Abstract
The purpose of this study is to investigate the prognostic value of tumor volume and radiation dose for predicting treatment outcomes in moderate-sized hepatocellular carcinoma (HCC).
A total of 72 patients with unresectable HCC ranging in size from 5 to 10 cm were treated with high-dose radiotherapy including hypofractionated radiotherapy (HRT) and stereotactic body radiotherapy (SBRT), in 3 institutions from 2003 to 2013. The HRT doses ranged from 33 to 60 Gy in 3 to 10 fractions. The primary endpoint was local progression-free survival (PFS); the secondary endpoints were overall PFS, overall survival (OS), and treatment toxicity.
The median follow-up period after radiotherapy was 12.8 months. The local PFS rates at 1 and 2 years were 57.0% and 39.0%, respectively, with a median of 13.6 months. The OS rates at 1 and 2 years were 70.1% and 45.2%, respectively, with a median of 21.1 months. A gross tumor volume (GTV) of 214 cm3 and a total dose of 105 Gy10 were identified as the optimal cutoff values of radiotherapeutic factors for local PFS. Patients with GTV ≤ 214 cm3 and total dose >105 Gy10 had significant higher 2-year local PFS and OS than patients with GTV >214 cm3 and total dose ≤ 105 Gy10 (P = .020 for local PFS, P = .009 for OS).
The optimal cutoff values of GTV ≤ 214 cm3 and total dose >105 Gy10 may be useful for predicting survival outcomes when treating moderate-sized HCC with high-dose radiotherapy.
Keywords: gross tumor volume, hepatocellular carcinoma, hypofractionated radiotherapy, radiation dose, stereotactic body radiotherapy
1. Introduction
Primary liver cancer is the sixth most frequently diagnosed cancer, but it is the third most common cause of cancer death worldwide.[1] Surgical resection remains the primary curable treatment for hepatocellular carcinoma (HCC), and results in 5-year survival rates of 60% to 70% in small HCC.[2] However, despite advances in early detection and diagnosis, the majority of patients are not suitable candidates for surgery because of extent of the tumor, or underlying inadequate liver function, and only 30% to 40% of patients may benefit from curative therapies.[3] For inoperable or unresectable diseases, modalities such as transplantation, chemoembolization, local ablation, systemic chemotherapy, and molecular target therapy are generally considered as treatment options.
Treatment options for patients with unresectable HCC > 5 cm diameter are limited. Transcatheter arterial chemoembolization (TACE) is the most commonly used alternative; however, tumor response rates are generally poor.[4] With the recent advances in radiotherapy (RT) techniques, including respiratory motion management and image-guided RT, the delivery of high-dose RT to tumors specifically while sparing the uninvolved normal liver or adjacent organ can be achieved. As a result, the role of RT for patients with HCC has been gradually expanded from palliative to curative. Recently, high-dose hypofractionated RT (HRT), or stereotactic body RT (SBRT), has emerged as a novel RT method in patients with small HCC. Although prospective studies remain sparse at present, many clinical studies have demonstrated HRT or SBRT to be feasible and effective for the treatment of small HCC.[3,5–8] However, there is concern about the safe delivery of high-dose RT using HRT or SBRT in treatment of large HCC, due to the limited amount of data available in such cases. Furthermore, there is no consensus on the optimal criteria of the radiotherapeutic factors such as tumor volume and total dose for its use.
In this study, we evaluate the prognostic value of gross tumor volume (GTV) and total dose for predicting survival outcomes and also present the results of high-dose RT including HRT and SBRT, for unresectable moderate-sized HCC.
2. Materials and methods
2.1. Patients
We retrospectively reviewed 72 patients with HCC who were treated with HRT or SBRT at 3 institutions between May 2003 and April 2013. The inclusion criteria were as follows: age >18 years; an Eastern Cooperative Oncology Group (ECOG) performances status ≤2; no previous abdominal RT; an initial diagnosis of primary HCC or recurrence; an inoperable disease status or refusal to undergo surgery; unsuitability for radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI); Child–Pugh (CP) class A or B disease; an incomplete response after TACE or unsuitable for TACE due to the lesion nonvisibility on hepatic angiogram; a greatest tumor dimension 5 to 10 cm; normal functional liver volume more than 1000 mL; and no evidence of an uncontrolled lesion at any other site. This study was reviewed and approved by the Institutional Review Boards of Incheon St. Mary's Hospital, the Catholic University of Korea (No. OC14RIGI0139) and by Korean Radiation Oncology Group (KROG 14–17).
