Labeling of Facial Motor Neurons. Facial neurons were labeled much as in the case of the PLL efferents, using GFP fluorescence of the islet-GFP line to visualize the nerve, except that the application of DiI was done on the facial branch distal to the place where it separates from the efferent branch.
Observation of Labeled Efferents. Microphotographs were taken on a Nikon Microphot microscope equipped with a Princeton Pentamax camera and with a focus controller from Applied Scientific Instrumentation (Eugene, OR). The driving software was iplab 3.6 for Macintosh. Z-series were routinely taken; they were deconvolved and stacked by using iplab 3.9 rapid deconvolution program. The images presented in Figs. 3 and 4 are not deconvolved, however, and are not treated in any way except for assembly of in-focus portions and adjustment of the look-up-table (contrast adjustment). The corresponding drawings were based partly on the deconvolved projections, in case these provided better detail, e.g., of dendritic branching. All image handling was done with iplab 3.9 running on a Macintosh G5, except for the final assembly of the figures, which was done with photoshop 7 (Adobe Systems, Mountain View, CA).
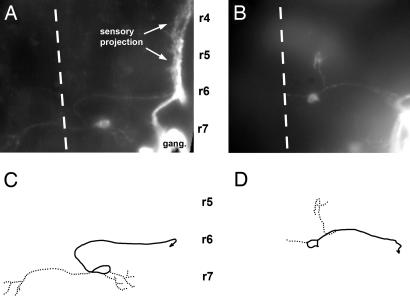
Fig. 3.
Central pathway of caudal PLL efferent neurons in 3-day-old embryos. (A and C) In the wild type. (B and D) In a sdf1a morphant embryo. A and B have been assembled by using a set of photographs taken at various focal planes; C and D have been drawn on the basis of A and B. Solid lines, axons; dotted lines, dendrites; dashed line, midline; r4, r5, r6, and r7, rhombomeres 4, 5, 6, and 7. In this and in the following figures, the approximate positions of rhombomeres shown on the figures are based on two criteria: the position of the otic vesicle, which forms along rhombomere 5 and extends approximately from rhombomere 4 to rhombomere 6, and the position of identified cranial nerves, specifically the V and VII nerves that exit the brain at the levels of rhombomeres 2 and 4, respectively, and were visualized by fluorescence in islet-GFP embryos, both in wild type and inmorphants.
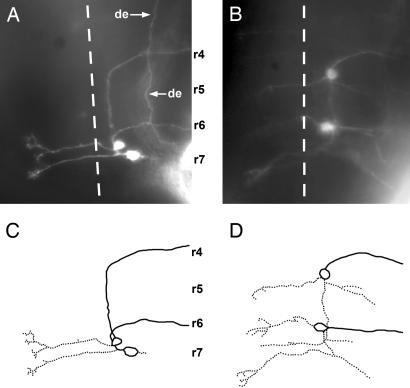
Fig. 4.
Central pathway of rostral and caudal PLL efferents in 3-day-old embryos. (A and C) Wild type. (B and D) sdf1a morphant. Symbols and abbreviations are as in Fig. 3. de, axon of a diencephalic efferent neuron.
Results
Peripheral Pathway of PLL Efferent Axons. The efferent neurons are easily visualized by labeling the neuromasts with the neuronal tracer DiI. Contrary to the sensory neurons, each of which innervates a single embryonic neuromast, efferent axons innervate several consecutive organs (unpublished observations). We first examined what drives the efferent axons peripherally to their target organs.
One obvious candidate for efferent axonal guidance is the migrating primordium itself, which is known to guide the afferent axons (6–8). We examined the relation between the leading edge of the efferent axons as seen in an islet-GFP transgenic line (14) and the trailing edge of the migrating primordium (Fig. 1A). The results show that the efferent fibers lag several somites behind the primordium and that, at a given stage, the distance between neurites and primordium is highly variable. The presence of a variable gap between primordium and efferent growth cone suggests that efferent extension and primordium migration are independent processes. Furthermore, the average speed of the efferent neurites is slower than that of the migrating primordium. We conclude that the efferent fibers are not driven directly by the migrating primordium.