2.2. Treatment
2.2.1. Hypofractionated radiotherapy
HRT was delivered using the TomoTherapy Hi-Art (TomoTherapy, Madison, WI). When performing the simulation with a contrast-enhanced liver dynamic computed tomography (CT), we used a custom-made double vacuum system (BodyFixW, Medical Intelligence, GmbH, Schwabmunchen, Germany) for immobilization and abdominal dampening. Two additional series of CT scans during inspiration and expiration were obtained to track the motion of the tumors and other internal organs. The GTV was defined as the tumor volume that was enhanced in the arterial phase and diluted in the delayed phase of CT scan. The internal target volume (ITV) was defined as the summation of the GTVs on the inspiratory and expiratory CT images, and the planning target volume (PTV) was defined with a 5 to 10 mm margin around the ITV. The median 50 Gy (range, 40–60 Gy) was delivered in 10 fractions during 2 weeks to the 95% isodose volume of the PTV. No more than 30% of the normal liver received >27 Gy, and no more than 50% of normal liver received >24 Gy. The volume receiving >37 Gy was limited to <1 cm3 for the stomach and duodenum. The maximal dose was kept <34 Gy for the spinal cord. In the kidney, no more than 33% of the renal volume received >18 Gy. A megavoltage CT scan was acquired before each treatment on the tomotherapy unit. The displacement of tumors and internal organs from their original position on the simulation CT was automatically or manually corrected.
2.2.2. Stereotactic body radiotherapy
SBRT was administrated using the CyberKnife (Accuray Inc, Sunnyvale, CA) or RapidArc image-guided SBRT systems (Varian Medical Systems, Inc, Palo Alto, CA). Patients were immobilized using a customized external vacuum-type immobilizer (Vac-Loc; Med- Tec, Inc, Orange City, IA). Abdominal compression using 4 belts was used to minimize breathing-related tumor motion. Six-gold fiducials or lipiodol deposits in the tumor were used to delineate the tumors. A thin-slice CT image was taken with a 2 mm slice thickness at 3 seconds per slice. These relatively slow CT images included the respiratory movement of the target; therefore, the tumor volume used during planning was larger than the GTV and was referred to as the ITV. PTV was defined as a 2 to 4 mm margin around the ITV. A dose of 46.5 Gy (median; range, 33–60 Gy) was prescribed for an isodose line administration (70%–80% of the maximum dose) that covered at least 97% of the PTV and SBRT was delivered in 3 to 5 fractions over 1 to 2 weeks. The adopted normal tissue constraint was that of at least 700 mL of normal liver, and should not receive a total dose ≥17 Gy. For the spinal cord, the maximum dose should not exceed 22 Gy in 3 fractions and the volume of irradiated volume of spinal cord received >18 Gy in 3 fractions should be allowed to be .25 mL or less. For the esophagus, the maximum dose should not exceed 24 Gy. In addition, RT dosages to the stomach, intestine, and kidneys were restricted to the lowest level possible. Daily image guidance using orthogonal x-ray imaging or on-board CT was used to ensure accurate treatment delivery.
2.3. Follow-up and response evaluation
All patients were examined by a radiation oncologist to assess acute toxicity during their RT. Medical history, physical examinations, blood test, and CT or magnetic resonance imaging (MRI) were performed regularly at 2- or 3-month intervals after completion of the RT. Treatment-related toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 4.0). Radiation-induced hepatic toxicity (RIHT) was defined as an increase of at least 2 points in the CP score within 3 months after the completion of RT.[9]
The treatment response was assessed by CT scans using the Response Evaluation Criteria in Solid Tumor (RECIST) 3 months after the completion of RT. Local failure was defined as a recurrence in the treated lesion, and intrahepatic failure was defined as a recurrence within the liver outside the treated lesion. Any recurrence beyond the liver was defined as distant metastasis.