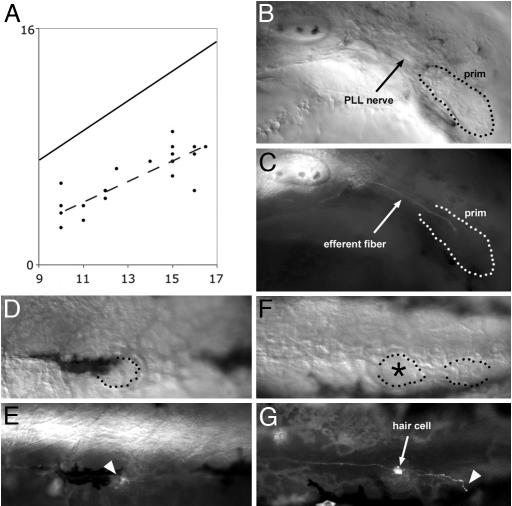
Fig. 1.
Peripheral growth of the efferent axons. (A) plot of the position of the leading efferent growth cones (ordinate) as a function of the developmental stage of the embryo (abscissa). The developmental stage is expressed as the position reached by the leading edge of the primordium, which is more accurately defined than its trailing edge (15). Both the position reached by the leading efferent neurite (ordinate) and the position reached by the leading edge of the primordium (abscissa) are expressed as somite number. Because the primordium extends over 2.5 somites on average, the diagonal line represents the average position of the trailing edge of the primordium. In this sample of 19 embryos, the leading efferent neurite lags anywhere between 3 and 9 somites behind the trailing edge of the primordium. The dashed line is the least-squares regression line for the positions of the efferent leading edges. (B and C) In a 32 hours after fertilization (haf) sdf1a morphant embryo, where the primordium fails to migrate (B), the efferent fiber reaches the trailing region of the primordium and stops there (C). When the primordium eventually differentiates into one or two ectopic neuromasts, these are invariably innervated by efferent neurites (not shown). (D–G) Efferent innervation in 3-day-old Zath1 morphant embryos. (D and E) A neuromast that comprises no hair cells (dotted outline) is nevertheless contacted by an efferent fiber (arrowhead). (F and G) Two terminal neuromasts, one of which (asterisk) comprises a hair cell and the other not. The neuromast with no hair cell is contacted by an efferent fiber (arrowhead). (Final magnification: B and C, ×150; D–G, ×130.)
The inactivation of sdf1a by morpholino-antisense results in a complete or nearly complete absence of migration of the PLL primordium between 20 and 40 haf and in the formation of one (or occasionally two) neuromast at an ectopic position at 48 haf (7). We examined the effect of the absence of SDF1 on the efferent axons in islet-GFP embryos where all motor neurons, including the lateral line efferents, express GFP (13). We observed that in 48-haf morphants, GFP-expressing efferent fibers are visible at the level of the ectopically located neuromasts, where they appear to connect their targets appropriately (data not shown). In younger embryos (32 haf), PLL efferent fibers are seen in the rostral region of the stationary primordium (Fig. 1 B and C). The fact that the efferent axons invariably reach the primordium and innervate ectopically located neuromast(s) suggests that they have been guided along the lateral line nerve that links the ganglion to the primordium at all times. A similar guidance of efferent by afferent fibers has been reported in the inner ear (15).