2.4. Statistical analysis
The primary endpoint was local progression-free survival (PFS); the secondary endpoints were overall PFS, overall survival (OS), and treatment-related toxicities. Local PFS, PFS, and OS were estimated by using the Kaplan-Meier method, with differences compared by using the log-rank test. Statistical significance was defined as a P value <.05; nonsignificant trend or borderline significance were defined as a P value <.1. The cut-off values of GTV and total dose were calculated using maximally selected chi-square test and these radiotherapeutic factors and clinical factors were also evaluated with the Kaplan–Meier survival analysis. Analysis of data was performed using SPSS software (Version 12.0; SPSS Inc, Chicago, IL) and R version 3.1.2 (R Development Core Team, Vienna, Austria).
3. Results
3.1. Patients
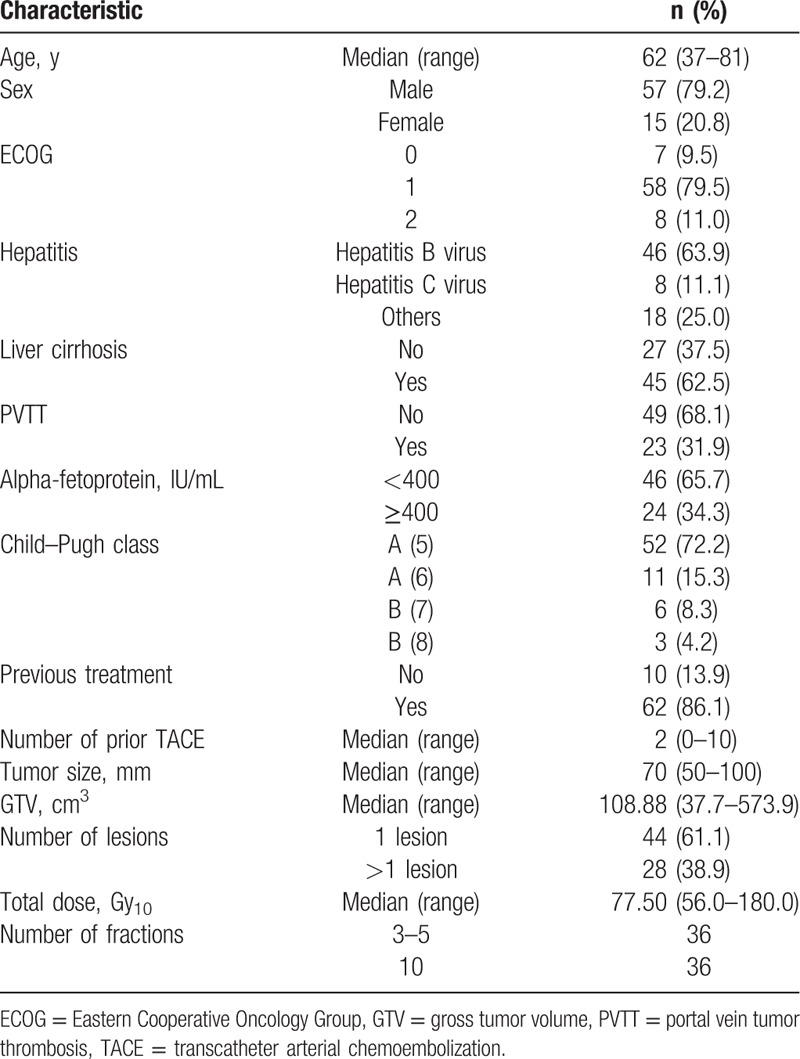
The patient and treatment characteristics of 72 patients are summarized in Table 1. The previous treatments of the 62 patients (86%) who were administered RT as salvage treatments were as follows: surgery in 4 patients, TACE in 58, RFA in 3, and PEI in 3 patients. The median tumor size was 7 cm. Thirty-six patients received HRT and the remaining were received SBRT. The median total dose translated to a biologically effective dose (BED) was 77.5 Gy10 (range, 56.0–180.0 Gy10) with an α/β ratio of 10.
Table 1.
Patient and treatment characteristics (n = 72).
3.2. Tumor response, patterns of failure, and survival outcome
The median follow-up duration after completion of RT was 12.8 months (range, .2–82.4 months). The tumor response at 3 months after RT based on the change in the maximum tumor size on CT or MRI was evaluated in 71 patients. Of these, 3 (4.2%) achieved a complete response and 35 (48.6%) had a partial response, yielding an overall response rate of 52.8%, according to the RECIST criteria. Stable disease was observed in 27 patients (37.5%), and progressive disease in 6 patients (8.3%). One patient who had liver transplantation within the 3-month period after RT was not evaluated. Intrahepatic outfield failure was the main cause of failure (37 of 72 patients). Eighteen patients presented with local recurrence and 16 patients had distant metastasis, most frequently at the lung (13 patients). Local PFS rates at 1 and 2 years were 57.0% and 39.0%, respectively, with a median of 13.6 months. The overall PFS rates at 1 and 2 years were 41.5% and 15.8%, respectively, with a median of 9.4 months. OS rates at 1 and 2 years were 70.1% and 45.2%, respectively, with a median of 21.1 months.
3.3. Variables affect survival outcome
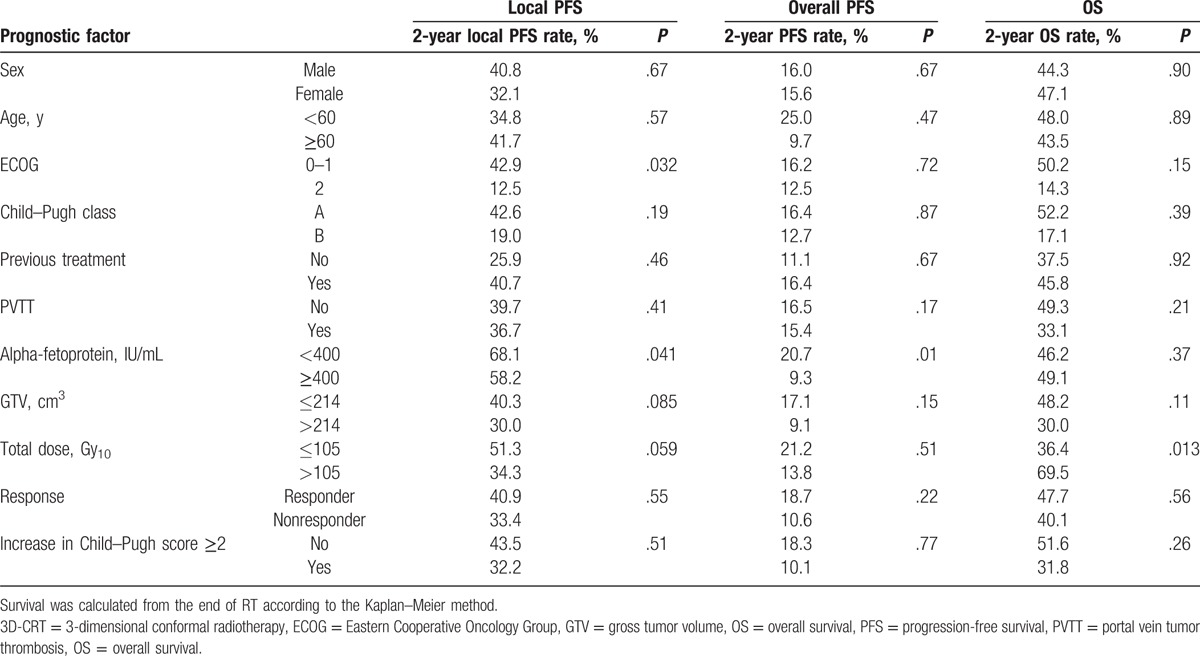
After completion of these analyses, we selected a GTV of 214 cm3 and a total dose of 105 Gy10 were identified as prognostic factors to affect local PFS by using a maximally selected chi-square test. The radiotherapeutic factors and clinical factors were also evaluated by Kaplan–Meier survival analysis. For local PFS, the ECOG (P = .032) and AFP (P = .041) were identified as significant prognostic factors and GTV (P = .085) and total dose (P = .059) showed borderline significance. For overall PFS, the AFP (P = .010) were identified as significant prognostic factors. For OS, total dose (P = .013) was identified as significant prognostic factors (Table 2).