Another factor that may play a role in the homing or stabilization of efferent fibers is the expression of neurotrophins by hair cells. In the inner ear, there is a marked loss of cochlear sensory neurons upon inactivation of the neurotrophin NT-3 or of its receptor, the tyrosine receptor kinase TrkC, whereas vestibular neurons are lost upon inactivation of BDNF (brain-derived neurotrophic factor) or of its receptor, TrkC (reviewed in ref. 16). Furthermore, the replacement of NT-3 by BDNF redirects vestibular neurons toward the cochlea, suggesting that the expression of neurotrophins may not only stabilize but also guide the afferent fibers (17). Neuromasts do not express NT-3, but they express BDNF and TrkC (18). We investigated a potentially attractive role of hair cells by examining the efferent innervation of neuromasts that were deprived of hair cells because of the inactivation of the proneural gene Zath1. The presence of hair cells was ascertained by 4-Di-2-ASP labeling. Neuromasts with no hair cells are more difficult to identify under Nomarski optics than normal neuromasts, but in several cases we could observe that efferent fibers make contact with neuromasts that comprise no hair cells (Fig. 1 D–G). We cannot rule out, however, that BDNF expression by the support cells plays a role in the establishment or maintenance of efferent innervation.
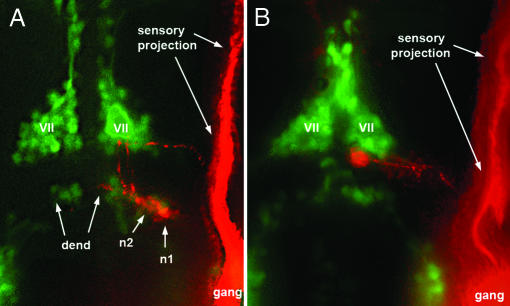
Central Pathway of Caudal PLL Efferents. Within the hindbrain, the PLL efferent neurons form two clusters, the rostral and caudal efferent nuclei (10, 11). Labeling of the PLL neuromasts always results in a labeling of caudal efferents and less frequently in the labeling of both rostral and caudal efferents (11, 19). The axons of the caudal neurons follow a tortuous pathway (Fig. 2A) that is first directed medially, then anteriorly along the midline and then laterally before exiting the hindbrain and extending posteriorly toward the PLL ganglion. Previous descriptions of the zebrafish PLL efferents were done on 5-day-old larvae (10). Because several classes of hindbrain motor neurons are known to undergo complex migrations, we examined the morphology of PLL efferent at earlier times, using the islet-GFP line to visualize the axons at the level of the ganglion and to label them with the neuronal tracer DiI. At 3 or more days after fertilization, the cell bodies of the caudal efferents are localized in rhombomere r7 (Fig. 2 A). At earlier times, however, we found that the cell bodies are often located in r6 (Fig. 2B). Although there are no precise markers for the r6/r7 boundary at this stage, the position of the efferent cell bodies relative to the major motor nuclei (as visualized in the islet-GFP transgenic line) leaves no doubt that the neurons of the caudal nucleus undergo a posterior migration after they have extend their axons laterally to exit the hindbrain (Fig. 2). We conclude that the majority of PLL efferents of the caudal nucleus are born in r6 and migrate during embryogenesis to their final position in r7.
Fig. 2.
Localization of caudal PLL efferent neurons relative to the facial motor nucleus as visualized in islet-GFP embryos. (A) Two efferent neurons, n1 and n2, occupy normal positions in a 3-day-old larva. GFP labeling is in green, DiI labeling is in red. (B) in a 48-haf embryo, the cell body is located at the posterior edge of the facial nucleus (VII) and has not yet reached its final, more posterior, position. The labeled efferent neurons appear red rather than yellow because the islet-GFP labeling was relatively faint and because the DiI labeling was enhanced to allow visualization of the axon (and partly of the dendrite, dend). gang, Ganglion.
SDF1 Is Required for the Migration of Caudal PLL Efferents. It has been noted previously that sdf1 is heterogeneously expressed in the hindbrain (7). We examined whether this expression of sdf1a could play a role in the posterior migration of the PLL efferent neurons by labeling the efferent fibers in islet-GFP morphant embryos. In 3-day-old sdf1a morphants, we observed that the somata of the PLL efferents are ectopically located. When only one type of efferent is labeled (caudal efferent), their cell bodies are found in a position that corresponds to r6 in the wild type, and their axonal pathway lacks the hairpin loop that is characteristic of the normal pathway (Fig. 3 B and D). This result confirms that caudal efferents are born in r6 and migrate toward r7 during normal development, and it shows that this migration requires the presence of the SDF1 chemokine.