Table 2.
Prognostic factors affecting outcomes.
3.4. Prognostic group based on GTV and total dose
Patients were divided into favorable (GTV ≤ 214 cm3 and total dose >105 Gy10), intermediate (GTV ≤ 214 cm3 and total dose ≤ 105 Gy10), and unfavorable prognostic groups (GTV >214 cm3 and total dose ≤ 105 Gy10) based on combination of GTV of 214 cm3 and a total dose of 105 Gy10. There was no patient who met the criteria of intermediate group (GTV >214 cm3 and total dose >105 Gy10). Table 3 showed local PFS, overall PFS, OS in these 3 groups. The favorable prognostic group consisted of 21 patients (29.2%), and showed 2 years local PFS rate and OS rate of 51.3% and 72.8%, respectively. The unfavorable prognostic group consisted of 11 patients (15.3%), and showed 2 years local PFS rate and OS rate of 30.0% (P = .020 for local PFS, P = .009 for OS) (Fig. 1).
Table 3.
Comparison of outcomes according to the combination of radiotherapeutic factors.
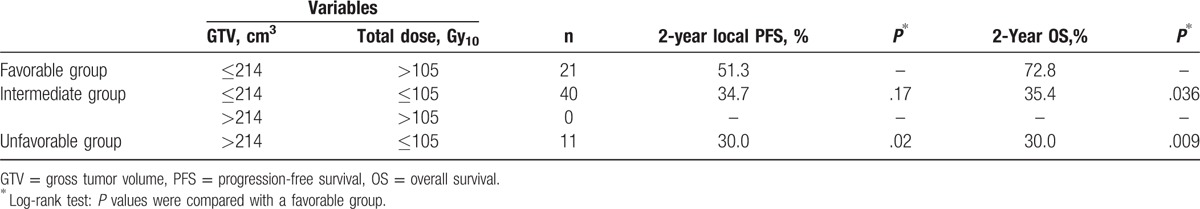
Figure 1.
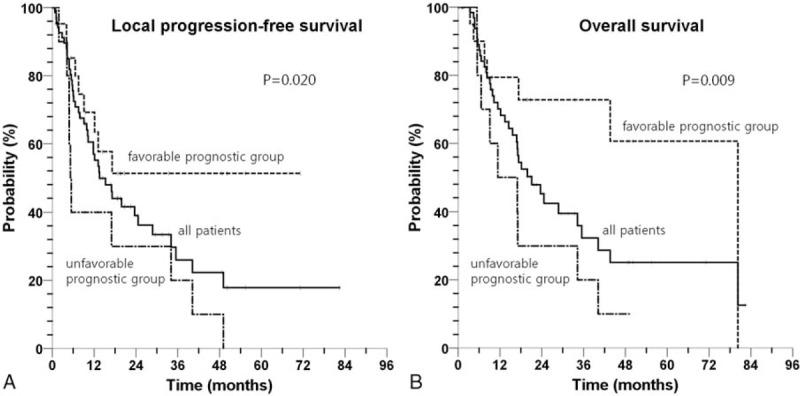
Kaplan–Meier survival curve of local progression-free survival (A) and overall survival (B) according to prognostic groups defined based on combination of a GTV of 214 cm3 and a total dose of 105 Gy10 (favorable prognostic group: GTV ≤ 214 cm3 and total dose >105 Gy10, unfavorable prognostic group: GTV > 214 cm3 and total dose ≤105 Gy10).
3.5. Treatment-related toxicity
All of our patients completed the planned RT without interruption associated with RT-related acute toxicity. An increase of at least 2 points in the CP score occurred in 21 patients (29.1%) within 3 months after the completion of RT. This radiation-related hepatic toxicity did not affect the patients’ survival outcomes (Table 2). The most common acute toxicities were grade 1or 2 constitutional symptoms (65%) and all improved without requiring specific management. One patient who received 51 Gy in 3 fractions experienced grade 4 gastrointestinal toxicity of gastroduodenal perforation and recovered after primary repair.
4. Discussion
Treatment options for patients with inoperable HCC lesions >5 cm are limited. These patients exceed the Milan criteria for liver transplantation (single HCC <5 cm or number ≤ 3 HCC < 3 cm).[2] For alternative curative therapy such as RFA, HCC >3 cm are usually too bulky, resulting in incomplete tumor ablation and a higher rate of local recurrence.[10] Large HCC has been found to result in more frequent microvascular invasion and a higher tumor grade, both of which usually lead to poor outcomes.[11] Yin et al[12] reported a local recurrence rate of 31.2% in tumors measuring 5 to 7 cm after RFA. According to the treatment plan of the Barcelona Liver Cancer Clinic algorithm, lesions >3 cm are intermediate or advanced stage HCCs and the recommendation is treatment with TACE, sorafenib, or inclusion in randomized control trials.[2] The challenge with large HCC is the poor response rate to standard treatments; therefore, the application of other local treatments such as RT might help improve treatment outcomes.[13] However, there is no consensus on optimal cut-off value of radiotherapeutic factors affecting treatment outcome in inoperable HCC lesions >5 cm. We found that a GTV of 214 cm3 and a total dose of 105 Gy10 as cut-off values of significant radiotherapeutic factors associated local PFS. Based on these factors, patients were divided into a favorable and an unfavorable prognostic group. Local PFS and OS were better in the favorable group than that in the unfavorable group (2-year local PFS rate: 51.3% vs 30.0%; 2-year OS rate: 72.8% vs 30.0%).
Previously, we reported that a PTV of 279 or 304 cm3 and a total dose of 60 Gy10 to be significantly associated with local PFS for patients with unresectable HCC who received total dose of 40 to 60 Gy with fraction size of 1.8 to 5 Gy.[14] The PTV of 279 and 304 cm3 were comparable with the GTV of approximately 6.5 to 7 cm. The present study included HCC ranging in size from 5 to 10 cm and our result of GTV of 214 cm3 are comparable with tumor diameter of 7 to 7.5 cm. Dawson[15] reported that the best outcomes after RT are found in patients with fewer than 3 lesion that are <6 cm in size with intact liver function. As for SBRT, Huang et al[16] reported that OS is significantly lower in patients with tumors >4 cm.
Historically, the role of RT has been limited to palliation in the treatment of HCC because of the liver's low tolerance to RT and the risk of RIHT.[7] However, with the invention of 3-dimensional conformal RT (3D-CRT), it is possible to minimize the irradiation of normal liver, and therefore facilitate an increased RT dose without a significant increase in toxicity.[17] In conventionally fractionated RT, improved local control (LC) and OS has been observed with doses exceeding 50 Gy.[18] Furthermore, achieving the intrahepatic tumor control after local therapy including 3D-CRT, HRT, and SBRT has been reported as the significant predictor of survival.[19] This means that a higher RT dose was shown to achieve a higher response rate and a higher survival rate.[2,18,20] Reports on dose-response relationship in HRT or SBRT for HCC are limited. Jang et al[7] revealed a dose-response relationship for LC and OS with SBRT and suggested SBRT dose of 54 Gy in 3 fractions (151.2 Gy10) with 2-year LC rate of 100% and 2-year OS rate of 71%.