Central Pathway of Rostral PLL Efferents. We have never been able to label specifically the rostral efferents. A comparison between embryos where both rostral and caudal efferents are labeled and embryos where only the latter are labeled reveals that the axons from rostral efferents follow a pathway similar to that of the caudal efferents except that they turn laterally at a much more anterior position (refs. 10 and 11 and Fig. 4A). By analogy to the case of the caudal efferents, where the migration of the cell body results in a posteriorward extension of the axon along the midline, one would expect the rostral efferents to be born in r4 and to migrate posteriorward toward r6. We have not been able to detect efferent cell bodies in r4 in normal embryos. When SDF1 is inactivated through the injection of morpholino-antisense, however, we observe that when two types of efferent neurons are labeled (caudal and rostral), one has its cell body in r6 and the other one in r4, and both have their axons directed laterally (Fig. 4 B and D). We conclude that the rostral efferent neurons are actually born in r4 and migrate toward r6 under the influence of SDF1, and that this migration takes place before the efferent axon reaches the ganglion and can be labeled with DiI.
Central Pathway of Facial Motor Neurons. Because the migration of the PLL primordium depends on SDF1, it could be that the abnormal behavior of efferent cell bodies in the sdf1a morphants is due to the block in primordium migration and not to a direct effect of sdf1a inactivation on the efferent neurons. To evaluate this possibility, we examined another population of hindbrain motor neurons, the neurons of the facial (VII) nucleus. The facial motor neurons, which innervate muscles derived from the second branchial arch, are known to migrate from r4 to r6 in the mammalian hindbrain (20). In the fish, the axons of the facial and the PLL efferent exit the hindbrain together and separate soon after leaving the central nervous system. We specifically labeled the facial neurons by applying DiI to the facial nerve after the latter has separated from the PLL nerve. The central projection is very similar to that of the rostral PLL efferents in that the axons exit the hindbrain at the level of r4 and the cell bodies are present in r6 (compare Figs. 5B and 4A). Facial neurons also resemble the rostral efferents in that their migration is completed earlier than that of the caudal efferents (21).
Fig. 5.
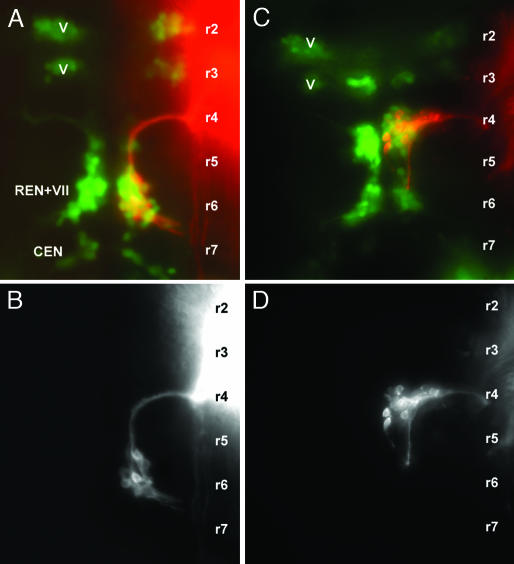
Central pathways of facial motor neurons in 2-day-old wild type (A and B) and sdf1a morphant embryo (C and D). The facial neurons were labeled with DiI and are shown either alone (B and D) or in an islet-GFP background (A and C; red, DiI; green, GFP). The approximate localization of the rhombomeres (r1, r2,...) was defined as in Fig. 3. V, trigeminal motor nucleus; VII, facial motor nucleus; REN, rostral efferent nucleus of the lateral line system; CEN, caudal efferent nucleus of the lateral line system.