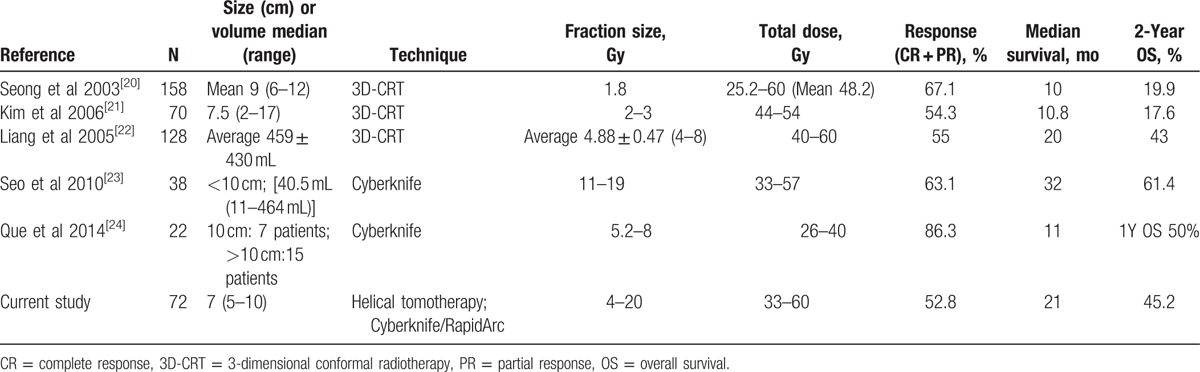
Although much progress has been made in RT and several studies have shown a dose-response relationship, there is no consensus with regard to the optimal dose-fractionation schedule on HCC. Currently, a variety of RT schedules are performed, such as hyperfractionation in a b.i.d. schedule, conventional fractionation, and hypofractionation.[18]Table 4 summarizes the treatment outcomes of the literature where RT (conventional RT vs HRT) was used, with the different fraction size for moderate- to large-sized unresectable HCC. Studies using conventional fractionated 3D-CRT for moderate- to large-sized HCC have relatively favorable response rates of 54% to 67% in the literature, but unsatisfactory survival outcomes.[20,21] Recent studies of hypofractionated 3D-CRT or SBRT have shown a higher response rate (range, 55%–86%) and higher survival rates (range, 11–32 months) compared to those of conventional fractionated 3D-CRT.[22–24] Liang et al[22] reported results of hypofractionated 3D-CRT, mostly in 4 to 6 Gy range per fraction and a total dose of 40 to 60 Gy. They found a tumor response rate of 55% and OS rates at 1 and 2 years of 65% and 45%, respectively. However, 15% of the patients developed RIHT. Seo et al[23] performed SBRT in 38 patients with <10 cm HCC. They found a 63% tumor response rate and a 61% 2-year OS rate. No RIHT or grade 4 toxicity was observed. Our study evaluated patients who had unresectable moderate-sized (5–10 cm) HCC treated with HRT or SBRT. The tumor response rate was 52.8%, and the 2-year OS was 45.2%, with a median survival of 21 months. Although caution must be taken regarding the interpretation of our clinical results due to the heterogeneity of patient and treatment characteristics, our results were more favorable in comparison to those of previous studies.
Table 4.
Comparison of the literature for radiotherapy in patients with moderate- to large-sized hepatocellular carcinoma.
In our study, intrahepatic outfield failure was the major pattern of failure, similar to previous findings.[8,25] This high intrahepatic recurrence rate may be explained by the multifocal nature of HCC in the cirrhotic liver and the advanced tumor stage. Most of our patients received locoregional therapies before RT, such as resection, TACE, RFA, or PEI. Fortunately, recent advances in systemic multikinase inhibitor therapy such as sorafenib, have been found to extend the survival in patients with HCC, and may enhance tumor radiation sensitivity.[26,27]
There were several limitations to this study, including its retrospective nature. The patients were not controlled in regard to variable prognostic factors, in particular, treatment undergone before RT. In addition, RT dose-fractionation schedules were mainly determined by physician's preference according to the tumor and patient characteristics and were not standardized. Furthermore, our patients were treated with different treatment technique including helical tomotherapy, CyberKnife, or RapidArc. The value of radiotherapeutic factors can be varied according to treatment planning technique.
5. Conclusions
In conclusion, higher-dose RT using HRT or SBRT can be delivered safely and showed feasibility, including substantial tumor regression and prolonged survival in moderate-sized HCC compared to conventional fractionated RT. The optimal cutoff values of GTV ≤ 214 cm3 and total dose >105 Gy10 may be useful for predicting survival outcomes after RT. Further prospective randomized trials are required to confirm the optimal criteria of radiotherapeutic factors and to evaluate the efficacy of a combination of HRT or SBRT.
Acknowledgments
The authors thank the clinical research coordinating center of catholic medical center for statistical assistance that greatly improved the manuscript.