In sdf1a morphants, the morphology of the facial neurons is dramatically altered (Fig. 5D) and resembles that of rostral efferent neurons in morphant embryos (compare Figs. 5D and 4B). The alteration in the behavior of hindbrain motor neurons can also be appreciated from the change in the overall pattern of islet expression (green in Fig. 5A, wild type, and 5C, sdfIa morphant). The two groups that give rise to the motor component of the trigeminal (V) nerve are known to remain in their rhombomere of origin in the wild type (22) and can be used as markers for r2 and r3. In contrast, the small group present in r7, and which corresponds at least in part to the caudal efferent nucleus (CEN) of the PLL, is completely absent in the morphant. The large group present in r6, which includes the facial nucleus and the rostral efferent nucleus of the PLL (VII + REN), is massively reduced, and a new group, most likely corresponding to the facial motor neurons and rostral efferent neurons that failed to migrate, is now detectable in r4.
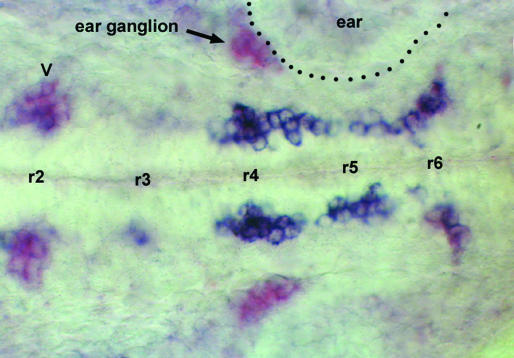
cxcr4b Is Expressed in Migrating Neurons. CXCR4 is the only known receptor for SDF1, and one would therefore expect it to be expressed in the migrating PLL efferents and facial motor neurons. We examined the pattern of expression of cxcr4b and found that at 25 haf, the gene is coexpressed with islet in two streams of cells extending from r4 to r6 on either side of the midline (Fig. 6). cxcr4 expression is reduced in the final stretch of the migration. It may be that this final step relies on the persistence of receptor protein after the cxcr4b gene has been turned off. Alternatively, it may be that this lateral (and dorsal) migration depends on another guidance system.
Fig. 6.
Expression of cxcr4b (blue) and of islet (purple) in a 25-haf embryo. Two streams of doubly labeled cells extend from r4 to r6 on either side of the midline. At the level of r6 the streams turn laterally and begin to loose cxcr4b expression, such that the purple labeling becomes more visible. In a few of the clusters that express islet-1, cxcr4b is expressed at a low level (trigeminal motor neurons in r2, labeled V in the figure) or not at all (statoacoustical ganglion apposed to the ear vesicle, labeled ear ganglion in the figure).
Discussion
In the goldfish as well as in the zebrafish, the cell bodies of lateral line efferent neurons form two closely apposed hindbrain nuclei, the rostral and caudal efferent nuclei (11). Our data indicate that the rostral neurons mostly arise from r4 and migrate toward r6. Their axons extend laterally in their rhombomere of origin (r4) until they reach the PLL afferent axons, which they then follow peripherally. The caudal neurons mostly arise from r6 and later migrate to r7. Their axons extend laterally in their rhombomere of origin and exit the brain near the PLL nerve, which they subsequently follow. The place of origin of the efferent neurons (r4 or r6) together with their subsequent migration under the control of SDF1 account for most features of their axonal pathways. Some aspects are yet to be fully accounted for, although, in particular it is not clear why the neurons born in r4 stop migrating in r6, whereas those born in r6 stop in r7. This difference may be related to the fact that the migration of r4 neurons occurs earlier than the migration of the r6 efferents.