Footnotes
Abbreviations: BED = biologically effective dose, CP = Child–Pugh, CT = computed tomography, 3D-CRT = 3-dimensional conformal radiotherapy, ECOG = Eastern Cooperative Oncology Group, GTV = gross tumor volume, HCC = hepatocellular carcinoma, HRT = hypofractionated radiotherapy, ITV = internal target volume, LC = local control, MRI = magnetic resonance imaging, OS = overall survival, PEI = percutaneous ethanol injection, PFS = progression-free survival, PTV = planning target volume, RECIST = Response Evaluation Criteria in Solid Tumor, RFA = radiofrequency ablation, RIHT = radiation-induced hepatic toxicity, RT = radiotherapy, SBRT = stereotactic body radiotherapy, TACE = transcatheter arterial chemoembolization.
The authors have no conflicts of interest to disclose.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013;87:22–32. [DOI] [PubMed] [Google Scholar]
- [3].Sanuki N. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol 2014;20:3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collettini F, Schnapauff D, Poellinger A, et al. Hepatocellular carcinoma: computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5-7 cm) and very large (>7 cm) tumours. Eur Radiol 2011;22:1101–9. [DOI] [PubMed] [Google Scholar]
- [5].Yoon SM, Lim Y-S, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One 2013;8:e79854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choi BO. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jap J Clin Oncol 2006;36:154–8. [DOI] [PubMed] [Google Scholar]
- [7].Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol 2013;8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park JH, Yoon SM, Lim YS, et al. Two-week schedule of hypofractionated radiotherapy as a local salvage treatment for small hepatocellular carcinoma. J Gastroenterol Hepatol 2013;28:1638–42. [DOI] [PubMed] [Google Scholar]
- [9].Son SH, Jang HS, Lee H, et al. Determination of the alpha/beta ratio for the normal liver on the basis of radiation-induced hepatic toxicities in patients with hepatocellular carcinoma. Radiat Oncol 2013;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ayav A, Germain A, Marchal F, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg 2010;200:435–9. [DOI] [PubMed] [Google Scholar]
- [11].Pawlik TM, Delman KA, Vauthey J-N, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplant 2005;11:1086–92. [DOI] [PubMed] [Google Scholar]
- [12].Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 2009;115:1914–23. [DOI] [PubMed] [Google Scholar]
- [13].Kim KH, Kim MS, Chang JS, et al. Therapeutic benefit of radiotherapy in huge (≥10 cm) unresectable hepatocellular carcinoma. Liver Int 2014;34:784–94. [DOI] [PubMed] [Google Scholar]
- [14].Son SH, Jang HS, Sung SY, et al. Identifying the optimal criteria of radiotherapeutic parameters for patients with unresectable locally advanced hepatocellular carcinoma. Oncotarget 2015;6:42372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dawson LA. Overview: where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol 2011;21:241–6. [DOI] [PubMed] [Google Scholar]
- [16].Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355–61. [DOI] [PubMed] [Google Scholar]
- [17].Kim JY, Yoo EJ, Jang JW, et al. Hypofractionated radiotheapy using helical tomotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Radiat Oncol 2013;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seong J, Lee IJ, Shim SJ, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 2009;29:147–52. [DOI] [PubMed] [Google Scholar]
- [19].Jung SM, Jang JW, You CR, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol 2012;27:684–9. [DOI] [PubMed] [Google Scholar]
- [20].Seong J, Park H, Han K, et al. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 2003;55:329–36. [DOI] [PubMed] [Google Scholar]
- [21].Kim TH, Kim DY, Park J-W, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol 2006;29:568–75. [DOI] [PubMed] [Google Scholar]
- [22].Liang S-X, Zhu X-D, Lu H-J, et al. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer 2005;103:2181–8. [DOI] [PubMed] [Google Scholar]
- [23].Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 2010;102:209–14. [DOI] [PubMed] [Google Scholar]
- [24].Que JY, Lin LC, Lin KL, et al. The efficacy of stereotactic body radiation therapy on huge hepatocellular carcinoma unsuitable for other local modalities. Radiat Oncol 2014;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:e603–7. [DOI] [PubMed] [Google Scholar]
- [26].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [27].Yu W, Gu K, Yu Z, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett 2013;329:109–17. [DOI] [PubMed] [Google Scholar]