The trilobite mutation has recently been shown to prevent the caudal migration of branchiomotor neurons (23). This mutation affects the fish homolog of the vang/stbm gene of Drosophila, which has been implicated in the establishment of planar cell polarity. The fish gene is required not only for branchiomotor neuron migration but also for cell movements during gastrulation (24), and it may therefore be a link between the general anteroposterior cell polarity system and the SDF1/CXCR4-mediated migration of motor hindbrain neurons along the anteroposterior axis.
Inner ear and more generally octavolateral efferents are thought to have segregated from the general pool of facial branchiomotor neurons early during craniate evolution (25, 26). Our observation that both the rostral lateral line efferents and the facial motor neurons migrate from r4 to r6 in the zebrafish supports the idea that in a first stage of evolution the octavolateral efferents segregated functionally from the branchiomotor population while still migrating to r6. Octavolateral efferents may have later lost the capability to migrate posteriorward and remained in their segment of origin, as observed in frogs, birds, and mammals (26).
Primitive teleosts such as osteoglossomorphs have only one hindbrain efferent nucleus, which corresponds to the rostral nucleus of the zebrafish (27, 28). The efferent axons course to an anterior level that probably corresponds to r4 (27), where they exit the hindbrain and subsequently innervate both the anterior and posterior lateral line systems. It appears, therefore, that the formation of an additional pool of lateral line efferent neurons in r6 and their subsequent migration to r7 is a derived feature of euteleosts. Whether these newly evolved r6 efferents are functionally distinct from their older r4 counterparts is not known. In line with a common origin for the facial motor neurons and rostral efferents, one can imagine that the caudal efferents arose as a subset of the glossopharyngeal (IX) branchiomotor neuron, which also migrate from r6 to r7. It would be interesting to see whether trilobite mutations, which are reported to prevent specifically the migration of the facial and glossopharyngeal branchiomotor neurons (23), also prevent the migration of both rostral and caudal lateral line efferents.
We have previously described the role of the SDF1/CXCR4 system in the posterior migration of the PLL primordium from the otic region to the tip of the tail (7). Here we show that the same ligand/receptor system is involved in the posterior migration of the lateral line efferents within the hindbrain. We cannot exclude that the migration of the neurons somehow depends on the migration of the primordium and that the effect of sdf1a inactivation on the efferents is therefore indirect. This explanation seems unlikely, however, because the efferent fibers are in most cases not in direct contact with the migrating primordium. Furthermore, we observed that the facial motor neurons are affected in exactly the same way as the rostral efferent neurons. The SDF1/CXCR4 system has also been implicated in the long-distance migration of the primordial germ cells toward the gonadal ridge, both in fish and in mice. All of these migration events are aligned along the anteroposterior axis, suggesting that the SDF1/CXCR4 system may be preferentially associated to migrations along this axis, much as the slit/robo and netrin/DCC systems are usually associated to guidance along the mediolateral axis, and the reelin system to guidance along the apicobasal axis of the central nervous system.
Acknowledgments
We thank A. Sarrazin and M. Allende for making the Zath1 morpholino available to us ahead of publication, B. Fritzsch for help with the comparative aspects of octavolateral efferent development, H. Okamoto for providing the islet-GFP line, and N. Cubedo for expert fish care. D.S. is the recipient of a Région Languedoc-Roussillon Institut National de la Santé et de la Recherche Médicale doctoral fellowship. This work was supported by the Association pour la Recherche sur le Cancer.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PLL, posterior lateral line system; haf, hours after fertilization.
References
- 1.Dijkgraaf, S. (1989) in The Mechanosensory Lateral Line, eds. Coombs, S. Görner, P. & Münz, H. (Springer, New York), pp. 7–14.
- 2.Ariëns Kappers, C. U., Huber, G. C. & Crosby, E. C. (1960) The Comparative Anatomy of the Nervous System of Vertebrates, Including Man (Hafner, New York), pp. 433–439.
- 3.Jörgensen, J. M. (1989) in The Mechanosensory Lateral Line, eds. Coombs, S. Görner, P. & Münz, H. (Springer, New York), pp. 115–145.
- 4.Fritzsch, B. (2001) in Nature Encyclopedia of Life Sciences (Nature Publishing Group, London). Available at www.els.net.
- 5.Pichon, F. & Ghysen, A. (2004) Evol. Dev. 6, 187–193. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe, W. K. (1985) J. Comp. Neurol. 238, 218–224. [DOI] [PubMed] [Google Scholar]
- 7.David, N., Sapède, D., St-Etienne, L., Thisse, C., Thisse, B., Dambly-Chaudière, C., Rosa, F. & Ghysen, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16297–16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmour, D., Knaut, H., Maischein, H. M. & Nüsslein-Volhard, C. (2004) Nat. Neurosci. 7, 491–492. [DOI] [PubMed] [Google Scholar]
- 9.Harrison, R. (1904) Arch. Mikrosk. Anat. Entwikl. Mech. 63, 35–149. [Google Scholar]
- 10.Metcalfe, W. K., Kimmel C. B. & Schabtach, E. (1985) J. Comp. Neurol. 233, 377–389. [DOI] [PubMed] [Google Scholar]
- 11.Zottoli, S. J. & Van Horne, C. (1983) J. Comp. Neurol. 219, 100–111. [DOI] [PubMed] [Google Scholar]
- 12.Fritzsch, B. (1999) in The Ear of Fishes and Amphibians, eds. Popper, A. N. & Fay, R. R. (Springer, New York), pp. 15–42.
- 13.Westerfield, M. (1994) The Zebrafish Book (Univ. of Oregon Press, Eugene).
- 14.Higashijima, S.-i., Hotta, Y. & Okamoto, H. (2000) J. Neurosci. 20, 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma, Q., Anderson, D. J. & Fritzsch, B. (2000) J. Assoc. Res. Otolaryngol. 1, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritzsch, B., Pirvola, U. & Ylikoskin, J. (1999) Cell Tissue Res. 295, 369–382. [DOI] [PubMed] [Google Scholar]
- 17.Tessarollo, L., Coppola, V. & Fritzsch, B. (2004) J. Neurosci. 24, 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germana, A., Catania, S., Cavallaro, M., Gonzalez-Martinez, T., Ciriaco, E., Hannestad, J. & Vega, J. A. (2002) J. Anat. 200, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bricaud, O., Chaar, V., Dambly-Chaudière, C. & Ghysen, A. (2001) J. Comp. Neurol. 434, 253–261. [DOI] [PubMed] [Google Scholar]
- 20.Auclair, F., Valdès, N. & Marchand, R. (1996) J. Comp. Neurol. 369, 451–461. [DOI] [PubMed] [Google Scholar]
- 21.McClintock, J. M., Kheirbek, M. A. & Prince, V. E. (2002) Development (Cambridge, U.K.) 129, 2339–2354. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar, A. (2004) Dev. Dyn. 229, 143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bingham, S., Higashijima, S., Okamoto, H. & Chandrasekhar, A. (2002) Dev. Biol. 242, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessen, J. R., Topczewski, J., Bingham, S., Sepich, D. S., Marlow, F., Chandrasekhar, A. & Solnica-Krezel, L. (2002) Nat. Cell Biol. 4, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts, B. L. & Meredith, G. E. (1992) in The Evolutionary Biology of Hearing, eds. Webster, D. B., Popper, A. N. & Fay, R. R. (Springer, New York), pp. 182–210.
- 26.Fritzsch, B. (1999) in The Efferent Auditory System: Basic Science and Clinical Applications, ed. Berlin, C. I. (Singular, San Diego), pp. 31–59.
- 27.Bell, C. C. (1981) J. Comp. Neurol. 195, 391–414. [DOI] [PubMed] [Google Scholar]
- 28.Wagner, T. & Schwartz, T. (1997) Anat. Embryol. (Berlin) 194, 271–278. [DOI] [PubMed] [Google Scholar]